Enhancing Medication Safety through Implementing the Qatar Tool for Reducing Inappropriate Medication (QTRIM) in Ambulatory Older Adults
Abstract
1. Plain Language Summary
2. Introduction
3. Materials and Methods
3.1. Study Design and Setting
3.2. Intervention Development
3.3. Implementation Process
3.4. Data Collection and Documentation
3.5. Key Performance Indicator (KPI) Measures
4. Results
4.1. Process Measures (Clinical Interventions Documentation)
4.1.1. Rumailah Hospital Geriatric Outpatient Pharmacy
4.1.2. Challenges Documented in Clinical Intervention
4.2. Outcome Measures
RH OP and Dermatology OP PIM Prescription Dispensing Rate/1000 Orders
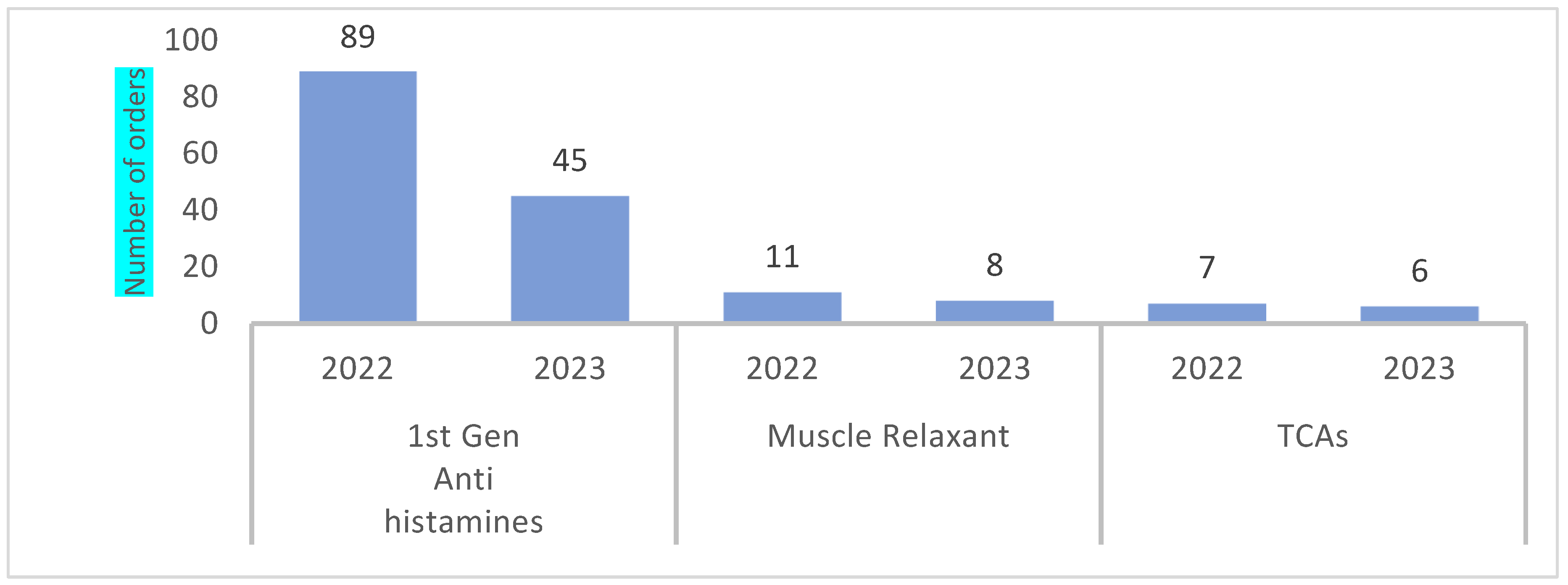
4.3. Distribution of PIMs by Prescribers Locations
5. Discussion
5.1. Multifaceted Approaches
5.2. Integrating with EHRs
5.3. Challenges and Future Plan
5.4. Performance Quality Indicators
5.5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelton, P.S.; Fritsch, M.A.; Scott, M.A. Assessing medication appropriateness in the elderly: A review of available measures. Drugs Aging 2000, 16, 437–450. [Google Scholar] [CrossRef]
- Cadogan, C.A.; Ryan, C.; Hughes, C.M. Appropriate Polypharmacy and Medicine Safety: When Many is not Too Many. Drug Saf. 2016, 39, 109–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rantsi, M.; Hyttinen, V.; Jyrkkä, J.; Vartiainen, A.-K.; Kankaanpää, E. Process evaluation of implementation strategies to reduce potentially inappropriate medication prescribing in older population: A scoping review. Res. Soc. Adm. Pharm. 2022, 18, 2367–2391. [Google Scholar] [CrossRef]
- Poudel, A.; Peel, N.; Mitchell, C.; Nissen, L.; Hubbard, R. A systematic review of prescribing criteria to evaluate appro-priateness of medications in frail older people. Rev. Clin. Gerontol. 2014, 24, 304–318. [Google Scholar] [CrossRef]
- Tian, F.; Chen, Z.; Zeng, Y.; Feng, Q.; Chen, X. Prevalence of Use of Potentially Inappropriate Medications among Older Adults Worldwide: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2326910. [Google Scholar] [CrossRef]
- Alyazeedi, A.; Algendy, A.F.; Sharabash, M.; Karawia, A. Prevalence, determinants, and associated risk of potentially inappropriate prescribing for older adults in Qatar: A national retrospective study. Clin. Interv. Aging 2019, 14, 1889–1899. [Google Scholar] [CrossRef]
- American Geriatrics Society. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medi-cation Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Gallagher, P.; Lang, P.O.; Cherubini, A.; Topinková, E.; Cruz-Jentoft, A.; Errasquín, B.M.; Mádlová, P.; Gasperini, B.; Hilde, B.; Baeyens, J.-P.; et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur. J. Clin. Pharmacol. 2011, 67, 1175–1188. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy; WHO/UHC/SDS/2019.11; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Farrell, B.; Raman-Wilms, L.; Sadowski, C.A.; Mallery, L.; Turner, J.; Gagnon, C.; Cole, M.; Grill, A.; Isenor, J.E.; Mangin, D.; et al. A Proposed Curricular Framework for an Interprofessional Approach to Deprescribing. Med. Sci. Educ. 2023, 33, 551–567. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Plácido, A.I.; Tavares, A.B.; Azevedo, D.; Mateos-Campos, R.; Figueiras, A.; Herdeiro, M.T.; Roque, F. Potentially Inappropriate Medication Prescribing in Older Adults According to EU (7)-Potentially Inappropriate Medication List: A Nationwide Study in Portugal. Curr. Ther. Res. Clin. Exp. 2022, 97, 100681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mucherino, S.; Casula, M.; Galimberti, F.; Guarino, I.; Olmastroni, E.; Tragni, E.; Orlando, V.; Menditto, E.; EDU.RE.DRUG Group. The Effectiveness of Interventions to Evaluate and Reduce Healthcare Costs of Potentially Inappropriate Prescriptions among the Older Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6724. [Google Scholar] [CrossRef]
- World Health Organization. Medication without Harm: WHO’s Third Global Patient Safety Challenge; WHO: Geneva, Switzerland, 2017.
- Rodrigues, D.A.; Plácido, A.I.; Mateos-Campos, R.; Figueiras, A.; Herdeiro, M.T.; Roque, F. Effectiveness of Interventions to Reduce Potentially Inappropriate Medication in Older Patients: A Systematic Review. Front. Pharmacol. 2022, 12, 777655. [Google Scholar] [CrossRef]
- Hovstadius, B.; Petersson, G.; Hellström, L.; Ericson, L. Trends in inappropriate drug therapy prescription in the elderly in Sweden from 2006 to 2013: Assessment using national indicators. Drugs Aging 2014, 31, 379–386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friebe, M.P.; LeGrand, J.R.; Shepherd, B.E.; Breeden, E.A.; Nelson, S.D. Reducing Inappropriate Outpatient Medication Prescribing in Older Adults across Electronic Health Record Systems. Appl. Clin. Inform. 2020, 11, 865–872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niehoff, K.M.; Rajeevan, N.; Charpentier, P.A.; Miller, P.L.; Goldstein, M.K.; Fried, T.R. Development of the Tool to Reduce Inappropri-ate Medications (TRIM): A Clinical Decision Support System to Improve Medication Prescribing for Older Adults. Pharmacotherapy 2016, 36, 694–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Indicators for Evaluation of Drug Therapy in the Elderly; The National Board of Health and Welfare: Stockholm, Sweden, 2003; p. 76.
- Komagamine, J.; Sugawara, K.; Hagane, K. Characteristics of elderly patients with polypharmacy who refuse to participate in an in-hospital deprescribing intervention: A retrospective cross-sectional study. BMC Geriatr. 2018, 18, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallagher, P.; Ryan, C.; Byrne, S.; Kennedy, J.; O’Mahony, D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2013, 51, 946–955. [Google Scholar]
- Linsky, A.M.; Kressin, N.R.; Stolzmann, K.; Pendergast, J.; Rosen, A.K.; Bokhour, B.G.; Simon, S.R. Direct-to-consumer strategies to promote deprescribing in primary care: A pilot study. BMC Prim. Care 2022, 23, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Barry, M. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Barry, P.J.; Gallagher, P.; Ryan, C.; O’Mahony, D.; Gallagher, J. START (screening tool to alert doctors to the right treatment)—An evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing 2012, 41, 242–248. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Schmader, K.E.; Samsa, G.P.; Weinberger, M.; Uttech, K.M.; Lewis, I.K.; Cohen, H.J. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 2011, 52, 631–638. [Google Scholar] [CrossRef] [PubMed]





| Class | Items | Rationale for Not Prescribing | Potential Alternatives |
|---|---|---|---|
| TCA | Amitriptyline | Strong anticholinergic properties and potential for sedation and orthostatic hypotension, syncope, bradycardia, syndrome of antidiuretic hormone secretion (SIADH), or hyponatremia. | Neuropathic pain: gabapentin, pregabalin, and duloxetine (mainly if depression exists). Depression: SNRIs, SSRIs except paroxetine, and low dose of mirtazapine. |
| Imipramine | Migraine and headache prevention; for other migraine prophylaxis, consider targeting other comorbidities (antidepressants, antihypertensives, and anticonvulsants). | ||
| Clomipramine, Trimipramine | |||
| Muscle Relaxants | Orphenadrine | Older adults poorly tolerate most muscle relaxants due to anticholinergic effects caused by some muscle relaxants, risk of sedation, delirium, and an increased risk of fracture. In addition, efficacy is questionable at doses tolerated by geriatric patients. | Non-pharmacological interventions, such as paracetamol; local applications/injections. And, other pain medications according to pain type, location, duration, and intensity. |
| NSAIDs | Avoid due to increased risk of gastrointestinal bleeding/peptic ulcer disease and acute kidney injury in older adults. Indomethacin is more likely than other NSAIDs to have adverse CNS effects. Of all the NSAIDs, indomethacin has the most damaging effects. | Non-pharmacological interventions, such as paracetamol; LAs, local injections, and other pain medications according to pain type, location, duration, and intensity. | |
| Ketorolac | If there is no practical alternative, use low-dose selective COXII-NSAIDs (such as celecoxib and etoricoxib) for the shortest period, along with PPI. | ||
| Indomethacin | |||
| First-Generation Antihistamines | Chlorpheniramine, cyproheptadine, and diphenhydramine (oral, hydroxyzine, clemastine, and promethazine | Potent anticholinergic properties, resulting in an increased risk of confusion/delirium, dry mouth, and constipation; use should also be avoided due to reduced clearance with advanced age and tolerance associated with use as a hypnotic. | Allergy: non-sedating, non-anticholinergic antihistamines like desloratadine and levocetirizine. Sleep disturbances: see BZD below. Nausea: treat the cause; consider ondansetron if indicated. |
| Dystonia including EPS: diphenhydramine injection. | |||
| Insulins | Aspart, glulisine, lispro, and regular in the absence of basal/intermediate insulin for chronic DM management | There is a higher risk of hypoglycemia associated with sliding-scale insulin without improvements in hyperglycemia, regardless of the care setting. | Tailored diabetes management plan considering antidiabetics with low hypoglycemic risk, such as metformin, gliptins, and cardioprotective agents, particularly for cardiac patients (gliflozins and GLP1 agonists). Consider adding basal/intermediate insulin to fast-/short-acting insulin. Consider decreasing the current dose of insulin when starting the new antidiabetics. |
| SSRIs (Selective Serotonin Reuptake Inhibitors) | Paroxetine | Strong anticholinergic properties and potential for sedation and orthostatic hypotension, falls or fractures, ataxia, impaired psychomotor function, syncope, and cause or exacerbate syndrome of inappropriate antidiuretic hormone secretion or hyponatremia. | Consider non-pharmacological interventions, other SSRIs, SNRIs, and low-dose mirtazapine,3 with appropriate monitoring of falls, ECG, and electrolytes. |
| Anticholinergics Antiparkinsonians | Procyclidine | Strong anticholinergic properties, and not recommended for the prevention of extrapyramidal symptoms with antipsychotics. In the treatment of Parkinson‘s disease, more effective agents are available. | Parkinson’s: Consider adding or adjusting dopaminergic medications (particularly levodopa/carbidopa if indicated). |
| Trihexyphenidyl | Dystonia including EPS: the HMC formulary includes a diphenhydramine injection. | ||
| Benztropine (oral) | |||
| Antispasmodics | Clidinium chlordiazepoxide, diphenoxylate and atropine, and scopolamine (excludes ophthalmic) | Highly anticholinergic properties and uncertain effectiveness as an antispasmodic. Chlordiazepoxide increases the risk of impaired cognition, delirium, falls, and fractures and has a slower metabolism in older adults. | Treating the cause: if an antispasmodic is required, consider a drug with lower anticholinergic properties, such as mebeverine. |
| Benzodiazepines | Alprazolam | Increased risk of impaired cognition, delirium, falls, fractures, and motor vehicle accidents with benzodiazepine use. | Sleep disturbance: non-pharmacological interventions, such as melatonin, low doses of mirtazapine (7.5–15 mg/d), and low-dose trazodone (25–50 mg/d). |
| Temazepam | Anxiety: antidepressants with an anxiolytic profile (SSRIs except paroxetine). | ||
| Cardiovascular Medications | Nifedipine immediate release and methyldopa | Nifedipine: the potential to cause hypotension and risk for precipitating myocardial ischemia. | Sustained-release nifedipine or other CCB may be used if indicated; other safer antihypertensive initiation or intensification (such as CBB, and ACEIs/ARBs). |
| Methyldopa: high risk of CNS ADRs and risk of bradycardia and orthostatic hypotension. |
| Process Measure | Definition | The percentage of PIM orders dispensed from the pharmacy with documented pharmacist intervention. |
| Numerator | The number of monthly PIM orders dispensed from the pharmacy with documented pharmacist intervention. | |
| Denominator | The total number of PIM orders dispensed from the pharmacy to older adults in a calendar month. | |
| Outcome Measure | Definition | The rate of PIMs dispensed from the pharmacy to older adults in a calendar month. |
| Numerator | The number of monthly PIM orders dispensed from the pharmacy to older adults in a calendar month multiplied by a standard population. | |
| Denominator | The total number of orders dispensed by a pharmacy to older adults in a calendar month. |
| Year Total Orders | 2022 | 2023 | Total | |
|---|---|---|---|---|
| N = 220 | N = 117 | N = 337 | ||
| Age, Mean (SD) | 70 (7.13) | 70 (6.90) | 70 (7.04) | |
| Sex, n (%) | ||||
| Female | 100 (45%) | 55 (47%) | 155 (46%) | |
| Male | 120 (55%) | 62 (53%) | 182 (54%) | |
| Dispense Location, n (%) | ||||
| RH Dermatology OP Pharmacy | 114 (52%) | 63 (54%) | 177 (53%) | |
| RH OP Pharmacy | 106 (48%) | 54 (46%) | 160 (47%) | |
| PIM, n (%) | PIM | |||
| ALPRAZolam | 4 (2%) | 3 (3%) | 7 (2%) | |
| Hyoscine N Butyl Bromide | 12 (5%) | 6 (5%) | 18 (5%) | |
| PARoxetine | 2 (1%) | 1 (1%) | 3 (1%) | |
| Amitriptyline | 29 (13%) | 14 (12%) | 43 (13%) | |
| Chlorpheniramine | 3 (1%) | 2 (2%) | 5 (1%) | |
| ClomiPRAMINE | 7 (3%) | 1 (1%) | 8 (2%) | |
| Cyproheptadine | 1 (0%) | 1 (1%) | 2 (1%) | |
| DiphenhydrAMINE/NH4Cl/Na Citrate/menthoL | 8 (4%) | 0 (0%) | 8 (2%) | |
| HydrOXYzine | 106 (48%) | 54 (46%) | 160 (47%) | |
| Imipramine | 1 (0%) | 1 (1%) | 2 (1%) | |
| Paracetamol-orphenadrine450/35 | 47 (21%) | 32 (27%) | 79 (23%) | |
| Promethazine hydrochloride | 0 (0%) | 2 (2%) | 2 (1%) | |
| Race, n (%) | ||||
| Arab | 147 (67%) | 83 (71%) | 230 (68%) | |
| Asian | 43 (20%) | 25 (21%) | 68 (20%) | |
| Black | 20 (9%) | 6 (5%) | 26 (8%) | |
| Persian | 5 (2%) | 1 (1%) | 6 (2%) | |
| White | 5 (2%) | 2 (2%) | 7 (2%) | |
| Therapeutic Class, n (%) | Therapeutic Class | |||
| First-Gen Antihistamines | 118 (54%) | 59 (50%) | 177 (53%) | |
| Antispasmodics | 12 (5%) | 6 (5%) | 18 (5%) | |
| Benzodiazepines | 4 (2%) | 3 (3%) | 7 (2%) | |
| Muscle Relaxants | 47 (21%) | 32 (27%) | 79 (23%) | |
| SSRIs | 2 (1%) | 1 (1%) | 3 (1%) | |
| TCAs | 37 (17%) | 16 (14%) | 53 (16%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyazeedi, A.; Sherbash, M.; Algendy, A.F.; Stewart, C.; Soiza, R.L.; Alhail, M.; Aldarwish, A.; Stewart, D.; Awaisu, A.; Ryan, C.; et al. Enhancing Medication Safety through Implementing the Qatar Tool for Reducing Inappropriate Medication (QTRIM) in Ambulatory Older Adults. Healthcare 2024, 12, 1186. https://doi.org/10.3390/healthcare12121186
Alyazeedi A, Sherbash M, Algendy AF, Stewart C, Soiza RL, Alhail M, Aldarwish A, Stewart D, Awaisu A, Ryan C, et al. Enhancing Medication Safety through Implementing the Qatar Tool for Reducing Inappropriate Medication (QTRIM) in Ambulatory Older Adults. Healthcare. 2024; 12(12):1186. https://doi.org/10.3390/healthcare12121186
Chicago/Turabian StyleAlyazeedi, Ameena, Mohamed Sherbash, Ahmed Fouad Algendy, Carrie Stewart, Roy L. Soiza, Moza Alhail, Abdulaziz Aldarwish, Derek Stewart, Ahmed Awaisu, Cristin Ryan, and et al. 2024. "Enhancing Medication Safety through Implementing the Qatar Tool for Reducing Inappropriate Medication (QTRIM) in Ambulatory Older Adults" Healthcare 12, no. 12: 1186. https://doi.org/10.3390/healthcare12121186
APA StyleAlyazeedi, A., Sherbash, M., Algendy, A. F., Stewart, C., Soiza, R. L., Alhail, M., Aldarwish, A., Stewart, D., Awaisu, A., Ryan, C., & Myint, P. K. (2024). Enhancing Medication Safety through Implementing the Qatar Tool for Reducing Inappropriate Medication (QTRIM) in Ambulatory Older Adults. Healthcare, 12(12), 1186. https://doi.org/10.3390/healthcare12121186










