The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
The Role of Steroid Hormones in Hepatic Inflammation and Fibrosis
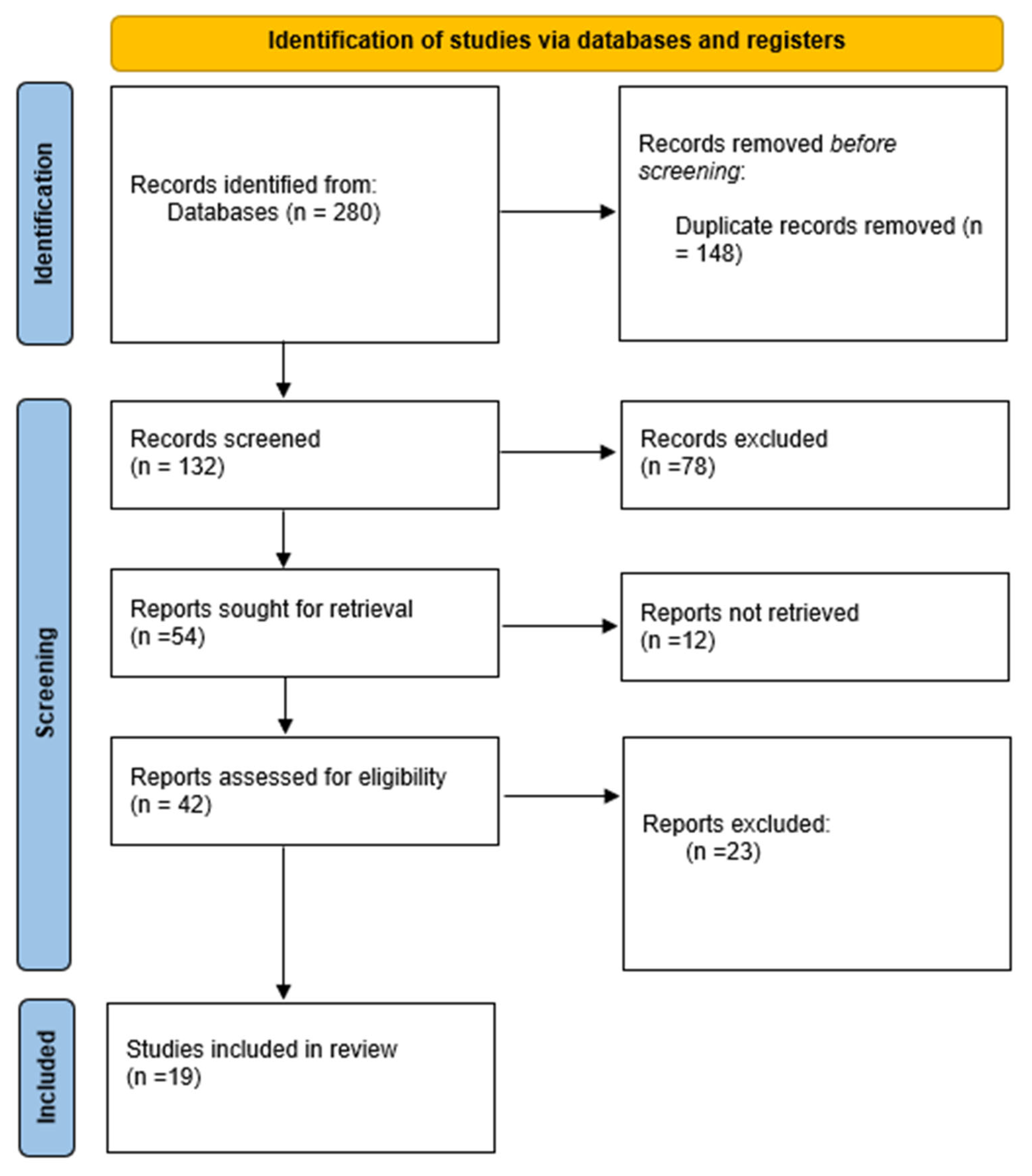
2. Materials and Methods
3. Results
3.1. Sample and Outcomes Regarding Menopausal Status
3.2. Insulin Resistance, BMI and Other Factors
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| References Study/Country Where the Study Was Performed | Number of Patients | Recruitment | Results |
|---|---|---|---|
| De Souza Bruno et al., 2014 [66] Cross-sectional study Brazil | 188 women (age ≥ 45 years and amenorrhea ≥ 12 months) Adjustments in age and weight | Population composed of postmenopausal women, aged 45–70 years, attending a public outpatient center in south-eastern Brazil from July 2011 to August 2012. | Of the 188 women, 73 (38.8%) had NAFLD. Blood pressure, waist circumference, body mass index, LDL cholesterol, triglycerides and glucose were significantly higher in NAFLD patients compared with women without NAFLD (control group) (p < 0.05). HOMA-IR values indicated insulin resistance only in the NAFLD group (6.1 ± 4.6 vs. 2.4 ± 1.4 in control group, p < 0.05). |
| Wang et al., 2015 [40] Cross-sectional study China | 9360 women Adjusted for abdominal obesity, diabetes, lipid profile, and systolic blood pressure | Between February 2014 and June 2014, 6899 subjects who were 18 to 93 years old were recruited in the SPECT-China study from 16 sites in Shanghai, Zhejiang, and Jiangxi Province. | The prevalence of NAFLD increased from 5.3% to 18.8% in women younger than 45 years versus women aged 45 to 55 years and rose to 27.8% in women older than 55 years. In obese women, the prevalence of NAFLD was 48.4%. |
| Gutierrez-Grobe et al., 2010 [41] Cross-sectional study Mexico | 197 who agreed to participate were divided into groups, comprising 93 with NAFLD and without NAFLD MS analysis of weight, height, BMI, waist, waist to hip ratio and percentage of body fat | Patients that went for check-ups at the unit of the Diagnostic Clinic at the Medica Sur Clinic & Foundation (University Hospital) between February 2009 and December 2009. | Of the 197 patients, 93(47.2%) had NAFLD and 104 (52.8%) did not have NAFLD. The prevalence of NAFLD in premenopausal, postmenopausal, and PCOS patients was 32.2, 57.9, and 62%, respectively. Age, BMI, hip to waist ratio, fasting glucose, HOMA-IR, and insulin were significantly higher in NAFLD patients. Women without NAFLD had significantly higher levels of serum estradiol (100 ± 95.4) compared with NAFLD patients (55.5 ± 66.6) p = 0.001. By group with and without NAFLD: premenopausal (55.44 ± 93.3 vs. 128.56 ± 109.22), postmenopausal (44.98 ± 51.41 vs. 42.72 ± 51.48) and PCOS women (64.9 ± 53.3 vs. 101.36 ± 80.89) had significantly different hormone profile. |
| Ryu et al., 2015 [42] Cross-sectional analysis Korea | 1559 women aged 44–56 years Adjusting for age, center, BMI, smoking status, alcohol intake, physical activity, and educational level | The study population consisted of women, aged 44–56 years old, who underwent a comprehensive health examination at the Kangbuk Samsung Hospital Total Healthcare Center in Seoul and Suwon, South Korea from November 2012 to March 2013. | Of the 1559 women, 334 had NAFLD. A higher prevalence of NAFLD was observed across menopausal stages (p for a trend < 0.05). After adjusting for age, center, BMI, smoking status, alcohol intake, physical activity, educational level, parity, and age at menarche, the odds ratios (95% CIs) for NAFLD comparing early transition, late transition, and postmenopause to pre-menopause were 1.07 (0.68–1.67), 1.87 (1.23–2.85), and 1.67 (1.01–2.78), respectively. |
| Florentino et al., 2013 [43] Cross-sectional study Brazil | 251 postmenopausal women | From April 2009 to April 2011, 251 women were included and evaluated coming from the National Health System from Northeast of Brazil, who reported the use of HRT(G1) or who denied the use (G2). | Prevalence of NAFLD was 37.1% (93/251) in postmenopausal women, 26, 4% (14/53) in the group with hormone replacement therapy and 39, 9% (79/198) without hormone replacement therapy. Insulin resistance (homeostatic model assessment of insulin resistance ≥ 3) (p < 0.05) was higher in the group of women with NAFLD diagnosis who did not refer to the use of hormone replacement therapy. |
| Park et al., 2020 [55] Cross-sectional study Korea | 4354 postmenopausal women Early, normal, and late menopause were defined as age at menopause <45 years, 45–54 years, and ≥55 years, respectively. Age, BMI, ALT, high FBG, HDL, LDL, TG, HOMA-IR, HT, GGT, HTN | Women who participated in the 2010–2012 Korea National Health and Nutrition Examination Survey. | When compared with normal menopausal women, early or late menopausal women had no significant differences in the odds ratios (ORs) of NAFLD: OR = 1.05, 95% confidence interval (CI), 0.83–1.32 and OR = 1.02, 95% CI, 0.75–1.39, respectively. The prevalence of advanced fibrosis was 2.1% (95% CI, 0.7–6.4%), 2.2% (95% CI, 1.3–3.8%), and 3.9% (95% CI, 1.2–12.2%) in early, normal, and late menopausal women, respectively. |
| Veronese et al., 2018 [56] Cross-sectional study Italy | 752 women in menopause and 535 in pre-menopause | The years from menopause were not associated with the severity of liver steatosis in NAFL (p for trend = 0.74; Spearman correlation = 0.04; 95%CI: −0.09 to 0.17), whereas all the indexes of adiposity and the number of metabolic syndrome factors were associated with a higher liver steatosis score. | |
| Park et al., 2006 [45] Cross-sectional study Korea Sonography | 6648 subjects, all of whom were older than 20 years of age (3530 men and 3118 women) | Subjects who underwent health screenings at Kangbuk Samsung Hospital, at the Sungkyunkwan University School of Medicine, from January to December 2003. | Factors associated with significantly increased risks of NAFLD among women included age, menopause, estrogen medication, and low HDL-C. |
| Hamaguchi et al., 2012 [44] Prospective study Japan | 1829 women and 2572 men Adjusted for age, metabolic syndrome, and weight gain. | All participants who were examined in health checkup programs between January and December 2001. | The incidence of NAFLD was 3.5% (28/802) in premenopausal women, 7.5% (4/53) in menopausal women, 6.1% (24/392) in postmenopausal women, and 5.3% (11/206) in women receiving hormone replacement therapy. |
| Volzke et al., 2007 [46] Cross-sectional population-based survey in northeast Germany. | 808 women aged 40–59 years without seropositivity to hepatitis B antigen and anti-hepatitis C virus. | Participants were identified from the population-based survey in Northeast Germany during June 2012 to February 2013. Adjusted for age, smoking, BMI, DM, W/H ratio, LDL, HT | Descriptive statistics showed the association between menopausal status and liver-related characteristics, which remained stable after adjustment for age. |
| Arshad et al., 2019 [47] | 13,559 female subjects | NHANES Adjusting for age and BMI | Unadjusted NAFLD prevalence increased from 18.54% (1988–1994) to 21.36% (1999–2006) to 24.86% (2007–2014) (Arshad et al., 2019). The age-standardized prevalence of NAFLD in this cohort increased from 20.96% (1988–1994) to 26.19% (2007–2014). The age-adjusted prevalence of cardiovascular disease (CVD), 5-year all-cause mortality, and 5-year cardiovascular mortality were significantly higher in women with NAFLD in this cohort compared with women without NAFLD. |
| Setroame et al., 2020 [39] Cross-sectional study Ghana | 185 participants: 88 premenopausal and 97 postmenopausal women | A cross-sectional study was conducted at the Out Patient Department (OPD) of Ho Teaching Hospital (HTH) and Ho Municipal Hospital in Ho, Ghana, from November 2018 to January 2019 | The prevalence of NAFLD among postmenopausal women was 49.48% higher than the 29.55% observed among premenopausal women. |
| Chen et al., 2019 [48] Cross-sectional study China | Menstrual period (n = 3404) Menopause transition period (n = 724) Postmenopausal period (n = 1915) | Employees and retirees recruited from the Jidong and Kailuan communities (Tangshan City, northern China) from 2010 to 2014. Age, BMI, DM, HTN, HLD, TG, LDL, HDL, WC | The adjusted odds ratios (ORs) with 95% confidence interval (CI) for NAFLD among participants in the menopause transition period and postmenopausal period were 1.10 (0.89–1.37) and 1.28 (1.04–1.58), respectively, compared with the women with menstrual periods. |
| Chung et al., 2015 [49] Retrospective cohort study | Premenopausal women: 728 Postmenopausalwomen: 695 | Women who underwent abdominal ultrasonography (US) and blood samplings at the Seoul National University Hospital Gangnam Healthcare Center, Seoul, Republic of Korea, for routine health check-ups in 2010 were recruited. Age, HTN, DM, HDL, smoking, HOMA, TG, W/H ratio, ALT | The prevalence of NAFLD was higher in postmenopausal women than in premenopausal women (27.2% versus 14.4%, p < 0.001). In premenopausal women, low HDL-cholesterol, central obesity, and homeostasis model assessment-estimated insulin resistance showed a significant association with the increased risk of NAFLD in multivariate analysis. In postmenopausal women, the presence of diabetes, triglyceridemia, and central obesity showed a significant association with the risk of NAFLD. The presence of menopause and hormone replacement therapy in postmenopausal women was not risk factors for NAFLD. |
| Bao et al., 2020 [50] Cross-sectional study China | 4323 female individuals were enrolled | Subjects who underwent routine health examinations at the Health Management Center of the West China Hospital of Sichuan University from January 2018 through December 2018. Finding persists among different obesity statuses | Regardless of whether the subjects were non-obese or obese, the prevalence of NAFLD was lower in non-menopausal subjects than in postmenopausal subjects (non-obese: 20.74% vs. 45.26%, respectively, p < 0.0001). |
| Sanghavi et al., 2013 [51] USA | 1018 Women aged 30–65 from the Dallas Heart Study (DHS) 48% were postmenopausal | Multiethnic population-based sample adjusting for age, race, lipids, smoking, blood pressure, diabetes, HOMA-IR, CRP and BMI | Postmenopausal status was associated with an increased prevalence of hepatic steatosis (34% vs. 24%, p < 0.001). |
| Yang et al., 2014 [52] Cross-sectional study USA | 541 adult patients diagnosed with NASH 36.5% postmenopausal women | Among the 541 patients, 338 patients (62%) were enrolled at Duke Liver Clinic while 203 patients (38%) were enrolled in the Duke Metabolic and Weight Loss Surgery Program. | Postmenopausal women and men had 1.6 [95%CI, 1.0–2.4], 1.7 [95%CI, 1.1–2.6]-fold increased risk of having greater severity of liver fibrosis than premenopausal women, respectively. BMI was 44.6 and 39.2 for pre- and postmenopausal woman, respectively. |
| Yoneda et al., 2011 [53] Multicenter retrospective cohort study | 419 women with biopsy-proven NAFLD were included; the study comprised 90 premenopausal and 329 postmenopausal patients. | Japan Study Group of NAFLD (JSG-NAFLD) database | Even in non-obese NAFLD patients, postmenopausal women still had more severe fibrosis compared to the premenopausal subjects (P50.0266; odds ratio [OR]: 2.173), after adjusting for hepatic inflammation, ballooning hepatocytes, BMI, impaired glucose tolerance/diabetes, and hypertension. |
References
- Adams, L.A.; Lymp, J.F.; Sauver, J.S.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006, 4, S5–S10. [Google Scholar]
- Loria, P.; Adinolfi, L.E.; Bellentani, S.; Bugianesi, E.; Grieco, A.; Fargion, S.; Gasbarrini, A.; Loguercio, C.; Lonardo, A.; Marchesini, G.; et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig. Liver Dis. 2010, 42, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Abdemalek, F. Nonalcoholic fatty liver disease in women. Women’s Health 2009, 5, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef]
- Al-Safi, Z.A.; Polotsky, A.J. Obesity and menopause. Best. Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. Adv. Clin. Chem. 2015, 72, 1–75. [Google Scholar]
- Mccullough, A.J. Pathophysiology of Nonalcoholic Steatohepatitis. J. Clin. Gastroenterol. 2006, 40, S17–S29. [Google Scholar]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017, 42, 92–108. [Google Scholar] [CrossRef]
- Lindheim, S.R.; Buchanan, T.A.; Duffy, D.M.; Vijod, M.A.; Kojima, T.; Stanczyk, F.Z.; Lobo, R.A. Comparison of estimates of insulin sensitivity in pre- and postmenopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. J. Soc. Gynecol. Investig. 1994, 1, 150–154. [Google Scholar] [CrossRef]
- Walton, C.; Godsland, I.F.; Proudler, A.J.; Wynn, V.; Stevenson, J.C. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur. J. Clin. Investig. 1993, 23, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogens and Female Liver Health. Steroids 2018, 133, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hart-Unger, S.; Arao, Y.; Hamilton, K.J.; Lierz, S.L.; Malarkey, D.E.; Hewitt, S.C.; Freemark, M.; Korach, K.S. Hormone Signaling and Fatty Liver in Females: Analysis of Estrogen Receptor Alpha Mutant Mice. Int. J. Obes. 2017, 41, 945–954. [Google Scholar] [CrossRef]
- Zhu, L.; Martinez, M.N.; Emfinger, C.H.; Palmisano, B.T.; Stafford, J.M. Estrogen Signaling Prevents Diet-Induced Hepatic Insulin Resistance in Male Mice with Obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1188–E1197. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Q.; Huang, T.; Tan, W.; Qu, L.; Chen, T.; Pan, H.; Chen, L.; Liu, J.; Wong, C.W.; et al. Dysfunction of Estrogen-Related Receptor Alpha-Dependent Hepatic Vldl Secretion Contributes to Sex Disparity in Nafld/Nash Development. Theranostics 2020, 10, 10874–10891. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S. Non-Alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Pan, H.J.; Lee, C.H. Prevention of Tamoxifen-Related Nonalcoholic Fatty Liver Disease in Breast Cancer Patients. Clin. Breast Cancer 2018, 18, e677–e685. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Yu, I.C.; Wang, R.S.; Chen, Y.T.; Liu, N.C.; Altuwaijri, S.; Hsu, C.L.; Ma, W.L.; Jokinen, J.; Sparks, J.D.; et al. Increased Hepatic Steatosis and Insulin Resistance in Mice Lacking Hepatic Androgen Receptor. Hepatology 2008, 47, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.L.; Lai, H.C.; Yeh, S.; Cai, X.; Chang, C. Androgen Receptor Roles in Hepatocellular Carcinoma, Fatty Liver, Cirrhosis and Hepatitis. Endocr. Relat. Cancer 2014, 21, R165–R182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Gray, N.E.; Kuo, T.; Harris, C.A. Regulation of Triglyceride Metabolism by Glucocorticoid Receptor. Cell Biosci. 2012, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, M.S. Pathogenesis of Nonalcoholic Steatohepatitis and Hormone-Based Therapeutic Approaches. Front. Endocrinol. 2018, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. Inflammation: Friend or Foe for Animal Production? Poult. Sci. 2018, 97, 510–514. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Kamei, Y.; Xu, L.; Heinzel, T.; Torchia, J.; Kurokawa, R.; Gloss, B.; Lin, S.C.; Heyman, R.A.; Rose, D.W.; Glass, C.K.; et al. A Cbp Integrator Complex Mediates Transcriptional Activation and Ap-1 Inhibition by Nuclear Receptors. Cell 1996, 85, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver Fibrosis: Pathophysiology, Pathogenetic Targets and Clinical Issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Donghia, R.; Guerra, V.; Procino, F.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; De Pergola, G.; Romanelli, F.; et al. Performance of Fatty Liver Index in Identifying Non-Alcoholic Fatty Liver Disease in Population Studies. A Meta-Analysis. J. Clin. Med. 2021, 10, 1877. [Google Scholar] [CrossRef]
- Setroame, A.M.; Affrim, P.K.; Abaka-Yawson, A.; Kwadzokpui, P.K.; Adrah, F.E.; Bless, H.; Mohammed, L.; Bawah, A.T.; Alidu, H.W. Prevalence of Metabolic Syndrome and Nonalcoholic Fatty Liver Disease among Premenopausal and Postmenopausal Women in Ho Municipality: A Cross-Sectional Study. BioMed Res. Int. 2020, 2020, 2168381. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, M.; Hu, Z.; Shrestha, U.K. Prevalence of nonalcoholic fatty liver disease and its metabolic risk factors in women of different ages and body mass index. Menopause 2015, 22, 667–673. [Google Scholar] [CrossRef]
- Gutierrez-Grobe, Y.; Ponciano-Rodríguez, G.; Ramos, M.H.; Uribe, M.; Méndez-Sánchez, N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann. Hepatol. 2010, 9, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Suh, B.S.; Chang, Y.; Kwon, M.J.; Yun, K.E.; Jung, H.S.; Kim, C.W.; Kim, B.K.; Kim, Y.J.; Choi, Y.; et al. Menopausal stages and non-alcoholic fatty liver disease in middle-aged women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 190, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Florentino, G.S.d.A.; Cotrim, H.P.; Vilar, C.P.; Florentino, A.V.d.A.; Guimaraes, G.M.A.; Barreto, V.S.T. Nonalcoholic fatty liver disease in menopausal women. Arq. Gastroenterol. 2013, 50, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Ohbora, A.; Takeda, N.; Fukui, M.; Kato, T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J. Gastroenterol. 2012, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jeon, W.K.; Kim, S.H.; Kim, H.J.; I Park, D.; Cho, Y.K.; Sung, I.K.; I Sohn, C.; Keum, D.K.; I Kim, B. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2006, 21 Pt 1, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Völzke, H.; Schwarz, S.; E Baumeister, S.; Wallaschofski, H.; Schwahn, C.; Grabe, H.J.; Kohlmann, T.; John, U.; Dören, M. Menopausal status and hepatic steatosis in a general female population. Gut 2007, 56, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Arshad, T.; Golabi, P.; Paik, J.; Mishra, A.; Younossi, Z.M. Prevalence of Nonalcoholic Fatty Liver Disease in the Female Population. Hepatol. Commun. 2018, 3, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Q.; Ai, P.; Liu, H.; Chen, X.; Xu, X.; Ding, G.; Li, Y.; Feng, X.; Wang, X.; et al. Association between Serum Uric Acid and Non-Alcoholic Fatty Liver Disease according to Different Menstrual Status Groups. Can. J. Gastroenterol. Hepatol. 2019, 2019, 2763093. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Yim, J.Y.; Kim, D.; Lim, S.H.; Yang, J.I.; Kim, Y.S.; Yang, S.Y.; Kwak, M.S.; Kim, J.S.; Cho, S.H. The influence of metabolic factors for nonalcoholic Fatty liver disease in women. BioMed Res. Int. 2015, 2015, 131528. [Google Scholar] [CrossRef]
- Bao, T.; Ying, Z.; Gong, L.; Du, J.; Ji, G.; Li, Z.; Gao, W.; Jiang, X.; Yang, H.; Huang, Y.; et al. Association between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in Nonobese Postmenopausal Women: A Cross-sectional Study. Sci. Rep. 2020, 10, 10072. [Google Scholar] [CrossRef]
- Sanghavi, M.; Turer, A.; Neeland, I.; Ayers, C.; de Lemos, J.; Khera, A. Postmenopausal status is independently associated with advanced hepatic steatosis: The dallas heart study. J. Am. Coll. Cardiol. 2013, 61 (Suppl. S10), E1429. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Fujii, H.; Sumida, Y.; Hyogo, H.; Itoh, Y.; Ono, M.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Japan Study Group of Nonalcoholic Fatty Liver Disease. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. 2011, 46, 1300–1306. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Charoenngam, N.; Ponvilawan, B.; Mariano, M.; Thongpiya, J.; Yingchoncharoen, P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Menopause 2023, 30, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; Kim, H.K.; Jang, H.J.; et al. Lack of association between early menopause and non-alcoholic fatty liver disease in postmenopausal women. Climacteric 2020, 23, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Notarnicola, M.; Osella, A.R.; Cisternino, A.M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Rotolo, O.; Zinzi, I.; Chiloiro, M.; et al. Menopause Does Not Affect Fatty Liver Severity In Women: A Population Study in a Mediterranean Area. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Venetsanaki, V.; Polyzos, S.A. Menopause and Non-Alcoholic Fatty Liver Disease: A Review Focusing on Therapeutic Perspectives. Curr. Vasc. Pharmacol. 2019, 17, 546–555. [Google Scholar] [CrossRef]
- Lavoie, J.M.; Pighon, A. NAFLD, estrogens, and physical exercise: The Animal Model. J. Nutr. Metab. 2012, 2012, 914938. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, P.; Paquette, A.; Brochu, M.; Prud’homme, D.; Rabasa-Lhoret, R.; Lavoie, J.M. Resistance training prevents liver fat accumulation in ovariectomized rats. Maturitas 2008, 59, 259–267. [Google Scholar] [CrossRef]
- Yoneda, M.; Thomas, E.; Sumida, Y.; Eguchi, Y.; Schiff, E.R. The influence of menopause on the development of hepatic fibrosis in nonobese women with nonalcoholic fatty liver disease. Hepatology 2014, 60, 1792. [Google Scholar] [CrossRef]
- McKenzie, J.; Fisher, B.M.; Jaap, A.J.; Stanley, A.; Paterson, K.; Sattar, N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: A randomized placebo- controlled trial. Clin. Endocrinol. 2006, 65, 40–44. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid profile differences during menopause: A review with meta-analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Macut, D.; Tziomalos, K.; Božić-Antić, I.; Bjekić-Macut, J.; Katsikis, I.; Papadakis, E.; Andrić, Z.; Panidis, D. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum. Reprod. 2016, 31, 1347–1353. [Google Scholar] [CrossRef]
- de Souza Bruno, A.; Rodrigues, M.H.; Alvares, M.C.B.; Nahas-Neto, J.; Nahas, E.P. Non-alcoholic fatty liver disease and its associated risk factors in Brazilian postmenopausal women. Climacteric 2014, 17, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Nystrom, M.; Noworolski, S.M.; Chu, P.; Mohanty, A.; Merriman, R. MRI steatosis grading: Development and initial validation of a color mapping system. AJR Am. J. Roentgenol. 2012, 198, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the Regulation of Estrogen Biosynthesis—Some New Perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Mirkin, S.; Bernick, B.; Constantine, G.D. Systemic estradiol levels with low-dose vaginal estrogens. Menopause 2020, 27, 361. [Google Scholar] [CrossRef]
- Simpson, E.R.; Misso, M.; Hewitt, K.N.; Hill, R.A.; Boon, W.C.; Jones, M.E.; Kovacic, A.; Zhou, J.; Clyne, C.D. Estrogen—The Good, the Bad, and the Unexpected. Endocr. Rev. 2005, 26, 322–330. [Google Scholar] [CrossRef]
- Turola, E.; Petta, S.; Vanni, E.; Milosa, F.; Valenti, L.; Critelli, R.; Miele, L.; Maccio, L.; Calvaruso, V.; Fracanzani, A.L.; et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. DMM Dis. Models Mech. 2015, 8, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Florentino, G.; Cotrim, H.P.; Florentino, A.; Padilha, C.; Medeiros-Neto, M.; Bragagnoli, G.; Schwingel, P. Hormone replacement therapy in menopausal women: Risk factor or protection to nonalcoholic fatty liver disease? Ann. Hepatol. 2012, 11, 147–149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntikoudi, A.; Spyrou, A.; Evangelou, E.; Dokoutsidou, E.; Mastorakos, G. The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease. Healthcare 2024, 12, 1081. https://doi.org/10.3390/healthcare12111081
Ntikoudi A, Spyrou A, Evangelou E, Dokoutsidou E, Mastorakos G. The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease. Healthcare. 2024; 12(11):1081. https://doi.org/10.3390/healthcare12111081
Chicago/Turabian StyleNtikoudi, Anastasia, Alketa Spyrou, Eleni Evangelou, Eleni Dokoutsidou, and George Mastorakos. 2024. "The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease" Healthcare 12, no. 11: 1081. https://doi.org/10.3390/healthcare12111081
APA StyleNtikoudi, A., Spyrou, A., Evangelou, E., Dokoutsidou, E., & Mastorakos, G. (2024). The Effect of Menopausal Status, Insulin Resistance and Body Mass Index on the Prevalence of Non-Alcoholic Fatty Liver Disease. Healthcare, 12(11), 1081. https://doi.org/10.3390/healthcare12111081







