Mid-Term Results of an Italian Multicentric Experience with the RoadsaverTM Dual-Layer Carotid Stent System
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. In-Hospital Outcomes
3.2. Thirty-Day and Medium-Term Outcomes
4. Discussion
- -
- The reporting of an intraoperative complication rate of less than 1.7% using the Roadsaver stent associated with a CPD;
- -
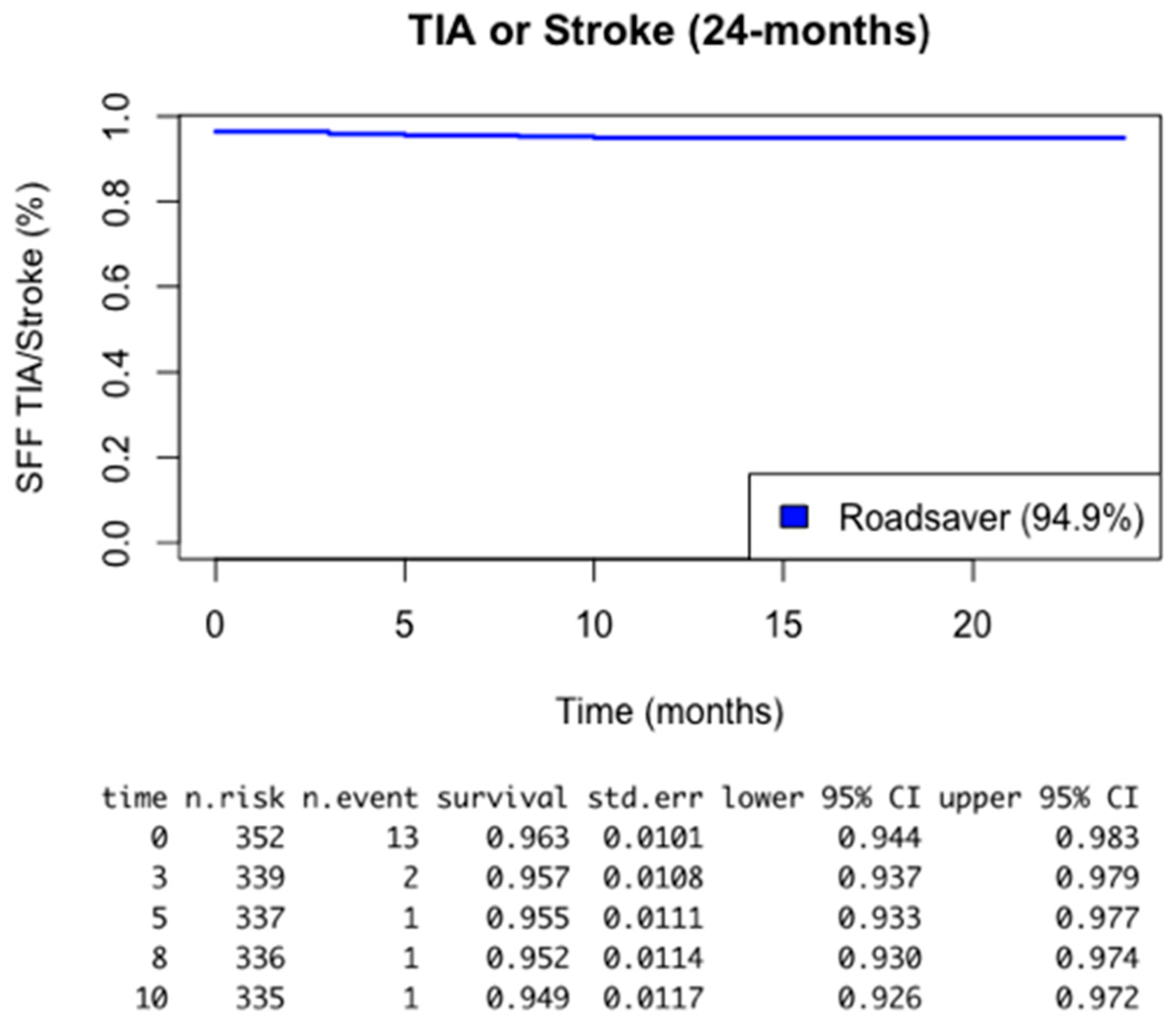
- Reporting, at an average follow-up of 35 months, a cumulative stroke rate of 2.2% and a transient ischemic attack rate of 2.8%; lower than many reported experiences in the literature, as indicated in Figure 1.
4.1. Future Research
- -
- To assess more accurately the extent to which brain protective devices may interfere with the number of intraoperative strokes
- -
- To evaluate transcervical carotid access as a safer and simpler alternative to femoral artery access
- -
- To assess more accurately the origin and evolution of the morphology for each type of plaque following implantation of different types of stents and whether this may have any influence on the degree of restenosis and distant postoperative events.
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pini, R.; Faggioli, G.; Paraskevas, K.I.; Campana, F.; Sufali, G.; Rocchi, C.; Palermo, S.; Gallitto, E.; Gargiulo, M. Carotid Artery Stenting with Double-Layer Stent: A Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2022. [Google Scholar] [CrossRef]
- Parlani, G.; De Rango, P.; Norgiolini, L.; Cao, P. Timing of Complications during Carotid Artery Stenting. How Can they Be Predicted? Acta Chir. Belg. 2006, 106, 367–371. [Google Scholar] [CrossRef]
- McCabe, D.J.H.; Pereira, A.C.; Clifton, A.; Bland, J.M.; Brown, M.M. Restenosis After Carotid Angioplasty, Stenting, or Endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). Stroke 2005, 36, 281–286. [Google Scholar] [CrossRef]
- Hrbáč, T.; Fiedler, J.; Procházka, V.; Jonszta, T.; Roubec, M.; Pakizer, D.; Václavík, D.; Netuka, D.; Heryán, T.; Školoudík, D. Comparison of carotid endarterectomy and repeated carotid angioplasty and stenting for in-stent restenosis (CERCAS trial): A randomised study. Stroke Vasc. Neurol. 2023, 8, 2075. [Google Scholar] [CrossRef]
- Brott, T.G.; Hobson, R.W.; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Park, K.Y.; Kim, D.I.; Kim, B.M.; Nam, H.S.; Kim, Y.D.; Heo, J.H.; Kim, D.J. Incidence of embolism associated with carotid artery stenting: Open-cell versus closed-cell stents. J. Neurosurg. 2013, 119, 642–647. [Google Scholar] [CrossRef]
- Timaran, C.H.; Rosero, E.B.; Higuera, A.; Ilarraza, A.; Modrall, J.G.; Clagett, G.P. Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J. Vasc. Surg. 2011, 54, 1310–1316.e1. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- White, C.J.; Brott, T.G.; Gray, W.A.; Heck, D.; Jovin, T.; Lyden, S.P.; Metzger, D.C.; Rosenfield, K.; Roubin, G.; Sachar, R.; et al. Carotid Artery Stenting: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Orso, M.; Alba, G.; Bevilacqua, S.; Capoccia, L.; Cappelli, A.; Giampaolo, C.; Carlo, C.; Marina, D.; Walter, D.; et al. Guideline on carotid surgery for stroke prevention: Updates from the Italian Society of Vascular and Endovascular Surgery. A trend towards personalized medicine. J. Cardiovasc. Surg. 2022, 63, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Nazari, P.; Golnari, P.; Hurley, M.C.; Shaibani, A.; Ansari, S.A.; Potts, M.B.; Jahromi, B.S. Carotid Stenting without Embolic Protection Increases Major Adverse Events: Analysis of the National Surgical Quality Improvement Program. AJNR Am. J. Neuroradiol. 2021, 42, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Mathias, M.d.K.; Jäger, M.d.H.; Hennigs, M.d.S.; Gissler, M.d.H.M. Endoluminal treatment of internal carotid artery stenosis. World J. Surg. 2001, 25, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Wholey, M.H.; Wholey, M.H.; Jarmolowski, C.R.; Eles, G.; Levy, D.; Buecthel, J. Endovascular stents for carotid artery occlusive disease. J. Endovasc. Surg. 1997, 4, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; White, C.J.; Hopkins, L.N.; Katzen, B.T.; Safian, R.; Wholey, M.H.; Gray, W.A.; Ciocca, R.; Bachinsky, W.B.; Ansel, G.; et al. Carotid artery revascularization in high-surgical-risk patients using the Carotid WALLSTENT and FilterWire EX/EZ: 1-year outcomes in the BEACH Pivotal Group. J. Am. Coll. Cardiol. 2008, 51, 427–434. [Google Scholar] [CrossRef][Green Version]
- Castriota, F.; de Campos Martins, E.C.; Liso, A.; Biamino, G.; Cremonesi, A. Technical Evolution of Carotid Stents. Interv. Cardiol. Rev. 2008, 3, 74. [Google Scholar] [CrossRef]
- Peluso, A.; Turchino, D.; Petrone, A.; Giribono, A.M.; Bracale, R.; Del Guercio, L.; Bracale, U.M. Standard Carotid Endarterectomy versus Carotid Artery Stenting with Closed-Cell Stent Design and Distal Embolic Protection: Does the age matter? Transl. Med. UniSa 2019, 19, 60. [Google Scholar]
- Bosiers, M.; Deloose, K.; Verbist, J.; Peeters, P. The Impact of Embolic Protection Device and Stent Design on the Outcome of CAS. Perspect. Vasc. Surg. Endovasc. Ther. 2008, 20, 272–279. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Campbell, J.E.; Hariri, N.; AbuRahma, J.; Dean, L.S.; Bates, M.C.; Nanjundappa, A.; Stone, P.A.; O’vil, A. Clinical Outcome of Carotid Artery Stenting According to Provider Specialty and Volume. Ann. Vasc. Surg. 2017, 44, 361–367. [Google Scholar] [CrossRef]
- Modrall, J.G.; Tsai, S.; Ramanan, B.; Kirkwood, M.L.; Ali, M.; Rectenwald, J.E.; Timaran, C.H.; Rosero, E.B. Defining the threshold surgeon volume associated with improved patient outcomes for carotid endarterectomy. J. Vasc. Surg. 2020, 72, 209–218.e1. [Google Scholar] [CrossRef]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical results in 1415 patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef]
- Mas, J.-L.; Chatellier, G.; Beyssen, B.; Branchereau, A.; Moulin, T.; Becquemin, J.-P.; Larrue, V.; Lièvre, M.; Leys, D.; Bonneville, J.-F.; et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 2006, 355, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Smout, J.; Macdonald, S.; Weir, G.; Stansby, G. Carotid artery stenting: Relationship between experience and complication rate. Int. J. Stroke 2010, 5, 477–482. [Google Scholar] [CrossRef]
- Lin, P.H.; Bush, R.L.; Peden, E.; Zhou, W.; Kougias, P.; Henao, E.; Mohiuddin, I.; Lumsden, A.B. What Is the Learning Curve for Carotid Artery Stenting with Neuroprotection? Analysis of 200 Consecutive Cases at an Academic Institution. Perspect. Vasc. Surg. Endovasc. Ther. 2005, 17, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mantese, V.A.; Timaran, C.H.; Chiu, D.; Begg, R.J.; Brott, T.G. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)—Stenting versus Carotid Endarterectomy for Carotid Disease. Stroke 2010, 41, S31. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.; Malinowski, K.; Rosenfield, K.; Capoccia, L.; Speziale, F.; de Donato, G.; Setacci, C.; Wissgott, C.; Sirignano, P.; Tekieli, L.; et al. Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4819. [Google Scholar] [CrossRef]
- Bracale, U.M.; Peluso, A.; Di Mauro, E.; del Guercio, L.; Di Taranto, M.D.; Giannotta, N.; Ielapi, N.; Provenzano, M.; Andreucci, M.; Serra, R. Carotid Endarterectomy versus Carotid Artery Stenting with Double-Layer Micromesh Carotid Stent: Contemporary Results of a Single-Center Retrospective Study. Ann. Vasc. Surg. 2022, 82, 41–46. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Sagris, M.; Giannopoulos, S.; Tzoumas, A.; Kokkinidis, D.G.; Texakalidis, P.; Koutsias, G.; Volteas, P.; Li, J.; Rafael, D. Malgor Embolic Protection Devices for Carotid Artery Stenting: A Network Meta-Analysis. J. Vasc. Surg. 2023, 77, 17S–18S. [Google Scholar] [CrossRef]
- Montorsi, P.; Caputi, L.; Galli, S.; Ravagnani, P.M.; Teruzzi, G.; Annoni, A.; Calligaris, G.; Fabbiocchi, F.; Trabattoni, D.; de Martini, S.; et al. Carotid Wallstent Versus Roadsaver Stent and Distal Versus Proximal Protection on Cerebral Microembolization During Carotid Artery Stenting. JACC Cardiovasc. Interv. 2020, 13, 403–414. [Google Scholar] [CrossRef]
- Luk, Y.; Chan, Y.C.; Cheng, S.W. Transcarotid Artery Revascularization as a New Modality of Treatment for Carotid Stenosis. Ann. Vasc. Surg. 2020, 64, 397–404. [Google Scholar] [CrossRef]
- de Borst, G.J. Transcarotid Artery Stenting: Hype or Hope? Stroke 2022, 53, 108–110. [Google Scholar] [CrossRef]
- Verzini, F.; Cao, P.; De Rango, P.; Parlani, G.; Maselli, A.; Romano, L.; Norgiolini, L.; Giordano, G. Appropriateness of learning curve for carotid artery stenting: An analysis of periprocedural complications. J. Vasc. Surg. 2006, 44, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- De Donato, G.; Setacci, F.; Sirignano, P.; Galzerano, G.; Cappelli, A.; Setacci, C. Optical coherence tomography after carotid stenting: Rate of stent malapposition, plaque prolapse and fibrous cap rupture according to stent design. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Tadros, R.O.; Malik, R.K.; Vouyouka, A.G.; Ellozy, S.H.; Marin, M.L.; Faries, P.L. A systematic review of carotid stent design and selection: Strategies to optimize procedural outcomes. Interv. Cardiol. 2013, 5, 203. [Google Scholar] [CrossRef]
- Fanelli, F.; Boatta, E.; Cannavale, A.; Corona, M.; Lucatelli, P.; Wlderk, A.; Cirelli, C.; Salvatori, F.M. Carotid artery stenting: Analysis of a 12-year single-center experience. J. Endovasc. Ther. 2012, 19, 749–756. [Google Scholar] [CrossRef]
- Righini, P.C.; Mazzaccaro, D.P.; Malacrida, G.; Modafferi, A.; Nano, G. Double Layer Micromesh Stents Versus Closed Cell Stents for the Endovascular Treatment of Carotid Stenosis in the Current Era. Eur. J. Vasc. Endovasc. Surg. 2019, 58, e544–e545. [Google Scholar] [CrossRef]
- Umemoto, T.; De Donato, G.; Pacchioni, A.; Reimers, B.; Ferrante, G.; Isobe, M.; Setacci, C. Optical coherence tomography assessment of newgeneration mesh-covered stents after carotid stenting. EuroIntervention 2017, 13, 1348–1355. [Google Scholar] [CrossRef]
- Musialek, P.; Stabile, E. Residual plaque prolapse with novel dual-layer carotid stents: Is it mesh-covered or not? EuroIntervention 2017, 13, 1266–1268. [Google Scholar] [CrossRef][Green Version]
- Stabile, E.; de Donato, G.; Musialek, P.; Deloose, K.; Nerla, R.; Sirignano, P.; Mazurek, A.; Mansour, W.; Fioretti, V.; Esposito, F.; et al. Use of Dual-Layered Stents for Carotid Artery Angioplasty: 1-Year Results of a Patient-Based Meta-Analysis. JACC Cardiovasc. Interv. 2020, 13, 1709–1715. [Google Scholar] [CrossRef]
| Overall (n = 353) | |
|---|---|
| Subject clinical characteristics | |
| Age (years) | 74.3 ± 8.3 |
| Males | 259 (73.3%) |
| Smoke | 206 (58.3%) |
| Hypertension (SAP > 140 mmHg and/or DAP > 90 mmHg) | 316 (89.5%) |
| Diabetes (glycemia > 125 mg/dL and/or use of HD/insulin) | 130 (36.8%) |
| Dyslipidemia (Tot. Chol. > 240 mg/dL and/or TGL > 150 mg/dL and/or use of LLD) | 292 (82.7%) |
| CAD | 148 (41.9%) |
| CKD (GFR < 60 mL/min/1.73 m2) | 48 (13.6%) |
| End-Stage Kidney Failure (intra- or extracorporeal dialysis) | 4 (1.4%) |
| Symptomatic stenosis | 21 (5.9%) |
| Contralateral carotid occlusion | 27 (7.6%) |
| Procedural characteristics | |
| Procedure for carotid restenosis | 47 (13.3%) |
| Stent length (mm) | 23.1 ± 4.1 |
| Stent diameter (mm) | 7.7 ± 0.6 |
| Cerebral Protection System (CPD): | |
| 159 (45%) |
| 103 (29.1%) |
| 59 (16.7%) |
| 25 (7%) |
| 7 (1.9%) |
| Overall (n = 353) | |
|---|---|
| Safety and Effectiveness Outcomes (24 months) | |
| TIA: | 10 (2.8%) |
| 6 (1.7%) |
| 4 (1.1%) |
| Stroke: | 8 (2.2%) |
| 6 (1.7%) |
| 2 (0.5%) |
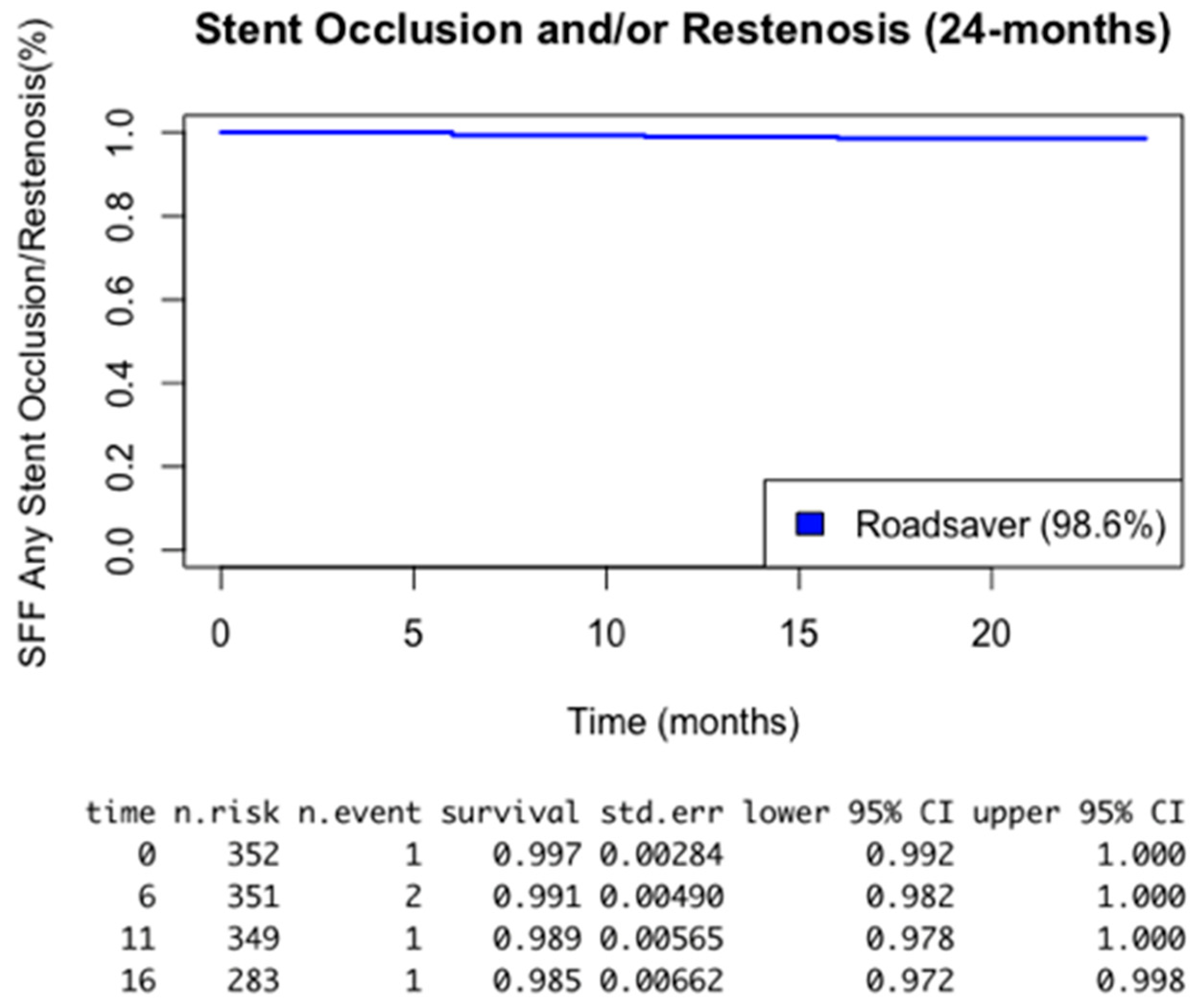
| Restenosis | 5 (1.4%) |
| All-cause death | 11 (3.1%) |
| Hospital stay (days) | 1.8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestri, O.; Accarino, G.; Turchino, D.; Squizzato, F.; Piazza, M.; Bastianon, M.; Di Gregorio, S.; Pratesi, G.; Antonello, M.; Costa, D.; et al. Mid-Term Results of an Italian Multicentric Experience with the RoadsaverTM Dual-Layer Carotid Stent System. Healthcare 2024, 12, 120. https://doi.org/10.3390/healthcare12010120
Silvestri O, Accarino G, Turchino D, Squizzato F, Piazza M, Bastianon M, Di Gregorio S, Pratesi G, Antonello M, Costa D, et al. Mid-Term Results of an Italian Multicentric Experience with the RoadsaverTM Dual-Layer Carotid Stent System. Healthcare. 2024; 12(1):120. https://doi.org/10.3390/healthcare12010120
Chicago/Turabian StyleSilvestri, Olga, Giulio Accarino, Davide Turchino, Francesco Squizzato, Michele Piazza, Martina Bastianon, Sara Di Gregorio, Giovanni Pratesi, Michele Antonello, Davide Costa, and et al. 2024. "Mid-Term Results of an Italian Multicentric Experience with the RoadsaverTM Dual-Layer Carotid Stent System" Healthcare 12, no. 1: 120. https://doi.org/10.3390/healthcare12010120
APA StyleSilvestri, O., Accarino, G., Turchino, D., Squizzato, F., Piazza, M., Bastianon, M., Di Gregorio, S., Pratesi, G., Antonello, M., Costa, D., Serra, R., & Bracale, U. M. (2024). Mid-Term Results of an Italian Multicentric Experience with the RoadsaverTM Dual-Layer Carotid Stent System. Healthcare, 12(1), 120. https://doi.org/10.3390/healthcare12010120









