Meniscal Allograft Transplants in Skeletally Immature Patients: A Systematic Review of Indications and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Eligibility Criteria and Search Strategy
2.3. Data Extraction
2.4. Methodological Study Quality Assessment
3. Results
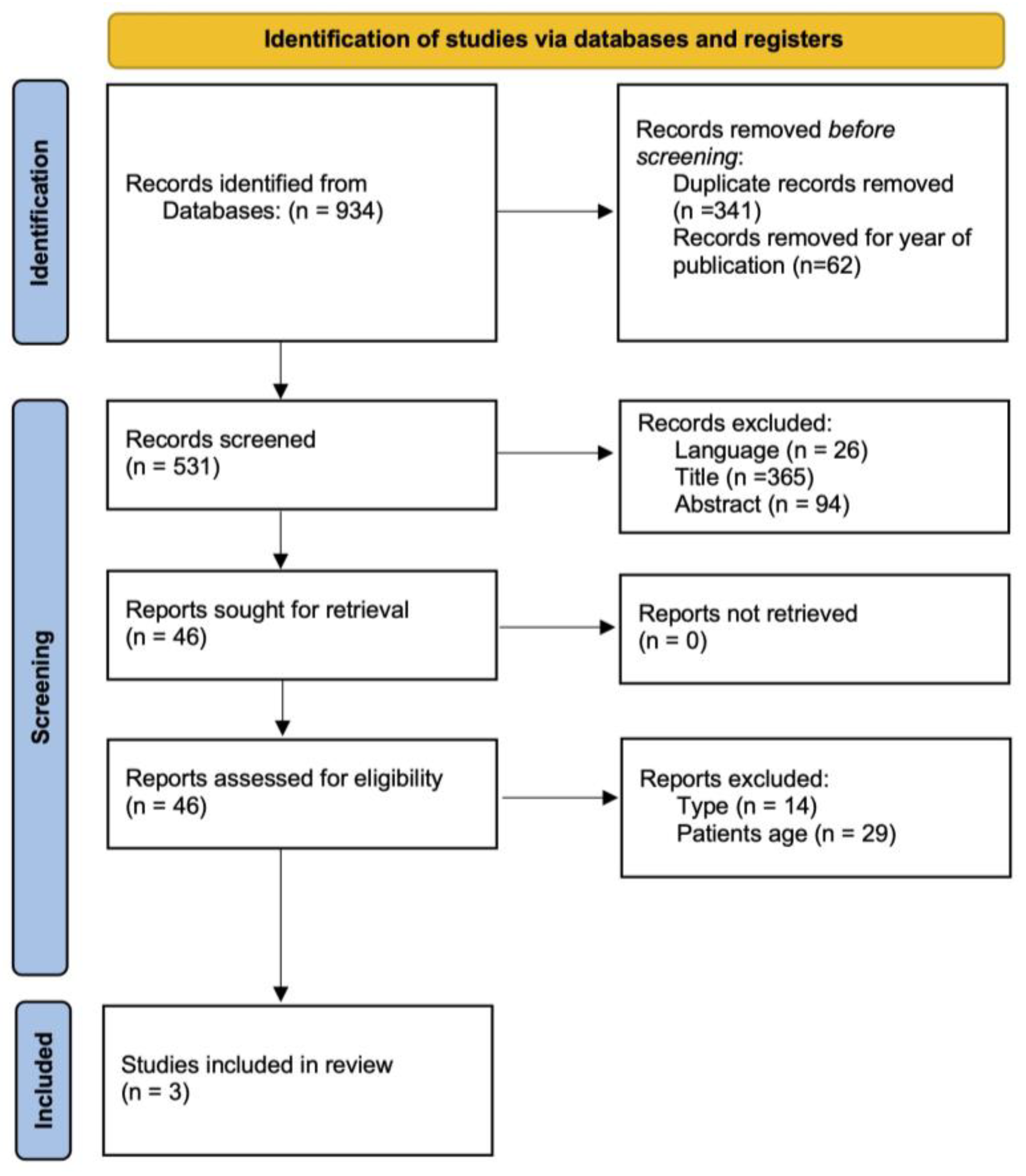
3.1. Study Selection
3.2. Study Characteristics
3.3. Patient Characteristics
3.4. Outcomes Reported
3.5. Complications Reported
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, H.E.; Lyons, M.M.; Patel, N.M. Epidemiology of Meniscal Allograft Transplantation at Children’s Hospitals in the United States. Orthop. J. Sports Med. 2021, 9, 23259671211034877. [Google Scholar] [CrossRef] [PubMed]
- Turati, M.; Boerci, L.; Piatti, M.; Zanchi, N.; Zatti, G.; Accadbled, F.; Bigoni, M. Updates on etiopathogenesis of musculoskeletal injuries in adolescent athletes. Minerva Pediatr. 2023, 75, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of traumatic meniscus tears: The 2019 ESSKA meniscus consensus. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, S.H.; Park, S.Y.; Jung, G.Y.; Chung, K.Y. Meniscus allograft transplantation for discoid lateral meniscus: Clinical comparison between discoid lateral meniscus and nondiscoid lateral meniscus. Arthroscopy 2014, 30, 724–730. [Google Scholar] [CrossRef]
- Ewing, J.W. ; Bristol-myers/zimmer Orthopaedic Symposi. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy; Raven Press: New York, NY, USA, 1990. [Google Scholar]
- Cole, B.J.; McCarty, L.P., 3rd; Kang, R.W.; Alford, W.; Lewis, P.B.; Hayden, J.K. Arthroscopic rotator cuff repair: Prospective functional outcome and repair integrity at minimum 2-year follow-up. J. Shoulder Elb. Surg. 2007, 16, 579–585. [Google Scholar] [CrossRef]
- Pereira, H.; Fatih Cengiz, I.; Gomes, S.; Espregueira-Mendes, J.; Ripoll, P.L.; Monllau, J.C.; Reis, R.L.; Oliveira, J.M. Meniscal allograft transplants and new scaffolding techniques. EFORT Open Rev. 2019, 4, 279–295. [Google Scholar] [CrossRef]
- Alford, W.; Cole, B.J. The indications and technique for meniscal transplant. Orthop. Clin. North Am. 2005, 36, 469–484. [Google Scholar] [CrossRef]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Chahla, J.; Cole, B.J. Meniscal Allograft Transplants. Clin. Sports Med. 2020, 39, 93–123. [Google Scholar] [CrossRef]
- Chahla, J.; Olivetto, J.; Dean, C.S.; Serra Cruz, R.; LaPrade, R.F. Lateral Meniscal Allograft Transplantation: The Bone Trough Technique. Arthrosc. Tech. 2016, 5, e371–e377. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Verdonk, P.C.; Reid, J.B., 3rd; Verdonk, R. Meniscus allograft transplantation: A current concepts review. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 476–492. [Google Scholar] [CrossRef]
- Tuca, M.; Luderowski, E.; Rodeo, S. Meniscal transplant in children. Curr. Opin. Pediatr. 2016, 28, 47–54. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Gaddi, D.; Mosca, A.; Piatti, M.; Munegato, D.; Catalano, M.; Di Lorenzo, G.; Turati, M.; Zanchi, N.; Piscitelli, D.; Chui, K.; et al. Acute Ankle Sprain Management: An Umbrella Review of Systematic Reviews. Front. Med. 2022, 9, 868474. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (accessed on 30 January 2023).
- Riboh, J.C.; Tilton, A.K.; Cvetanovich, G.L.; Campbell, K.A.; Cole, B.J. Meniscal Allograft Transplantation in the Adolescent Population. Arthroscopy 2016, 32, 1133–1140 e1131. [Google Scholar] [CrossRef]
- Kocher, M.S.; Tepolt, F.A.; Vavken, P. Meniscus transplantation in skeletally immature patients. J. Pediatr. Orthop. B 2016, 25, 343–348. [Google Scholar] [CrossRef]
- Middleton, S.; Asplin, L.; Stevenson, C.; Thompson, P.; Spalding, T. Meniscal allograft transplantation in the paediatric population: Early referral is justified. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1908–1913. [Google Scholar] [CrossRef]
- Cole, B.J.; Carter, T.R.; Rodeo, S.A. Allograft meniscal transplantation: Background, techniques, and results. Instr. Course Lect. 2003, 52, 383–396. [Google Scholar] [CrossRef]
- Bellisari, G.; Samora, W.; Klingele, K. Meniscus tears in children. Sports Med. Arthrosc. Rev. 2011, 19, 50–55. [Google Scholar] [CrossRef]
- Liu, J.N.; Gowd, A.K.; Redondo, M.L.; Christian, D.R.; Cabarcas, B.C.; Yanke, A.B.; Cole, B.J. Establishing Clinically Significant Outcomes After Meniscal Allograft Transplantation. Orthop. J. Sports Med. 2019, 7, 2325967118818462. [Google Scholar] [CrossRef]
- Jacobsen, J.S.; Knudsen, P.; Fynbo, C.; Rolving, N.; Warming, S. Reproducibility and responsiveness of a Danish Pedi-IKDC subjective knee form for children with knee disorders. Scand J. Med. Sci. Sports 2016, 26, 1408–1414. [Google Scholar] [CrossRef]
- MacKay, C.; Clements, N.; Wong, R.; Davis, A.M. A systematic review of estimates of the minimal clinically important difference and patient acceptable symptom state of the Western Ontario and McMaster Universities Osteoarthritis Index in patients who underwent total hip and total knee replacement. Osteoarthr. Cartil. 2019, 27, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.I. Meniscal allograft transplantation for symptomatic knee after meniscectomy of torn discoid medial meniscus: Report of three cases. Acta Orthop. Traumatol. Turc. 2018, 52, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gottliebsen, M.; Turati, M. The paediatric knee: Traumatic injuries. J. Child. Orthop. 2023, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.; Sepulveda, M.; Jesus Tuca, M.; Birrer, E. Discoid meniscus: Current concepts. EFORT Open Rev. 2020, 5, 371–379. [Google Scholar] [CrossRef]
- Smillie, I. The congenital discoid meniscus. J. Bone Jt. Surg. 1948, 30, 671–682. [Google Scholar] [CrossRef]
- Turati, M.; Anghilieri, F.M.; Accadbled, F.; Piatti, M.; Di Benedetto, P.; Moltrasio, F.; Zatti, G.; Zanchi, N.; Bigoni, M. Discoid meniscus in human fetuses: A systematic review. Knee 2021, 30, 205–213. [Google Scholar] [CrossRef]
- Asokan, A.; Ayub, A.; Ramachandran, M. Pediatric meniscal injuries: Current concepts. J. Child. Orthop. 2023, 17, 70–75. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, Y.S.; Ha, H.C.; Shim, J.S.; Lim, K.S. A novel magnetic resonance imaging classification of discoid lateral meniscus based on peripheral attachment. Am. J. Sports Med. 2009, 37, 1564–1569. [Google Scholar] [CrossRef]
- Deie, M.; Ochi, M.; Sumen, Y.; Kawasaki, K.; Adachi, N.; Yasunaga, Y.; Ishida, O. Relationship between osteochondritis dissecans of the lateral femoral condyle and lateral menisci types. J. Pediatr. Orthop. 2006, 26, 79–82. [Google Scholar] [CrossRef]
- Logan, C.A.; Tepolt, F.A.; Kocher, S.D.; Feroe, A.G.; Micheli, L.J.; Kocher, M.S. Symptomatic Discoid Meniscus in Children and Adolescents: A Review of 470 Cases. J. Pediatr. Orthop. 2021, 41, 496–501. [Google Scholar] [CrossRef]
- Mickiewicz, P.; Binkowski, M.; Bursig, H.; Wrobel, Z. Preservation and sterilization methods of the meniscal allografts: Literature review. Cell Tissue Bank 2014, 15, 307–317. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pr. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Lorenzo, P.; Bayliss, M.T.; Heinegard, D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 2004, 23, 381–391. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.S.; Go, E.B.; Lee, J.Y.; Kim, J.Y.; Lee, S.Y.; Lee, D.H. Proteomic Analysis of the Meniscus Cartilage in Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 8181. [Google Scholar] [CrossRef]
- Comins, J.; Brodersen, J.; Krogsgaard, M.; Beyer, N. Rasch analysis of the Knee injury and Osteoarthritis Outcome Score (KOOS): A statistical re-evaluation. Scand J. Med. Sci. Sports 2008, 18, 336–345. [Google Scholar] [CrossRef]
- Hansen, C.F.; Ostergaard Madsen, M.; Warming, S.; Rathcke, M.W.; Krogsgaard, M.; Christensen, K.B. KOOS-Child exhibits inadequate structural validity in a cohort of paediatric patients with ACL deficiency. Br. J. Sports Med. 2022, 56, 1284–1291. [Google Scholar] [CrossRef]
- Duckett, T.; Fox, C.M.; Hart, J.M.; Norte, G.E. Rationale for a Parsimonious Measure of Subjective Knee Function Among Individuals With Anterior Cruciate Ligament Reconstruction: A Rasch Analysis. J. Athl. Train. 2021, 56, 1340–1348. [Google Scholar] [CrossRef]
- Geri, T.; Piscitelli, D.; Meroni, R.; Bonetti, F.; Giovannico, G.; Traversi, R.; Testa, M. Rasch analysis of the Neck Bournemouth Questionnaire to measure disability related to chronic neck pain. J. Rehabil. Med. 2015, 47, 836–843. [Google Scholar] [CrossRef]
- Meroni, R.; Piscitelli, D.; Bonetti, F.; Zambaldi, M.; Guccione, A.A.; Pillastrini, P. Rasch analysis of the Italian version of fear avoidance beliefs questionnaire (FABQ-I). Disabil. Rehabil. 2015, 37, 151–157. [Google Scholar] [CrossRef]
- Collins, N.J.; Prinsen, C.A.; Christensen, R.; Bartels, E.M.; Terwee, C.B.; Roos, E.M. Knee Injury and Osteoarthritis Outcome Score (KOOS): Systematic review and meta-analysis of measurement properties. Osteoarthr. Cartil. 2016, 24, 1317–1329. [Google Scholar] [CrossRef]
- van der Velden, C.A.; van der Steen, M.C.; Leenders, J.; van Douveren, F.; Janssen, R.P.A.; Reijman, M. Pedi-IKDC or KOOS-child: Which questionnaire should be used in children with knee disorders? BMC Musculoskelet. Disord. 2019, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J. Man. Manip. Ther. 2008, 16, E82–E83. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hart, D.L.; Stratford, P.W.; Mioduski, J.E. Baseline dependency of minimal clinically important improvement. Phys. Ther. 2011, 91, 675–688. [Google Scholar] [CrossRef]
| Study | Sample Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adequate Case Definition | Representativeness of the Cases | Selection of Control | Definition of Control | Comparability of Cases | Controls Based on the Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | ||
| Riboh et al. (2016) [16] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||
| Kocher et al. (2016) [17] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | |||
| Middleton et al. (2019) [18] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||
| Study | Subjects | Surgical Technique | Lateral vs. Medial Meniscus | Associated Procedures |
|---|---|---|---|---|
| Riboh et al. (2016) [16] | n = 32 (23 F; 9 M) Mean age 15.4 years (13–16 years) | Bone-plug technique (bridge-in-slot technique [19]) | 5 medial menisci 27 lateral menisci | n = 18, 11 ACI; 2 ACL reconstructions; 1 OATS; 3 Osteochondral allografts; 1 HTO |
| Kocher et al. (2016) [17] | n = 3 (2 F; 1 M) Mean age 12.6 years (9–14 years) | Physeal-sparing bone-plug technique [17] | 1 medial meniscus 2 lateral menisci | - |
| Middleton et al. (2019) [18] | n = 23 (14 F; 9 M) Mean age 17 years (8–18 years) | Free-graft technique with sutures through bone tunnels tied over a bone bridge on the anteromedial tibia [20] | 4 medial menisci 19 lateral menisci | n = 6, 1 HTO; 1 ACL reconstruction; 1 ACL revision; 3 microfracture procedures |
| Study | Follow-Up | Pre-Operative Scores | Final Follow-Up | Subsequent Procedure |
|---|---|---|---|---|
| Riboh et al. (2016) [16] | Mean: 7.2 ± 3.2 years (range 2–15 years) | IKDC: 40.19 ± 18.98 | IKDC: 65.02 ± 17.70 | 7 reinterventions: 1 femoral osteotomy; 3 chondroplasty; 2 meniscectomy; 1 lysis of adhesions; 1 removal of loose body + ACLR |
| Lysholm: 43.80 ± 20.37 | Lysholm: 58.52 ± 17.92 | |||
| KOOS pain: 64.19 ± 23.20 KOOS symptoms: 59.73 ± 17.83 KOOS ADL: 75.38 ± 22.35 KOOS sports: 35.19 ± 22.89 KOOS QOL: 26.62 ± 16.96 | KOOS pain: 76.57 KOOS symptoms: 72.36 KOOS ADL: 90.09 KOOS sports: 62.61 KOOS QOL: 54.89 | |||
| Kocher et al. (2016) [17] | Mean: 31± 20 months | Pedi-IKDC: Not Reported | Pedi-IKDC: 68.3 ± 4 | 1 lysis of adhesion |
| Lysholm: Not Reported | Lysholm: 55.7 ± 22.3 | |||
| Tegner: Not Reported | Tegner: 7 | |||
| Middleton et al. (2019) [18] | Median: 3.8 years (range 0.2–7.8 years) | IKDC: 40.6 ± 12 | IKDC: 86.2± 3.2 | 8 reinterventions; 1 partial meniscectomy; 1 re-suture of partial tear; 1 chondroplasty PFJ; 1 arthroscopy; 4 removals of suture knots |
| Lysholm: 57.3 ± 18.2 | Lysholm 94.5 ± 2.1 | |||
| KOOS pain: 70 KOOS symptoms: 61 KOOS ADL: 81 KOOS sports: 43 KOOS QOL: 28 | KOOS pain: 85 KOOS symptoms: 78 KOOS ADL: 88 KOOS Sports: 69 KOOS QOL: 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turati, M.; Boerci, L.; Piatti, M.; Russo, L.; Rigamonti, L.; Buonanotte, F.; Courvoisier, A.; Zatti, G.; Piscitelli, D.; Bigoni, M. Meniscal Allograft Transplants in Skeletally Immature Patients: A Systematic Review of Indications and Outcomes. Healthcare 2023, 11, 1312. https://doi.org/10.3390/healthcare11091312
Turati M, Boerci L, Piatti M, Russo L, Rigamonti L, Buonanotte F, Courvoisier A, Zatti G, Piscitelli D, Bigoni M. Meniscal Allograft Transplants in Skeletally Immature Patients: A Systematic Review of Indications and Outcomes. Healthcare. 2023; 11(9):1312. https://doi.org/10.3390/healthcare11091312
Chicago/Turabian StyleTurati, Marco, Linda Boerci, Massimiliano Piatti, Laura Russo, Luca Rigamonti, Francesco Buonanotte, Aurelien Courvoisier, Giovanni Zatti, Daniele Piscitelli, and Marco Bigoni. 2023. "Meniscal Allograft Transplants in Skeletally Immature Patients: A Systematic Review of Indications and Outcomes" Healthcare 11, no. 9: 1312. https://doi.org/10.3390/healthcare11091312
APA StyleTurati, M., Boerci, L., Piatti, M., Russo, L., Rigamonti, L., Buonanotte, F., Courvoisier, A., Zatti, G., Piscitelli, D., & Bigoni, M. (2023). Meniscal Allograft Transplants in Skeletally Immature Patients: A Systematic Review of Indications and Outcomes. Healthcare, 11(9), 1312. https://doi.org/10.3390/healthcare11091312







