Elements Characterising Multicomponent Interventions Used to Improve Disease Management Models and Clinical Pathways in Acute and Chronic Heart Failure: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Inclusion and Exclusion Criteria
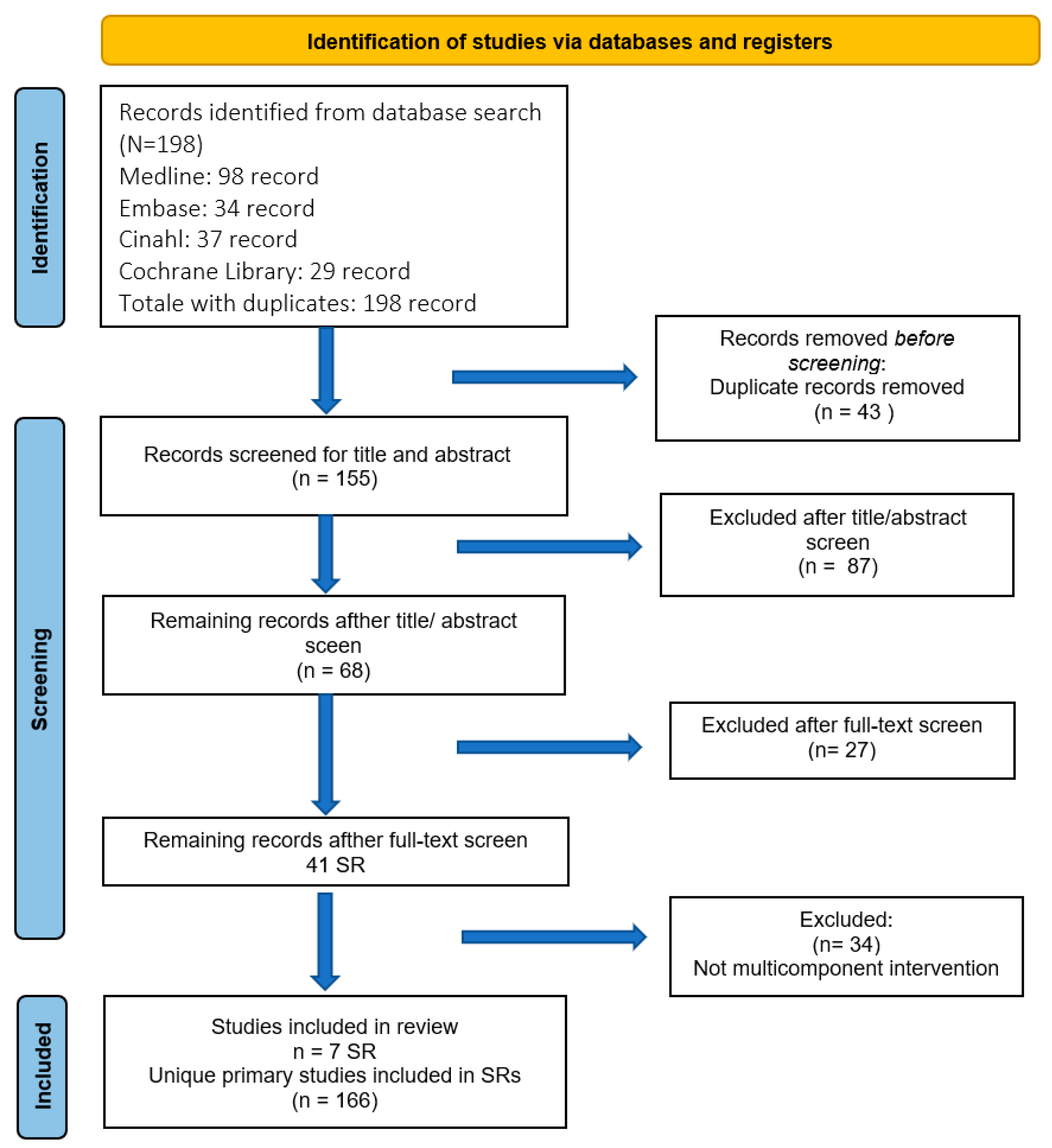
2.3. Information Sources, Search Strategy and Selection Process
2.4. Data Charting Process and Data Items
2.5. Classification of Interventions and Actions
- standard or common classification according to the European Society of Cardiology (ESC) guidelines [29]:
- -
- ≥50% (normal LVEF or HF with preserved EF (HFpEF))
- -
- 40–49% (HF with mid-range ejection fraction (HFmrEF)),
- -
- <40% (HF with reduced EF (HFrEF)),
- other classification containing LVEF cut-offs that overlap with ESC criteria, and
- not specified, in case of missing information on LVEF classification.
2.6. Synthesis of Results
3. Results
3.1. Selection of Sources of Evidence
3.2. Characteristics of Included Studies
3.3. Description of the Interventions
3.4. Type of Intervention Components by Level of LVEF Impairment, Setting, and Size
3.5. Team Organisation Structure by Setting and LVEF Impairment
3.6. Qualitative Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Riet, E.E.S.; Hoes, A.W.; Limburg, A.; Landman, M.A.J.; van der Hoeven, H.; Rutten, F.H. Prevalence of Unrecognized Heart Failure in Older Persons with Shortness of Breath on Exertion. Eur. J. Heart Fail. 2014, 16, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical Epidemiology of Heart Failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef]
- Ceia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.G.; on behalf of the EPICA Investigators. Prevalence of Chronic Heart Failure in Southwestern Europe: The EPICA Study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar] [CrossRef]
- van Riet, E.E.S.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.J.; Rutten, F.H. Epidemiology of Heart Failure: The Prevalence of Heart Failure and Ventricular Dysfunction in Older Adults over Time. A Systematic Review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef]
- Emmons-Bell, S.; Johnson, C.; Roth, G. Prevalence, Incidence and Survival of Heart Failure: A Systematic Review. Heart 2022, 108, 1351–1360. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal Trends and Patterns in Heart Failure Incidence: A Population-Based Study of 4 Million Individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Bodenheimer, T.; Wagner, E.H.; Grumbach, K. Improving Primary Care for Patients with Chronic Illness. JAMA 2002, 288, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- HRSA Health Resources and Services Administration Chronic Care Model. Available online: https://www.hrsa.gov/behavioral-health/chronic-care-model (accessed on 18 January 2023).
- Baptista, D.R.; Wiens, A.; Pontarolo, R.; Regis, L.; Reis, W.C.T.; Correr, C.J. The Chronic Care Model for Type 2 Diabetes: A Systematic Review. Diabetol. Metab. Syndr. 2016, 8, 7. [Google Scholar] [CrossRef]
- Davy, C.; Bleasel, J.; Liu, H.; Tchan, M.; Ponniah, S.; Brown, A. Effectiveness of Chronic Care Models: Opportunities for Improving Healthcare Practice and Health Outcomes: A Systematic Review. BMC Health Serv. Res. 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Stellefson, M.; Dipnarine, K.; Stopka, C. The Chronic Care Model and Diabetes Management in US Primary Care Settings: A Systematic Review. Prev. Chronic Dis. 2013, 10, E26. [Google Scholar] [CrossRef]
- Yeoh, E.K.; Wong, M.C.S.; Wong, E.L.Y.; Yam, C.; Poon, C.M.; Chung, R.Y.; Chong, M.; Fang, Y.; Wang, H.H.X.; Liang, M.; et al. Benefits and Limitations of Implementing Chronic Care Model (CCM) in Primary Care Programs: A Systematic Review. Int. J. Cardiol. 2018, 258, 279–288. [Google Scholar] [CrossRef]
- Clark, A.M.; Wiens, K.S.; Banner, D.; Kryworuchko, J.; Thirsk, L.; McLean, L.; Currie, K. A Systematic Review of the Main Mechanisms of Heart Failure Disease Management Interventions. Heart 2016, 102, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Feltner, C.; Jones, C.D.; Cené, C.W.; Zheng, Z.-J.; Sueta, C.A.; Coker-Schwimmer, E.J.L.; Arvanitis, M.; Lohr, K.N.; Middleton, J.C.; Jonas, D.E. Transitional Care Interventions to Prevent Readmissions for Persons with Heart Failure: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2014, 160, 774. [Google Scholar] [CrossRef] [PubMed]
- Gorthi, J.; Hunter, C.B.; Mooss, A.N.; Alla, V.M.; Hilleman, D.E. Reducing Heart Failure Hospital Readmissions: A Systematic Review of Disease Management Programs. Cardiol. Res. 2014, 5, 126–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, L.; Troster, S.M.; Cai, K.; Shack, A.; Chang, Y.-J.R.; Wang, D.; Kim, J.S.; Turial, D.; Bierman, A.S. Improving Heart Failure Outcomes in Ambulatory and Community Care: A Scoping Study. Med. Care Res. Rev. 2017, 74, 551–581. [Google Scholar] [CrossRef]
- Rice, H.; Say, R.; Betihavas, V. The Effect of Nurse-Led Education on Hospitalisation, Readmission, Quality of Life and Cost in Adults with Heart Failure. A Systematic Review. Patient Educ. Couns. 2018, 101, 363–374. [Google Scholar] [CrossRef]
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J. Disease Management Interventions for Heart Failure. Cochrane Database Syst. Rev. 2019, 2019, CD002752. [Google Scholar] [CrossRef]
- Van Spall, H.G.C.; Rahman, T.; Mytton, O.; Ramasundarahettige, C.; Ibrahim, Q.; Kabali, C.; Coppens, M.; Brian Haynes, R.; Connolly, S. Comparative Effectiveness of Transitional Care Services in Patients Discharged from the Hospital with Heart Failure: A Systematic Review and Network Meta-Analysis: Comparative Effectiveness of Transitional Care Services in Patients Hospitalized with Heart Failure. Eur. J. Heart Fail. 2017, 19, 1427–1443. [Google Scholar] [CrossRef]
- Ghizzardi, G.; Arrigoni, C.; Dellafiore, F.; Vellone, E.; Caruso, R. Efficacy of Motivational Interviewing on Enhancing Self-Care Behaviors among Patients with Chronic Heart Failure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Heart Fail. Rev. 2022, 27, 1029–1041. [Google Scholar] [CrossRef]
- Kadu, M.K.; Stolee, P. Facilitators and Barriers of Implementing the Chronic Care Model in Primary Care: A Systematic Review. BMC Fam. Pract. 2015, 16, 12. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.H. Chronic Disease Management: What Will It Take to Improve Care for Chronic Illness? Eff. Clin. Pract. 1998, 1, 2–4. [Google Scholar] [PubMed]
- Bonomi, A.E.; Wagner, E.H.; Glasgow, R.E.; VonKorff, M. Assessment of Chronic Illness Care (ACIC): A Practical Tool to Measure Quality Improvement. Health Serv. Res. 2002, 37, 791–820. [Google Scholar] [CrossRef]
- Sendall, M.; McCosker, L.; Crossley, K.; Bonner, A. A Structured Review of Chronic Care Model Components Supporting Transition between Healthcare Service Delivery Types for Older People with Multiple Chronic Diseases. Health Inf. Manag. 2017, 46, 58–68. [Google Scholar] [CrossRef]
- Si, D.; Bailie, R.; Weeramanthri, T. Effectiveness of Chronic Care Model-Oriented Interventions to Improve Quality of Diabetes Care: A Systematic Review; Centre for Reviews and Dissemination: York, UK, 2008. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless Pulmonary Artery Haemodynamic Monitoring in Chronic Heart Failure: A Randomised Controlled Trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Adamson, P.B.; Gold, M.R.; Bennett, T.; Bourge, R.C.; Stevenson, L.W.; Trupp, R.; Stromberg, K.; Wilkoff, B.L.; Costanzo, M.R.; Luby, A.; et al. Continuous Hemodynamic Monitoring in Patients with Mild to Moderate Heart Failure: Results of The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients with Chronic Heart Failure (REDUCEhf) Trial. Congest. Heart Fail. 2011, 17, 248–254. [Google Scholar] [CrossRef]
- Adlbrecht, C.; Huelsmann, M.; Berger, R.; Moertl, D.; Strunk, G.; Oesterle, A.; Ahmadi, R.; Szucs, T.; Pacher, R. Cost Analysis and Cost-Effectiveness of NT-ProBNP-Guided Heart Failure Specialist Care in Addition to Home-Based Nurse Care. Eur. J. Clin. Investig. 2011, 41, 315–322. [Google Scholar] [CrossRef]
- Ågren, S.; Evangelista, L.S.; Hjelm, C.; Strömberg, A. Dyads Affected by Chronic Heart Failure: A Randomized Study Evaluating Effects of Education and Psychosocial Support to Patients with Heart Failure and Their Partners. J. Card. Fail. 2012, 18, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ågren, S.; Evangelista, L.S.; Davidson, T.; Strömberg, A. Cost-Effectiveness of a Nurse-Led Education and Psychosocial Programme for Patients with Chronic Heart Failure and Their Partners. J. Clin. Nurs. 2013, 22, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Agrinier, N.; Altieri, C.; Alla, F.; Jay, N.; Dobre, D.; Thilly, N.; Zannad, F. Effectiveness of a Multidimensional Home Nurse Led Heart Failure Disease Management Program--a French Nationwide Time-Series Comparison. Int. J. Cardiol. 2013, 168, 3652–3658. [Google Scholar] [CrossRef] [PubMed]
- Aguado, O.; Morcillo, C.; Delàs, J.; Rennie, M.; Bechich, S.; Schembari, A.; Fernández, F.; Rosell, F. Long-Term Implications of a Single Home-Based Educational Intervention in Patients with Heart Failure. Heart Lung 2010, 39, S14–S22. [Google Scholar] [CrossRef]
- Agvall, B.; Alehagen, U.; Dahlström, U. The Benefits of Using a Heart Failure Management Programme in Swedish Primary Healthcare. Eur. J. Heart Fail. 2013, 15, 228–236. [Google Scholar] [CrossRef]
- Albert, N.M.; Buchsbaum, R.; Li, J. Randomized Study of the Effect of Video Education on Heart Failure Healthcare Utilization, Symptoms, and Self-Care Behaviors. Patient Educ. Couns. 2007, 69, 129–139. [Google Scholar] [CrossRef]
- Aldamiz-Echevarría Iraúrgui, B.; Muñiz, J.; Rodríguez-Fernández, J.A.; Vidán-Martínez, L.; Silva-César, M.; Lamelo-Alfonsín, F.; Díaz-Díaz, J.L.; Ramos-Polledo, V.; Castro-Beiras, A. Randomized controlled clinical trial of a home care unit intervention to reduce readmission and death rates in patients discharged from hospital following admission for heart failure. Rev. Esp. Cardiol. 2007, 60, 914–922. [Google Scholar] [CrossRef][Green Version]
- Angermann, C.E.; Störk, S.; Gelbrich, G.; Faller, H.; Jahns, R.; Frantz, S.; Loeffler, M.; Ertl, G. Competence Network Heart Failure Mode of Action and Effects of Standardized Collaborative Disease Management on Mortality and Morbidity in Patients with Systolic Heart Failure: The Interdisciplinary Network for Heart Failure (INH) Study. Circ. Heart Fail. 2012, 5, 25–35. [Google Scholar] [CrossRef]
- Antonicelli, R.; Testarmata, P.; Spazzafumo, L.; Gagliardi, C.; Bilo, G.; Valentini, M.; Olivieri, F.; Parati, G. Impact of Telemonitoring at Home on the Management of Elderly Patients with Congestive Heart Failure. J. Telemed. Telecare 2008, 14, 300–305. [Google Scholar] [CrossRef]
- Artinian, N.T.; Harden, J.K.; Kronenberg, M.W.; Vander Wal, J.S.; Daher, E.; Stephens, Q.; Bazzi, R.I. Pilot Study of a Web-Based Compliance Monitoring Device for Patients with Congestive Heart Failure. Heart Lung 2003, 32, 226–233. [Google Scholar] [CrossRef]
- Atienza, F.; Anguita, M.; Martinez-Alzamora, N.; Osca, J.; Ojeda, S.; Almenar, L.; Ridocci, F.; Vallés, F.; de Velasco, J.A. PRICE Study Group Multicenter Randomized Trial of a Comprehensive Hospital Discharge and Outpatient Heart Failure Management Program. Eur. J. Heart Fail. 2004, 6, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.; Williams, R.; Ross, L.; Moseley, L.; Hutchison, S. Randomised Controlled Trial of Cardiac Rehabilitation in Elderly Patients with Heart Failure. Eur. J. Heart Fail. 2005, 7, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.W.; DeWalt, D.A.; Schillinger, D.; Hawk, V.; Ruo, B.; Bibbins-Domingo, K.; Weinberger, M.; Macabasco-O’Connell, A.; Grady, K.L.; Holmes, G.M.; et al. The Effect of Progressive, Reinforcing Telephone Education and Counseling Versus Brief Educational Intervention on Knowledge, Self-Care Behaviors and Heart Failure Symptoms. J. Card. Fail. 2011, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Balk, A.H.; Davidse, W.; van Dommelen, P.; Klaassen, E.; Caliskan, K.; van der Burgh, P.; Leenders, C.M. Tele-Guidance of Chronic Heart Failure Patients Enhances Knowledge about the Disease. A Multi-Centre, Randomised Controlled Study. Eur. J. Heart Fail. 2008, 10, 1136–1142. [Google Scholar] [CrossRef]
- Barnason, S.; Zimmerman, L.; Nieveen, J.; Schmaderer, M.; Carranza, B.; Reilly, S. Impact of a Home Communication Intervention for Coronary Artery Bypass Graft Patients with Ischemic Heart Failure on Self-Efficacy, Coronary Disease Risk Factor Modification, and Functioning. Heart Lung 2003, 32, 147–158. [Google Scholar] [CrossRef]
- Bekelman, D.B.; Plomondon, M.E.; Carey, E.P.; Sullivan, M.D.; Nelson, K.M.; Hattler, B.; McBryde, C.F.; Lehmann, K.G.; Gianola, K.; Heidenreich, P.A.; et al. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 725–732. [Google Scholar] [CrossRef]
- Benatar, D.; Bondmass, M.; Ghitelman, J.; Avitall, B. Outcomes of Chronic Heart Failure. Arch. Intern. Med. 2003, 163, 347–352. [Google Scholar] [CrossRef]
- Berger, R.; Moertl, D.; Peter, S.; Ahmadi, R.; Huelsmann, M.; Yamuti, S.; Wagner, B.; Pacher, R. N-Terminal pro-B-Type Natriuretic Peptide-Guided, Intensive Patient Management in Addition to Multidisciplinary Care in Chronic Heart Failure a 3-Arm, Prospective, Randomized Pilot Study. J. Am. Coll. Cardiol. 2010, 55, 645–653. [Google Scholar] [CrossRef]
- Bernocchi, P.; Scalvini, S.; Galli, T.; Paneroni, M.; Baratti, D.; Turla, O.; La Rovere, M.T.; Volterrani, M.; Vitacca, M. A Multidisciplinary Telehealth Program in Patients with Combined Chronic Obstructive Pulmonary Disease and Chronic Heart Failure: Study Protocol for a Randomized Controlled Trial. Trials 2016, 17, 462. [Google Scholar] [CrossRef]
- Bernocchi, P.; Vitacca, M.; La Rovere, M.T.; Volterrani, M.; Galli, T.; Baratti, D.; Paneroni, M.; Campolongo, G.; Sposato, B.; Scalvini, S. Home-Based Telerehabilitation in Older Patients with Chronic Obstructive Pulmonary Disease and Heart Failure: A Randomised Controlled Trial. Age Ageing 2018, 47, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Black, J.T.; Romano, P.S.; Sadeghi, B.; Auerbach, A.D.; Ganiats, T.G.; Greenfield, S.; Kaplan, S.H.; Ong, M.K. BEAT-HF Research Group A Remote Monitoring and Telephone Nurse Coaching Intervention to Reduce Readmissions among Patients with Heart Failure: Study Protocol for the Better Effectiveness after Transition—Heart Failure (BEAT-HF) Randomized Controlled Trial. Trials 2014, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Blue, L.; Lang, E.; McMurray, J.J.; Davie, A.P.; McDonagh, T.A.; Murdoch, D.R.; Petrie, M.C.; Connolly, E.; Norrie, J.; Round, C.E.; et al. Randomised Controlled Trial of Specialist Nurse Intervention in Heart Failure. BMJ 2001, 323, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Bourge, R.C.; Abraham, W.T.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M.; Magalski, A.; Zile, M.R.; Smith, A.L.; Smart, F.W.; O’Shaughnessy, M.A.; et al. Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients with Advanced Heart Failure: The COMPASS-HF Study. J. Am. Coll. Cardiol. 2008, 51, 1073–1079. [Google Scholar] [CrossRef]
- Brandon, A.F.; Schuessler, J.B.; Ellison, K.J.; Lazenby, R.B. The Effects of an Advanced Practice Nurse Led Telephone Intervention on Outcomes of Patients with Heart Failure. Appl. Nurs. Res. 2009, 22, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.F.; Casper, G.R.; Burke, L.J.; Johnson, K.A.; Brown, R.; Valdez, R.S.; Sebern, M.; Perez, O.A.; Sturgeon, B. Technology-Enhanced Practice for Patients with Chronic Cardiac Disease: Home Implementation and Evaluation. Heart Lung 2010, 39, S34–S46. [Google Scholar] [CrossRef] [PubMed]
- Brotons, C.; Falces, C.; Alegre, J.; Ballarín, E.; Casanovas, J.; Catà, T.; Martínez, M.; Moral, I.; Ortiz, J.; Pérez, E.; et al. Randomized Clinical Trial of the Effectiveness of a Home-Based Intervention in Patients with Heart Failure: The IC-DOM Study. Rev. Española Cardiol. 2009, 62, 400–408. [Google Scholar] [CrossRef]
- Capomolla, S.; Febo, O.; Ceresa, M.; Caporotondi, A.; Guazzotti, G.; La Rovere, M.; Ferrari, M.; Lenta, F.; Baldin, S.; Vaccarini, C.; et al. Cost/Utility Ratio in Chronic Heart Failure: Comparison between Heart Failure Management Program Delivered by Day-Hospital and Usual Care. J. Am. Coll. Cardiol. 2002, 40, 1259–1266. [Google Scholar] [CrossRef]
- Çavuşoğlu, Y.; Zoghi, M.; Eren, M.; Bozçalı, E.; Kozdağ, G.; Şentürk, T.; Alicik, G.; Soylu, K.; Sarı, İ.; Berilgen, R.; et al. Post-Discharge Heart Failure Monitoring Program in Turkey: Hit-PoinT. Anatol. J. Cardiol. 2017, 17, 107–112. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in Patients with Heart Failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef]
- Chen, Y.; Funk, M.; Wen, J.; Tang, X.; He, G.; Liu, H. Effectiveness of a Multidisciplinary Disease Management Program on Outcomes in Patients with Heart Failure in China: A Randomized Controlled Single Center Study. Heart Lung 2018, 47, 24–31. [Google Scholar] [CrossRef]
- Clark, A.P.; McDougall, G.; Riegel, B.; Joiner-Rogers, G.; Innerarity, S.; Meraviglia, M.; Delville, C.; Davila, A. Health Status and Self-Care Outcomes after an Education-Support Intervention for People with Chronic Heart Failure. J. Cardiovasc. Nurs. 2015, 30, S3–S13. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Louis, A.A.; Rigby, A.S.; Janssens, U.; Balk, A.H.M.M. TEN-HMS Investigators Noninvasive Home Telemonitoring for Patients with Heart Failure at High Risk of Recurrent Admission and Death: The Trans-European Network-Home-Care Management System (TEN-HMS) Study. J. Am. Coll. Cardiol. 2005, 45, 1654–1664. [Google Scholar] [CrossRef]
- Cline, C.M.; Israelsson, B.Y.; Willenheimer, R.B.; Broms, K.; Erhardt, L.R. Cost Effective Management Programme for Heart Failure Reduces Hospitalisation. Heart 1998, 80, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H. CONNECT Investigators The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial: The Value of Wireless Remote Monitoring with Automatic Clinician Alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- das Cruz, F.D.; Issa, V.S.; Ayub-Ferreira, S.M.; Chizzola, P.R.; Souza, G.E.C.; Moreira, L.F.P.; Lanz-Luces, J.R.; Bocchi, E.A. Effect of a Sequential Education and Monitoring Programme on Quality-of-Life Components in Heart Failure. Eur. J. Heart Fail. 2010, 12, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Dansky, K.H.; Vasey, J.; Bowles, K. Use of Telehealth by Older Adults to Manage Heart Failure. Res. Gerontol. Nurs. 2008, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dansky, K.; Vasey, J. Managing Heart Failure Patients after Formal Homecare. Telemed. E-Health 2009, 15, 983–991. [Google Scholar] [CrossRef]
- Dar, O.; Riley, J.; Chapman, C.; Dubrey, S.W.; Morris, S.; Rosen, S.D.; Roughton, M.; Cowie, M.R. A Randomized Trial of Home Telemonitoring in a Typical Elderly Heart Failure Population in North West London: Results of the Home-HF Study. Eur. J. Heart Fail. 2009, 11, 319–325. [Google Scholar] [CrossRef]
- Davis, K.K.; Mintzer, M.; Dennison Himmelfarb, C.R.; Hayat, M.J.; Rotman, S.; Allen, J. Targeted Intervention Improves Knowledge but Not Self-Care or Readmissions in Heart Failure Patients with Mild Cognitive Impairment. Eur. J. Heart Fail. 2012, 14, 1041–1049. [Google Scholar] [CrossRef]
- de la Porte, P.W.F.B.-A.; Lok, D.J.A.; van Veldhuisen, D.J.; van Wijngaarden, J.; Cornel, J.H.; Zuithoff, N.P.A.; Badings, E.; Hoes, A.W. Added Value of a Physician-and-Nurse-Directed Heart Failure Clinic: Results from the Deventer-Alkmaar Heart Failure Study. Heart 2007, 93, 819–825. [Google Scholar] [CrossRef]
- de Souza, E.N.; Rohde, L.E.; Ruschel, K.B.; Mussi, C.M.; Beck-da-Silva, L.; Biolo, A.; Clausell, N.; Rabelo-Silva, E.R. A Nurse-Based Strategy Reduces Heart Failure Morbidity in Patients Admitted for Acute Decompensated Heart Failure in Brazil: The HELEN-II Clinical Trial. Eur. J. Heart Fail. 2014, 16, 1002–1008. [Google Scholar] [CrossRef]
- DeBusk, R.F.; Miller, N.H.; Parker, K.M.; Bandura, A.; Kraemer, H.C.; Cher, D.J.; West, J.A.; Fowler, M.B.; Greenwald, G. Care Management for Low-Risk Patients with Heart Failure: A Randomized, Controlled Trial. Ann. Intern. Med. 2004, 141, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Del Sindaco, D.; Pulignano, G.; Minardi, G.; Apostoli, A.; Guerrieri, L.; Rotoloni, M.; Petri, G.; Fabrizi, L.; Caroselli, A.; Venusti, R.; et al. Two-Year Outcome of a Prospective, Controlled Study of a Disease Management Programme for Elderly Patients with Heart Failure. J. Cardiovasc. Med. 2007, 8, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Dendale, P.; De Keulenaer, G.; Troisfontaines, P.; Weytjens, C.; Mullens, W.; Elegeert, I.; Ector, B.; Houbrechts, M.; Willekens, K.; Hansen, D. Effect of a Telemonitoring-Facilitated Collaboration between General Practitioner and Heart Failure Clinic on Mortality and Rehospitalization Rates in Severe Heart Failure: The TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) Study. Eur. J. Heart Fail. 2012, 14, 333–340. [Google Scholar] [CrossRef] [PubMed]
- DeWalt, D.A.; Malone, R.M.; Bryant, M.E.; Kosnar, M.C.; Corr, K.E.; Rothman, R.L.; Sueta, C.A.; Pignone, M.P. A Heart Failure Self-Management Program for Patients of All Literacy Levels: A Randomized, Controlled Trial [ISRCTN11535170]. BMC Health Serv. Res. 2006, 6, 30. [Google Scholar] [CrossRef]
- DeWalt, D.A.; Schillinger, D.; Ruo, B.; Bibbins-Domingo, K.; Baker, D.W.; Holmes, G.M.; Weinberger, M.; Macabasco-O’Connell, A.; Broucksou, K.; Hawk, V.; et al. Multisite Randomized Trial of a Single-Session Versus Multisession Literacy-Sensitive Self-Care Intervention for Patients with Heart Failure. Circulation 2012, 125, 2854–2862. [Google Scholar] [CrossRef]
- Domingo, M.; Lupón, J.; González, B.; Crespo, E.; López, R.; Ramos, A.; Urrutia, A.; Pera, G.; Verdú, J.M.; Bayes-Genis, A. Evaluation of a Telemedicine System for Heart Failure Patients: Feasibility, Acceptance Rate, Satisfaction and Changes in Patient Behavior: Results from the CARME (CAtalan Remote Management Evaluation) Study. Eur. J. Cardiovasc. Nurs. 2012, 11, 410–418. [Google Scholar] [CrossRef]
- Domingues, F.B.; Clausell, N.; Aliti, G.B.; Dominguez, D.R.; Rabelo, E.R. Education and Telephone Monitoring by Nurses of Patients with Heart Failure: Randomized Clinical Trial. Arq. Bras. Cardiol. 2011, 96, 233–239. [Google Scholar] [CrossRef]
- Doughty, R.N.; Wright, S.P.; Pearl, A.; Walsh, H.J.; Muncaster, S.; Whalley, G.A.; Gamble, G.; Sharpe, N. Randomized, Controlled Trial of Integrated Heart Failure Management: The Auckland Heart Failure Management Study. Eur. Heart J. 2002, 23, 139–146. [Google Scholar] [CrossRef]
- Ducharme, A.; Doyon, O.; White, M.; Rouleau, J.L.; Brophy, J.M. Impact of Care at a Multidisciplinary Congestive Heart Failure Clinic: A Randomized Trial. CMAJ 2005, 173, 40–45. [Google Scholar] [CrossRef]
- Duffy, J.R.; Hoskins, L.M.; Dudley-Brown, S. Improving Outcomes for Older Adults with Heart Failure: A Randomized Trial Using a Theory-Guided Nursing Intervention. J. Nurs. Care Qual. 2010, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.B.; Reilly, C.M.; Gary, R.; Higgins, M.K.; Culler, S.; Butts, B.; Butler, J. Randomized Clinical Trial of an Integrated Self-Care Intervention for Persons with Heart Failure and Diabetes: Quality of Life and Physical Functioning Outcomes. J. Card. Fail. 2015, 21, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Ekman, I.; Andersson, B.; Ehnfors, M.; Matejka, G.; Persson, B.; Fagerberg, B. Feasibility of a Nurse-Monitored, Outpatient-Care Programme for Elderly Patients with Moderate-to-Severe, Chronic Heart Failure. Eur. Heart J. 1998, 19, 1254–1260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eyre, V.; Lang, C.C.; Smith, K.; Jolly, K.; Davis, R.; Hayward, C.; Wingham, J.; Abraham, C.; Green, C.; Warren, F.C.; et al. Rehabilitation Enablement in Chronic Heart Failure-a Facilitated Self-Care Rehabilitation Intervention in Patients with Heart Failure with Preserved Ejection Fraction (REACH-HFpEF) and Their Caregivers: Rationale and Protocol for a Single-Centre Pilot Randomised Controlled Trial. BMJ Open 2016, 6, e012853. [Google Scholar] [CrossRef]
- Feldman, P.H.; Murtaugh, C.M.; Pezzin, L.E.; McDonald, M.V.; Peng, T.R. Just-in-Time Evidence-Based e-Mail “Reminders” in Home Health Care: Impact on Patient Outcomes. Health Serv. Res. 2005, 40, 865–885. [Google Scholar] [CrossRef]
- Feldman, P.H.; Peng, T.R.; Murtaugh, C.M.; Kelleher, C.; Donelson, S.M.; McCann, M.E.; Putnam, M.E. A Randomized Intervention to Improve Heart Failure Outcomes in Community-Based Home Health Care. Home Health Care Serv. Q. 2004, 23, 1–23. [Google Scholar] [CrossRef]
- Ferrante, D.; Varini, S.; Macchia, A.; Soifer, S.; Badra, R.; Nul, D.; Grancelli, H.; Doval, H. Long-Term Results after a Telephone Intervention in Chronic Heart Failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) Follow-Up. J. Am. Coll. Cardiol. 2010, 56, 372–378. [Google Scholar] [CrossRef]
- Finkelstein, J.; Dennison, C.R. A Pilot Study of Home Automated Telemanagement (HAT) System in African Americans with Congestive Heart Failure. In Proceedings of the 2010 Second International Conference on eHealth, Telemedicine, and Social Medicine, Washington, DC, USA, 10–16 February 2010; IEEE: St. Maarten, Netherlands Antilles, 2010; pp. 90–94. [Google Scholar]
- Flynn, K.J.; Powell, L.H.; Mendes de Leon, C.F.; Muñoz, R.; Eaton, C.B.; Downs, D.L.; Silver, M.A.; Calvin, J.E. Increasing Self-Management Skills in Heart Failure Patients: A Pilot Study. Congest. Heart Fail. 2005, 11, 297–302. [Google Scholar] [CrossRef][Green Version]
- Galbreath, A.D.; Krasuski, R.A.; Smith, B.; Stajduhar, K.C.; Kwan, M.D.; Ellis, R.; Freeman, G.L. Long-Term Healthcare and Cost Outcomes of Disease Management in a Large, Randomized, Community-Based Population with Heart Failure. Circulation 2004, 110, 3518–3526. [Google Scholar] [CrossRef]
- Gattis, W.A.; Hasselblad, V.; Whellan, D.J.; O’Connor, C.M. Reduction in Heart Failure Events by the Addition of a Clinical Pharmacist to the Heart Failure Management Team: Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch. Intern. Med. 1999, 159, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Scalvini, S.; Zanelli, E.; Corrà, U.; Longobardi, G.L.; Ricci, V.A.; Baiardi, P.; Glisenti, F. Multicenter Randomised Trial on Home-Based Telemanagement to Prevent Hospital Readmission of Patients with Chronic Heart Failure. Int. J. Cardiol. 2009, 131, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.R.; Piette, J.D.; Walsh, M.N.; Frank, T.A.; Jaski, B.E.; Smith, A.L.; Rodriguez, R.; Mancini, D.M.; Hopton, L.A.; Orav, E.J.; et al. Randomized Trial of a Daily Electronic Home Monitoring System in Patients with Advanced Heart Failure: The Weight Monitoring in Heart Failure (WHARF) Trial. Am. Heart J. 2003, 146, 705–712. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Lupón, J.; Herreros, J.; Urrutia, A.; Altimir, S.; Coll, R.; Prats, M.; Valle, V. Patient’s Education by Nurse: What We Really Do Achieve? Eur. J. Cardiovasc. Nurs. 2005, 4, 107–111. [Google Scholar] [CrossRef]
- González-Guerrero, J.L.; Alonso-Fernández, T.; García-Mayolín, N.; Gusi, N.; Ribera-Casado, J.M. Effect of A Follow-Up Program in Elderly Adults with Heart Failure with Cognitive Impairment after Hospital Discharge. J. Am. Geriatr. Soc. 2015, 63, 1950–1951. [Google Scholar] [CrossRef]
- Gotsman, I.; Zwas, D.; Zemora, Z.; Jabara, R.; Admon, D.; Lotan, C.; Keren, A. Clinical Outcome of Patients with Chronic Heart Failure Followed in a Specialized Heart Failure Center. Isr. Med. Assoc. J. 2011, 13, 468–473. [Google Scholar] [PubMed]
- Granger, B.B.; Sandelowski, M.; Tahshjain, H.; Swedberg, K.; Ekman, I. A Qualitative Descriptive Study of the Work of Adherence to a Chronic Heart Failure Regimen: Patient and Physician Perspectives. J. Cardiovasc. Nurs. 2009, 24, 308–315. [Google Scholar] [CrossRef]
- Hanchett, E.; Torrens, P.R. A Public Health Home Nursing Program for Outpatients with Heart Diseases. Public. Health Rep. 1967, 82, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.B.; Browne, G.B.; Roberts, J.; Tugwell, P.; Gafni, A.; Graham, I.D. Quality of Life of Individuals with Heart Failure: A Randomized Trial of the Effectiveness of Two Models of Hospital-to-Home Transition. Med. Care 2002, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.L.; Sisk, J.E.; Wang, J.J.; Tuzzio, L.; Casabianca, J.M.; Chassin, M.R.; Horowitz, C.; McLaughlin, M.A. Cost-Effectiveness of Nurse-Led Disease Management for Heart Failure in an Ethnically Diverse Urban Community. Ann. Intern. Med. 2008, 149, 540–548. [Google Scholar] [CrossRef]
- Heisler, M.; Halasyamani, L.; Resnicow, K.; Neaton, M.; Shanahan, J.; Brown, S.; Piette, J.D. “I Am Not Alone”: The Feasibility and Acceptability of Interactive Voice Response-Facilitated Telephone Peer Support Among Older Adults with Heart Failure. Congest. Heart Fail. 2007, 13, 149–157. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Ni, H.; Nauman, D.J.; Burgess, D.; Toy, W.; Wise, K.; Dutton, D.; Crispell, K.; Vossler, M.; Everett, J. Prospective Evaluation of an Outpatient Heart Failure Management Program. J. Card. Fail. 2001, 7, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Brooksby, I.; Lenaghan, E.; Ashton, K.; Hay, L.; Smith, R.; Shepstone, L.; Lipp, A.; Daly, C.; Howe, A.; et al. Effectiveness of Visits from Community Pharmacists for Patients with Heart Failure: HeartMed Randomised Controlled Trial. BMJ 2007, 334, 1098. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Yang, H.; Chan, L.S.; Or, K.; Lee, D.T.F.; Yu, C.M.; Woo, J. A Community Model of Group Rehabilitation for Older Patients with Chronic Heart Failure: A Pilot Study. Disabil. Rehabil. 2006, 28, 1491–1497. [Google Scholar] [CrossRef]
- Inglis, S.C.; Pearson, S.; Treen, S.; Gallasch, T.; Horowitz, J.D.; Stewart, S. Extending the Horizon in Chronic Heart Failure: Effects of Multidisciplinary, Home-Based Intervention Relative to Usual Care. Circulation 2006, 114, 2466–2473. [Google Scholar] [CrossRef][Green Version]
- Jaarsma, T.; Halfens, R.; Huijer Abu-Saad, H.; Dracup, K.; Gorgels, T.; van Ree, J.; Stappers, J. Effects of Education and Support on Self-Care and Resource Utilization in Patients with Heart Failure. Eur. Heart J. 1999, 20, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, T.; Halfens, R.; Tan, F.; Abu-Saad, H.H.; Dracup, K.; Diederiks, J. Self-Care and Quality of Life in Patients with Advanced Heart Failure: The Effect of a Supportive Educational Intervention. Heart Lung 2000, 29, 319–330. [Google Scholar] [CrossRef]
- Jaarsma, T.; Abu-Saad, H.H.; Dracup, K.; Halfens, R. Self-Care Behaviour of Patients with Heart Failure. Scand. J. Caring Sci. 2000, 14, 112–119. [Google Scholar] [CrossRef]
- Jaarsma, T.; Van Der Wal, M.H.L.; Hogenhuis, J.; Lesman, I.; Luttik, M.-L.A.; Veeger, N.J.G.M.; Van Veldhuisen, D.J. Design and Methodology of the COACH Study: A Multicenter Randomised Coordinating Study Evaluating Outcomes of Advising and Counselling in Heart Failure. Eur. J. Heart Fail. 2004, 6, 227–233. [Google Scholar] [CrossRef]
- Jaarsma, T.; van der Wal, M.H.L.; Lesman-Leegte, I.; Luttik, M.-L.; Hogenhuis, J.; Veeger, N.J.; Sanderman, R.; Hoes, A.W.; van Gilst, W.H.; Lok, D.J.A.; et al. Effect of Moderate or Intensive Disease Management Program on Outcome in Patients with Heart Failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch. Intern. Med. 2008, 168, 316–324. [Google Scholar] [CrossRef]
- Jerant, A.F.; Azari, R.; Martinez, C.; Nesbitt, T.S. A Randomized Trial of Telenursing to Reduce Hospitalization for Heart Failure: Patient-Centered Outcomes and Nursing Indicators. Home Health Care Serv. Q. 2003, 22, 1–20. [Google Scholar] [CrossRef]
- Jerant, A.F.; von Friederichs-Fitzwater, M.M.; Moore, M. Patients’ Perceived Barriers to Active Self-Management of Chronic Conditions. Patient Educ. Couns. 2005, 57, 300–307. [Google Scholar] [CrossRef]
- Karlsson, M.R.; Edner, M.; Henriksson, P.; Mejhert, M.; Persson, H.; Grut, M.; Billing, E. A Nurse-Based Management Program in Heart Failure Patients Affects Females and Persons with Cognitive Dysfunction Most. Patient Educ. Couns. 2005, 58, 146–153. [Google Scholar] [CrossRef]
- Kasper, E.K.; Gerstenblith, G.; Hefter, G.; Van Anden, E.; Brinker, J.A.; Thiemann, D.R.; Terrin, M.; Forman, S.; Gottlieb, S.H. A Randomized Trial of the Efficacy of Multidisciplinary Care in Heart Failure Outpatients at High Risk of Hospital Readmission. J. Am. Coll. Cardiol. 2002, 39, 471–480. [Google Scholar] [CrossRef]
- Khunti, K.; Stone, M.; Paul, S.; Baines, J.; Gisborne, L.; Farooqi, A.; Luan, X.; Squire, I. Disease Management Programme for Secondary Prevention of Coronary Heart Disease and Heart Failure in Primary Care: A Cluster Randomised Controlled Trial. Heart 2007, 93, 1398–1405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimmelstiel, C.; Levine, D.; Perry, K.; Patel, A.R.; Sadaniantz, A.; Gorham, N.; Cunnie, M.; Duggan, L.; Cotter, L.; Shea-Albright, P.; et al. Randomized, Controlled Evaluation of Short- and Long-Term Benefits of Heart Failure Disease Management within a Diverse Provider Network: The SPAN-CHF Trial. Circulation 2004, 110, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.S.; Scott, L.D.; Britton, A.S. The Use of Supportive-Educative and Mutual Goal-Setting Strategies to Improve Self-Management for Patients with Heart Failure. Home Healthc. Nurse 2007, 25, 502–510. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients with Chronic Heart Failure: The Telemedical Interventional Monitoring in Heart Failure Study. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef]
- Koelling, T.M.; Johnson, M.L.; Cody, R.J.; Aaronson, K.D. Discharge Education Improves Clinical Outcomes in Patients with Chronic Heart Failure. Circulation 2005, 111, 179–185. [Google Scholar] [CrossRef]
- Kommuri, N.V.A.; Johnson, M.L.; Koelling, T.M. Relationship between Improvements in Heart Failure Patient Disease Specific Knowledge and Clinical Events as Part of a Randomized Controlled Trial. Patient Educ. Couns. 2012, 86, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Amatruda, J.; Smith, G.L.; Mattera, J.A.; Roumanis, S.A.; Radford, M.J.; Crombie, P.; Vaccarino, V. Randomized Trial of an Education and Support Intervention to Prevent Readmission of Patients with Heart Failure. J. Am. Coll. Cardiol. 2002, 39, 83–89. [Google Scholar] [CrossRef]
- Kwok, T.; Lee, J.; Woo, J.; Lee, D.T.; Griffith, S. A Randomized Controlled Trial of a Community Nurse-Supported Hospital Discharge Programme in Older Patients with Chronic Heart Failure. J. Clin. Nurs. 2008, 17, 109–117. [Google Scholar] [CrossRef]
- LaFramboise, L.M.; Woster, J.; Yager, A.; Yates, B.C. A Technological Life Buoy: Patient Perceptions of the Health Buddy. J. Cardiovasc. Nurs. 2009, 24, 216–224. [Google Scholar] [CrossRef]
- Lainscak, M. Implementation of Guidelines for Management of Heart Failure in Heart Failure Clinic: Effects beyond Pharmacological Treatment. Int. J. Cardiol. 2004, 97, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote Monitoring Reduces Healthcare Use and Improves Quality of Care in Heart Failure Patients with Implantable Defibrillators: The Evolution of Management Strategies of Heart Failure Patients with Implantable Defibrillators (EVOLVO) Study. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef]
- Lang, C.C.; Smith, K.; Wingham, J.; Eyre, V.; Greaves, C.J.; Warren, F.C.; Green, C.; Jolly, K.; Davis, R.C.; Doherty, P.J.; et al. A Randomised Controlled Trial of a Facilitated Home-Based Rehabilitation Intervention in Patients with Heart Failure with Preserved Ejection Fraction and Their Caregivers: The REACH-HFpEF Pilot Study. BMJ Open 2018, 8, e019649. [Google Scholar] [CrossRef]
- Laramee, A.S.; Levinsky, S.K.; Sargent, J.; Ross, R.; Callas, P. Case Management in a Heterogeneous Congestive Heart Failure Population: A Randomized Controlled Trial. Arch. Intern. Med. 2003, 163, 809–817. [Google Scholar] [CrossRef]
- Ledwidge, M.; Barry, M.; Cahill, J.; Ryan, E.; Maurer, B.; Ryder, M.; Travers, B.; Timmons, L.; McDonald, K. Is Multidisciplinary Care of Heart Failure Cost-Beneficial When Combined with Optimal Medical Care? Eur. J. Heart Fail. 2003, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, M.E.; Denhaerynck, K.; Brunner-La Rocca, H.-P.; Burnand, B.; Conca-Zeller, A.; Bernasconi, A.T.; Mahrer-Imhof, R.; Froelicher, E.S.; De Geest, S. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF): A Randomised Controlled Trial Study of an Outpatient Inter-Professional Management Programme for Heart Failure Patients in Switzerland. Swiss Med. Wkly. 2011, 141, w13171. [Google Scholar] [CrossRef]
- Liljeroos, M.; Ågren, S.; Jaarsma, T.; Årestedt, K.; Strömberg, A. Long Term Follow-Up after a Randomized Integrated Educational and Psychosocial Intervention in Patient-Partner Dyads Affected by Heart Failure. PLoS ONE 2015, 10, e0138058. [Google Scholar] [CrossRef] [PubMed]
- Linné, A.B.; Liedholm, H. Effects of an Interactive CD-Program on 6 Months Readmission Rate in Patients with Heart Failure—A Randomised, Controlled Trial [NCT00311194]. BMC Cardiovasc. Disord. 2006, 6, 30. [Google Scholar] [CrossRef][Green Version]
- Liu, M.-H.; Wang, C.-H.; Huang, Y.-Y.; Tung, T.-H.; Lee, C.-M.; Yang, N.-I.; Wang, J.-S.; Kuo, L.-T.; Cherng, W.-J. Edema Index-Guided Disease Management Improves 6-Month Outcomes of Patients with Acute Heart Failure. Int. Heart J. 2012, 53, 11–17. [Google Scholar] [CrossRef] [PubMed]
- López Cabezas, C.; Falces Salvador, C.; Cubí Quadrada, D.; Arnau Bartés, A.; Ylla Boré, M.; Muro Perea, N.; Homs Peipoch, E. Randomized Clinical Trial of a Postdischarge Pharmaceutical Care Program vs Regular Follow-up in Patients with Heart Failure. Farm. Hosp. 2006, 30, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.; Hopp, F.; Subramanian, U.; Wiitala, W.; Welsh, D.E.; Larkin, A.; Stemmer, K.; Zak, C.; Vaitkevicius, P. Evaluation of a Nurse Practitioner Disease Management Model for Chronic Heart Failure: A Multi-Site Implementation Study. Congest. Heart Fail. 2012, 18, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lupón, J.; González, B.; Mas, D.; Urrutia, A.; Arenas, M.; Domingo, M.; Altimir, S.; Valle, V. Patients’ Self-Care Improvement with Nurse Education Intervention in Spain Assessed by the European Heart Failure Self-Care Behaviour Scale. Eur. J. Cardiovasc. Nurs. 2008, 7, 16–20. [Google Scholar] [CrossRef]
- Mao, C.-T.; Liu, M.-H.; Hsu, K.-H.; Fu, T.-C.; Wang, J.-S.; Huang, Y.-Y.; Yang, N.-I.; Wang, C.-H. Effect of Multidisciplinary Disease Management for Hospitalized Heart Failure under a National Health Insurance Programme. J. Cardiovasc. Med. 2015, 16, 616–624. [Google Scholar] [CrossRef] [PubMed]
- McCauley, K.M.; Bixby, M.B.; Naylor, M.D. Advanced Practice Nurse Strategies to Improve Outcomes and Reduce Cost in Elders with Heart Failure. Dis. Manag. 2006, 9, 302–310. [Google Scholar] [CrossRef]
- McDonald, K.; Ledwidge, M.; Cahill, J.; Quigley, P.; Maurer, B.; Travers, B.; Ryder, M.; Kieran, E.; Timmons, L.; Ryan, E. Heart Failure Management: Multidisciplinary Care Has Intrinsic Benefit above the Optimization of Medical Care. J. Card. Fail. 2002, 8, 142–148. [Google Scholar] [CrossRef]
- Mehralian, H.; Salehi, S.; Moghaddasi, J.; Amiri, M.; Rafiei, H. The Comparison of the Effects of Education Provided by Nurses on the Quality of Life in Patients with Congestive Heart Failure (CHF) in Usual and Home-Visit Cares in Iran. Glob. J. Health Sci. 2014, 6, 256–260. [Google Scholar] [CrossRef]
- Mejhert, M.; Kahan, T.; Persson, H.; Edner, M. Limited Long Term Effects of a Management Programme for Heart Failure. Heart 2004, 90, 1010–1015. [Google Scholar] [CrossRef]
- Miche, E.; Roelleke, E.; Zoller, B.; Wirtz, U.; Schneider, M.; Huerst, M.; Amelang, M.; Radzewitz, A. A Longitudinal Study of Quality of Life in Patients with Chronic Heart Failure Following an Exercise Training Program. Eur. J. Cardiovasc. Nurs. 2009, 8, 281–287. [Google Scholar] [CrossRef]
- Moertl, D.; Steiner, S.; Coyle, D.; Berger, R. Cost-Utility Analysis of Nt-Probnp-Guided Multidisciplinary Care in Chronic Heart Failure. Int. J. Technol. Assess. Health Care 2013, 29, 3–11. [Google Scholar] [CrossRef][Green Version]
- Mortara, A.; Pinna, G.D.; Johnson, P.; Maestri, R.; Capomolla, S.; La Rovere, M.T.; Ponikowski, P.; Tavazzi, L.; Sleight, P. HHH Investigators Home Telemonitoring in Heart Failure Patients: The HHH Study (Home or Hospital in Heart Failure). Eur. J. Heart Fail. 2009, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Mussi, C.M.; Ruschel, K.; de Souza, E.N.; Lopes, A.N.M.; Trojahn, M.M.; Paraboni, C.C.; Rabelo, E.R. Home Visit Improves Knowledge, Self-Care and Adhesion in Heart Failure: Randomized Clinical Trial HELEN-I. Rev. Lat. Am. Enferm. 2013, 21, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.D.; Brooten, D.A.; Campbell, R.L.; Maislin, G.; McCauley, K.M.; Schwartz, J.S. Transitional Care of Older Adults Hospitalized with Heart Failure: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2004, 52, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Albanese, M.C.; De Biaggio, P.; Caliandro, D.; Gregori, D.; Goss, P.; Miani, D.; Fresco, C.; Rossi, P.; Bulfoni, A.; et al. Lack of Improvement of Clinical Outcomes by a Low-Cost, Hospital-Based Heart Failure Management Programme. J. Cardiovasc. Med. 2006, 7, 614–622. [Google Scholar] [CrossRef]
- Oddone, E.Z.; Weinberger, M.; Giobbie-Hurder, A.; Landsman, P.; Henderson, W. Enhanced Access to Primary Care for Patients with Congestive Heart Failure. Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. Eff. Clin. Pract. 1999, 2, 201–209. [Google Scholar]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring after Discharge of Hospitalized Patients with Heart Failure: The Better Effectiveness after Transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef]
- Paradis, V.; Cossette, S.; Frasure-Smith, N.; Heppell, S.; Guertin, M.-C. The Efficacy of a Motivational Nursing Intervention Based on the Stages of Change on Self-Care in Heart Failure Patients. J. Cardiovasc. Nurs. 2010, 25, 130–141. [Google Scholar] [CrossRef]
- Pekmezaris, R.; Mitzner, I.; Pecinka, K.R.; Nouryan, C.N.; Lesser, M.L.; Siegel, M.; Swiderski, J.W.; Moise, G.; Younker, R.; Smolich, K. The Impact of Remote Patient Monitoring (Telehealth) upon Medicare Beneficiaries with Heart Failure. Telemed. J. E-Health 2012, 18, 101–108. [Google Scholar] [CrossRef]
- Phillips, C.O.; Singa, R.M.; Rubin, H.R.; Jaarsma, T. Complexity of Program and Clinical Outcomes of Heart Failure Disease Management Incorporating Specialist Nurse-Led Heart Failure Clinics. A Meta-Regression Analysis. Eur. J. Heart Fail. 2005, 7, 333–341. [Google Scholar] [CrossRef]
- Postmus, D.; Pari, A.A.A.; Jaarsma, T.; Luttik, M.L.; van Veldhuisen, D.J.; Hillege, H.L.; Buskens, E. A Trial-Based Economic Evaluation of 2 Nurse-Led Disease Management Programs in Heart Failure. Am. Heart J. 2011, 162, 1096–1104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Powell, L.H.; Calvin, J.E.; Richardson, D.; Janssen, I.; Mendes de Leon, C.F.; Flynn, K.J.; Grady, K.L.; Rucker-Whitaker, C.S.; Eaton, C.; Avery, E.; et al. Self-Management Counseling in Patients with Heart Failure: The Heart Failure Adherence and Retention Randomized Behavioral Trial. JAMA 2010, 304, 1331–1338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rainville, E.C. Impact of Pharmacist Interventions on Hospital Readmissions for Heart Failure. Am. J. Health Syst. Pharm. 1999, 56, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Butler, J.; Culler, S.D.; Gary, R.A.; Higgins, M.; Schindler, P.; Butts, B.; Dunbar, S.B. An Economic Evaluation of a Self-Care Intervention in Persons with Heart Failure and Diabetes. J. Card. Fail. 2015, 21, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.W.; Beckham, V.; Wittenberg, C.; Leven, C.L.; Freedland, K.E.; Carney, R.M. A Multidisciplinary Intervention to Prevent the Readmission of Elderly Patients with Congestive Heart Failure. N. Engl. J. Med. 1995, 333, 1190–1195. [Google Scholar] [CrossRef]
- Rich, M.W.; Vinson, J.M.; Sperry, J.C.; Shah, A.S.; Spinner, L.R.; Chung, M.K.; Davila-Roman, V. Prevention of Readmission in Elderly Patients with Congestive Heart Failure: Results of a Prospective, Randomized Pilot Study. J. Gen. Intern. Med. 1993, 8, 585–590. [Google Scholar] [CrossRef]
- Riegel, B.; Carlson, B.; Glaser, D.; Romero, T. Randomized Controlled Trial of Telephone Case Management in Hispanics of Mexican Origin with Heart Failure. J. Card. Fail. 2006, 12, 211–219. [Google Scholar] [CrossRef]
- Riegel, B.; Carlson, B.; Kopp, Z.; LePetri, B.; Glaser, D.; Unger, A. Effect of a Standardized Nurse Case-Management Telephone Intervention on Resource Use in Patients with Chronic Heart Failure. Arch. Intern. Med. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Riegel, B.; Dickson, V.V.; Hoke, L.; McMahon, J.P.; Reis, B.F.; Sayers, S. A Motivational Counseling Approach to Improving Heart Failure Self-Care: Mechanisms of Effectiveness. J. Cardiovasc. Nurs. 2006, 21, 232–241. [Google Scholar] [CrossRef]
- Ruschel, K.B.; Rabelo-Silva, E.R.; Rohde, L.E.; de Souza, E.N.; Mussi, C.M.; Polanczyk, C.A. Cost-Effectiveness of a Home Visit Program for Patients with Heart Failure in Brazil: Evidence from a Randomized Clinical Trial. Value Health Reg. Issues 2018, 17, 81–87. [Google Scholar] [CrossRef]
- Sales, V.L.; Ashraf, M.S.; Lella, L.K.; Huang, J.; Bhumireddy, G.; Lefkowitz, L.; Feinstein, M.; Kamal, M.; Caesar, R.; Cusick, E.; et al. Utilization of Trained Volunteers Decreases 30-Day Readmissions for Heart Failure. J. Card. Fail. 2013, 19, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.S.; Kline, S.E.; Schmalfuss, C.M.; Carver, H.M.; Aranda, J.M.; Pauly, D.F.; Hill, J.A.; Neugaard, B.I.; Chumbler, N.R. Early Outcomes of a Care Coordination-Enhanced Telehome Care Program for Elderly Veterans with Chronic Heart Failure. Telemed. E-Health 2005, 11, 20–27. [Google Scholar] [CrossRef]
- Schwarz, K.A.; Mion, L.C.; Hudock, D.; Litman, G. Telemonitoring of Heart Failure Patients and Their Caregivers: A Pilot Randomized Controlled Trial. Prog. Cardiovasc. Nurs. 2008, 23, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Leonard, K.J.; Cafazzo, J.A.; Barnsley, J.; Masino, C.; Ross, H.J. Perceptions and Experiences of Heart Failure Patients and Clinicians on the Use of Mobile Phone-Based Telemonitoring. J. Med. Internet Res. 2012, 14, e25. [Google Scholar] [CrossRef]
- Seto, E.; Leonard, K.J.; Cafazzo, J.A.; Barnsley, J.; Masino, C.; Ross, H.J. Mobile Phone-Based Telemonitoring for Heart Failure Management: A Randomized Controlled Trial. J. Med. Internet Res. 2012, 14, e31. [Google Scholar] [CrossRef] [PubMed]
- Shearer, N.B.C.; Cisar, N.; Greenberg, E.A. A Telephone-Delivered Empowerment Intervention with Patients Diagnosed with Heart Failure. Heart Lung 2007, 36, 159–169. [Google Scholar] [CrossRef]
- Shively, M.J.; Gardetto, N.J.; Kodiath, M.F.; Kelly, A.; Smith, T.L.; Stepnowsky, C.; Maynard, C.; Larson, C.B. Effect of Patient Activation on Self-Management in Patients with Heart Failure. J. Cardiovasc. Nurs. 2013, 28, 20–34. [Google Scholar] [CrossRef]
- Sisk, J.E.; Hebert, P.L.; Horowitz, C.R.; McLaughlin, M.A.; Wang, J.J.; Chassin, M.R. Effects of Nurse Management on the Quality of Heart Failure Care in Minority Communities: A Randomized Trial. Ann. Intern. Med. 2006, 145, 273–283. [Google Scholar] [CrossRef]
- Smeulders, E.S.T.F.; van Haastregt, J.C.M.; Ambergen, T.; Stoffers, H.E.J.H.; Janssen-Boyne, J.J.J.; Uszko-Lencer, N.H.K.M.; Gorgels, A.P.M.; Lodewijks-van der Bolt, C.L.B.; van Eijk, J.T.M.; Kempen, G.I.J.M. Heart Failure Patients with a Lower Educational Level and Better Cognitive Status Benefit Most from a Self-Management Group Programme. Patient Educ. Couns. 2010, 81, 214–221. [Google Scholar] [CrossRef]
- Smeulders, E.S.T.F.; Van Haastregt, J.C.M.; Ambergen, T.; Uszko-Lencer, N.H.K.M.; Janssen-Boyne, J.J.J.; Gorgels, A.P.M.; Stoffers, H.E.J.H.; Lodewijks-van der Bolt, C.L.B.; Van Eijk, J.T.M.; Kempen, G.I.J.M. Nurse-Led Self-Management Group Programme for Patients with Congestive Heart Failure: Randomized Controlled Trial. J. Adv. Nurs. 2010, 66, 1487–1499. [Google Scholar] [CrossRef]
- Soran, O.Z.; Piña, I.L.; Lamas, G.A.; Kelsey, S.F.; Selzer, F.; Pilotte, J.; Lave, J.R.; Feldman, A.M. A Randomized Clinical Trial of the Clinical Effects of Enhanced Heart Failure Monitoring Using a Computer-Based Telephonic Monitoring System in Older Minorities and Women. J. Card. Fail. 2008, 14, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Pearson, S.; Horowitz, J.D. Effects of a Home-Based Intervention among Patients with Congestive Heart Failure Discharged from Acute Hospital Care. Arch. Intern. Med. 1998, 158, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Horowitz, J.D. Home-Based Intervention in Congestive Heart Failure: Long-Term Implications on Readmission and Survival. Circulation 2002, 105, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Jenkins, A.; Buchan, S.; McGuire, A.; Capewell, S.; McMurray, J.J.J.V. The Current Cost of Heart Failure to the National Health Service in the UK. Eur. J. Heart Fail. 2002, 4, 361–371. [Google Scholar] [CrossRef]
- Stewart, S.; Marley, J.E.; Horowitz, J.D. Effects of a Multidisciplinary, Home-Based Intervention on Planned Readmissions and Survival among Patients with Chronic Congestive Heart Failure: A Randomised Controlled Study. Lancet 1999, 354, 1077–1083. [Google Scholar] [CrossRef]
- Stromberg, A. Nurse-Led Heart Failure Clinics Improve Survival and Self-Care Behaviour in Patients with Heart FailureResults from a Prospective, Randomised Trial. Eur. Heart J. 2003, 24, 1014–1023. [Google Scholar] [CrossRef]
- Thompson, D.R.; Roebuck, A.; Stewart, S. Effects of a Nurse-Led, Clinic and Home-Based Intervention on Recurrent Hospital Use in Chronic Heart Failure. Eur. J. Heart Fail. 2005, 7, 377–384. [Google Scholar] [CrossRef]
- Triller, D.M.; Hamilton, R.A. Effect of Pharmaceutical Care Services on Outcomes for Home Care Patients with Heart Failure. Am. J. Health Syst. Pharm. 2007, 64, 2244–2249. [Google Scholar] [CrossRef]
- Tsuchihashi-Makaya, M.; Matsuo, H.; Kakinoki, S.; Takechi, S.; Tsutsui, H. J-HOMECARE Investigators Rationale and Design of the Japanese Heart Failure Outpatients Disease Management and Cardiac Evaluation (J-HOMECARE). J. Cardiol. 2011, 58, 165–172. [Google Scholar] [CrossRef]
- Tsuchihashi-Makaya, M.; Matsuo, H.; Kakinoki, S.; Takechi, S.; Kinugawa, S.; Tsutsui, H. J-HOMECARE Investigators Home-Based Disease Management Program to Improve Psychological Status in Patients with Heart Failure in Japan. Circ. J. 2013, 77, 926–933. [Google Scholar] [CrossRef]
- Tsuyuki, R.T.; Fradette, M.; Johnson, J.A.; Bungard, T.J.; Eurich, D.T.; Ashton, T.; Gordon, W.; Ikuta, R.; Kornder, J.; Mackay, E.; et al. A Multicenter Disease Management Program for Hospitalized Patients with Heart Failure. J. Card. Fail. 2004, 10, 473–480. [Google Scholar] [CrossRef]
- van der Wal, M.H.L.; Jaarsma, T.; Moser, D.K.; van Gilst, W.H.; van Veldhuisen, D.J. Qualitative Examination of Compliance in Heart Failure Patients in The Netherlands. Heart Lung 2010, 39, 121–130. [Google Scholar] [CrossRef] [PubMed]
- van Veldhuisen, D.J.; Braunschweig, F.; Conraads, V.; Ford, I.; Cowie, M.R.; Jondeau, G.; Kautzner, J.; Aguilera, R.M.; Lunati, M.; Yu, C.M.; et al. Intrathoracic Impedance Monitoring, Audible Patient Alerts, and Outcome in Patients with Heart Failure. Circulation 2011, 124, 1719–1726. [Google Scholar] [CrossRef]
- Wakefield, B.J.; Ward, M.M.; Holman, J.E.; Ray, A.; Scherubel, M.; Burns, T.L.; Kienzle, M.G.; Rosenthal, G.E. Evaluation of Home Telehealth Following Hospitalization for Heart Failure: A Randomized Trial. Telemed. J. E-Health 2008, 14, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.N.; Albert, N.M.; Curtis, A.B.; Gheorghiade, M.; Heywood, J.T.; Liu, Y.; Mehra, M.R.; O’Connor, C.M.; Reynolds, D.; Yancy, C.W.; et al. Lack of Association between Electronic Health Record Systems and Improvement in Use of Evidence-Based Heart Failure Therapies in Outpatient Cardiology Practices. Clin. Cardiol. 2012, 35, 187–196. [Google Scholar] [CrossRef]
- Weintraub, A.; Gregory, D.; Patel, A.R.; Levine, D.; Venesy, D.; Perry, K.; Delano, C.; Konstam, M.A. A Multicenter Randomized Controlled Evaluation of Automated Home Monitoring and Telephonic Disease Management in Patients Recently Hospitalized for Congestive Heart Failure: The SPAN-CHF II Trial. J. Card. Fail. 2010, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowiecki, M.; Poprawski, K.; Nowicka, A.; Kandziora, M.; Piatkowska, A.; Jankowiak, M.; Michałowicz, B.; Stawski, W.; Dziamska, M.; Kaszuba, D.; et al. A New Programme of Multidisciplinary Care for Patients with Heart Failure in Poznań: One-Year Follow-Up. Kardiol. Pol. 2006, 64, 1063–1070, discussion 1071–1072. [Google Scholar]
- Wongpiriyayothar, A.; Piamjariyakul, U.; Williams, P.D. Effects of Patient Teaching, Educational Materials, and Coaching Using Telephone on Dyspnea and Physical Functioning among Persons with Heart Failure. Appl. Nurs. Res. 2011, 24, e59–e66. [Google Scholar] [CrossRef]
- Woodend, A.K.; Sherrard, H.; Fraser, M.; Stuewe, L.; Cheung, T.; Struthers, C. Telehome Monitoring in Patients with Cardiac Disease Who Are at High Risk of Readmission. Heart Lung 2008, 37, 36–45. [Google Scholar] [CrossRef]
- Wright, S.P.; Walsh, H.; Ingley, K.M.; Muncaster, S.A.; Gamble, G.D.; Pearl, A.; Whalley, G.A.; Sharpe, N.; Doughty, R.N. Uptake of Self-Management Strategies in a Heart Failure Management Programme. Eur. J. Heart Fail. 2003, 5, 371–380. [Google Scholar] [CrossRef]
- Yu, C.-M.; Wang, L.; Chau, E.; Chan, R.H.-W.; Kong, S.-L.; Tang, M.-O.; Christensen, J.; Stadler, R.W.; Lau, C.-P. Intrathoracic Impedance Monitoring in Patients with Heart Failure: Correlation with Fluid Status and Feasibility of Early Warning Preceding Hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.F.; Lee, D.T.F.; Stewart, S.; Thompson, D.R.; Choi, K.-C.; Yu, C.-M. Effect of Nurse-Implemented Transitional Care for Chinese Individuals with Chronic Heart Failure in Hong Kong: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015, 63, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A Systematic Review of Chronic Disease Management Interventions in Primary Care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, M.; Papangeli, I.; Simonson, B.; Akkad, A.D.; Hill, M.C.; Arduini, A.; Fleming, S.J.; Melanson, M.; Hayat, S.; Kost-Alimova, M.; et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 2022, 608, 174–180. [Google Scholar] [CrossRef] [PubMed]

| Description | Scope |
|---|---|
| Population | Adult patients with heart failure at any stage of the disease |
| Intervention | Multicomponent disease management interventions and clinical pathways to manage the chronic and acute phases of HF patients |
| Comparator | Standard care (routine or standard care, as defined by the primary studies) |
| Outcomes | Studies investigating any outcome of efficacy, effectiveness, and costs will be considered |
| Study design | All study designs were included, given the broad scope of the review. No limits were given on the duration of the intervention or the length of follow-up. |
| CCM Element | Description of the CCM Element | Intervention Components | Description and Example |
|---|---|---|---|
| Self-management support | Emphasis on the importance of the central role the patients have in managing their own care. | Educational interventions | Educational interventions on self-monitoring, medical management, decision making, or adoption and maintenance of health-promoting behaviours, divided into: - mHealth-based interventions (delivery of health messages, interventions, and verification of notions provided through education via mobile phones, tablets, and other wireless technologies), - eHealth (web-based computer-tailored interventions) and - face-to-face teaching sessions conducted by educators using written or printed materials |
| Motivational counselling and/or behavioural therapy/support | Telephonic or face-to-face motivational counselling sessions focused on self-monitoring and medical management, decision-making, or adoption and maintenance of health-promoting behaviours. | ||
| Family and caregiver education/support | Any kind of educational, motivational, behavioural intervention oriented towards a family member or caregiver. | ||
| Physical activity | Provision of individual or group physical activity lessons, instructions, or programs. | ||
| Self-monitoring and medical management tools | Distributed logs, notebooks, calendars, and dosette boxes or provided technological aids (electronic reminders, phone cues) for self-monitoring, for example, salt intake or weight control. | ||
| Telephone advice lines | Working hours or out-of-hours answerphone system providing advice/support service about self-management. | ||
| Decision support | Integration of evidence-based guidelines into daily clinical practice | Integrated CHF protocols into routine practice | Implementation of protocols or guidelines into daily clinical practice. |
| Provider education | Any kind of education or case discussions with care providers, usually nurses. | ||
| Linkages between primary and speciality care | Organisation or coordination of patient care activities and sharing of clinical information between different professionals involved in primary care and speciality health services. | ||
| Community resources and policies | Developing partnerships with community organisations that support and meet patients’ need | Linking patients to outside resources | Referring a patient to a local community health program, church-based support groups, and clinic-based support groups. |
| Logistic support | Providing transport to patients from home to the outpatient clinic or community intervention site. | ||
| Third sector involvement | Activities with community-based organisations, volunteer groups, self-help groups, centres for the elderly, etc. | ||
| Community-based self-management programs | Group intervention attended in the community aims to improve disease control and promote self-efficacy. | ||
| Social support | Social support provided by community-based organisations or involvement in social structures within the community. | ||
| Delivery system | Focus on teamwork and an expanded scope of practice for a team member to support chronic care | Patient care planning/discharge planning | Development of an individualised discharge plan or adaptation of recommendations and prescriptions for a patient prior to their discharge from the hospital. |
| Telemedicine/remote monitoring | Use of telecommunication equipment to remind patients or detect early signs and symptoms of heart failure. | ||
| Multidisciplinary team | Involvement of three or more providers from different healthcare specialities in patient care. | ||
| Advanced practice nurse involvement | Advanced practice nurses are involved in the provision of care services. | ||
| Nurse-led/Nurse case manager | The activities of management, assessment, planning, and coordination of patient care are carried out under the responsibility of the nurses. | ||
| Clinical information system | Developing information systems based on patient populations to provide relevant client data | Disease registry | Computer or web-based applications or systems used to capture, manage, and provide information about the specific condition to support organised care management of patients. |
| Monitoring indicators and feedback to the provider | Collecting and sharing biometric data and patient-reported insights with care teams who evaluate trends and intervene, if necessary. | ||
| Advising/reminders systems for providers | E-mails or messages sent to nurses that contain reminders, instructions, and/or guidelines. | ||
| System for sharing information between providers | Web-based medical records accessible to all health professionals involved in the care of the patient. |
| Severity (LVEF) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not Specified n = 120 | ESC Classification | Other Classification | Overall n = 166 | |||||||
| ≥50% N = 1 | 40–49% N = 4 | <40% N = 19 | ≥45% N = 4 | ≤45% N = 15 | ≤55% N = 3 | |||||
| Setting | Inpatient | 44 | 1 | 1 | 6 | / | 4 | / | 56 | |
| Outpatient | 34 | 1 | / | 10 | / | 7 | 1 | 53 | ||
| Primary care | 9 | / | 1 | / | 3 | / | 13 | |||
| Home | 87 | 1 | 3 | 13 | 4 | 7 | 3 | 118 | ||
| Intervention | Self-management support | m-Health education | 57 | / | 2 | 8 | 3 | 5 | / | 74 |
| e-Health education | 17 | / | / | 5 | / | 1 | / | 23 | ||
| Face-to-face didactic session | 84 | 1 | 3 | 12 | 4 | 13 | 3 | 120 | ||
| Motivation counselling and/or behavioural therapy/support | 35 | 1 | / | 6 | 2 | 3 | / | 47 | ||
| Family and caregiver education/support | 36 | / | 2 | 4 | 3 | 8 | 3 | 56 | ||
| Physical activity | 8 | / | / | 1 | 2 | 2 | 1 | 15 | ||
| Self-monitoring and medical management tools | 62 | 1 | 1 | 6 | 3 | 6 | / | 79 | ||
| Telephone advice lines | 41 | 1 | / | 5 | / | 3 | 2 | 52 | ||
| Overall | 109 | 1 | 4 | 16 | 4 | 15 | 3 | 152 | ||
| Decision support | Integrated CHF protocols into routine practice | 28 | / | 1 | 2 | / | 2 | / | 33 | |
| Provider education | 18 | / | / | 2 | 2 | 3 | / | 25 | ||
| Linkage between primary and speciality care | 19 | / | / | 5 | / | 3 | 2 | 29 | ||
| Overall | 50 | 0 | 1 | 9 | 2 | 5 | 2 | 69 | ||
| Community resource and policy | Linking patients to an outside resource | 4 | / | / | / | / | 1 | / | 5 | |
| Logistic support | / | / | 1 | / | 1 | / | 2 | |||
| Third sector involvement | 1 | / | / | / | / | 1 | / | 2 | ||
| Community-based self-management programs | 2 | / | / | / | / | / | / | 2 | ||
| Social support | 8 | / | / | 1 | / | 3 | / | 12 | ||
| Overall | 5 | 0 | 0 | 1 | 0 | 1 | 0 | 7 | ||
| Delivery system | Patient care planning/discharge planning | 25 | / | / | 2 | / | 2 | / | 29 | |
| Telemedicine/remote monitoring | 56 | / | 2 | 12 | / | 2 | 3 | 75 | ||
| Multidisciplinary team | 23 | / | / | 5 | / | 3 | 1 | 32 | ||
| Advanced practitioner nurse involvement | 37 | 1 | 2 | 7 | 3 | 7 | 1 | 58 | ||
| Nurse-led/nurse case manager | 25 | / | / | 4 | / | 4 | 2 | 35 | ||
| Overall | 97 | 1 | 3 | 14 | 3 | 11 | 3 | 132 | ||
| Clinical information system | Disease registry | / | / | / | / | / | / | / | / | |
| Monitoring indicators and feedback to the provider | 21 | / | 1 | 12 | / | 1 | 1 | 36 | ||
| Advising/reminding system for providers | 7 | / | / | 1 | / | / | / | 8 | ||
| Sharing information between providers | 12 | / | 2 | 3 | / | 2 | 1 | 20 | ||
| Overall | 35 | 0 | 2 | 12 | 0 | 2 | 2 | 53 | ||
| Inpatient n = 56 | Outpatient n = 53 | Primary Care n = 13 | Home n = 118 | Overall n = 166 | ||
|---|---|---|---|---|---|---|
| Self-management support | m-Health education | 23 | 17 | 6 | 58 | 104 |
| e-Health education | 6 | 8 | 1 | 19 | 34 | |
| Face-to-face didactic session | 49 | 43 | 10 | 84 | 186 | |
| Motivation counselling and/or behavioural therapy/support | 21 | 14 | 4 | 35 | 74 | |
| Family and caregiver education/support | 23 | 17 | 5 | 45 | 90 | |
| Physical activity | 3 | 4 | 2 | 8 | 17 | |
| Self-monitoring and medical management tools | 33 | 26 | 8 | 58 | 125 | |
| Telephone advice lines | 28 | 16 | 3 | 41 | 88 | |
| Overall | 53 | 49 | 12 | 108 | 222 | |
| Decision support | Integrated CHF protocols into routine practice | 11 | 13 | 4 | 22 | 50 |
| Provider education | 7 | 5 | 2 | 14 | 28 | |
| Linkage between primary and speciality care | 9 | 12 | 2 | 19 | 42 | |
| Overall | 22 | 23 | 6 | 45 | 76 | |
| Community resource and policy | Linking patients to outside resources | 1 | 2 | 1 | 2 | 6 |
| Logistic support | / | 1 | 1 | 1 | 3 | |
| Third sector involvement | 1 | / | 1 | 1 | 3 | |
| Community-based self-management programs | 1 | 1 | / | 1 | 3 | |
| Social support | / | / | / | / | / | |
| Overall | 2 | 3 | 1 | 4 | 10 | |
| Delivery system | Patient care planning/discharge planning | 23 | 6 | 1 | 26 | 56 |
| Telemedicine/remote monitoring | 26 | 20 | 5 | 64 | 115 | |
| Multidisciplinary team | 17 | 14 | 4 | 19 | 59 | |
| Advanced practitioner nurse involvement | 17 | 22 | 3 | 42 | 83 | |
| Nurse-led/nurse case manager | 12 | 14 | 3 | 29 | 58 | |
| Overall | 49 | 40 | 8 | 101 | 198 | |
| Clinical information system | Disease registry | / | / | / | / | / |
| Monitoring indicators and feedback to the provider | 8 | 13 | / | 30 | 51 | |
| Advising/reminding system for providers | 1 | 3 | / | 5 | 9 | |
| Sharing information between providers | 4 | 5 | 2 | 15 | 26 | |
| Overall | 11 | 17 | 4 | 40 | 72 | |
| Study Size | |||||||
|---|---|---|---|---|---|---|---|
| <100 n = 35 | 100–1000 n = 118 | >1000 n = 13 | Overall n = 166 | ||||
| Setting | Inpatient | 8 | 36 | 6 | 50 | ||
| Outpatient | 11 | 36 | 3 | 50 | |||
| Primary care | 2 | 10 | 12 | ||||
| Home | 26 | 77 | 11 | 114 | |||
| Intervention | Self-management support | m-Health education | 19 | 47 | 6 | 72 | |
| e-Health education | 5 | 15 | 2 | 22 | |||
| Face-to-face didactic session | 22 | 83 | 7 | 112 | |||
| Motivation counselling and/or behavioural therapy/support | 11 | 28 | 6 | 45 | |||
| Family and caregiver education/support | 9 | 39 | 5 | 53 | |||
| Physical activity | 4 | 8 | / | 12 | |||
| Self-monitoring and medical management tools | 15 | 51 | 6 | 72 | |||
| Telephone advice lines | 6 | 37 | 5 | 48 | |||
| Overall | 32 | 102 | 10 | 142 | |||
| Decision support | Integrated CHF protocols into routine practice | 3 | 23 | 3 | 29 | ||
| Provider education | 5 | 15 | 3 | 23 | |||
| Linkage between primary and speciality care | 1 | 23 | 4 | 28 | |||
| Overall | 9 | 47 | 7 | 63 | |||
| Community resource and policy | Linking patients to an outside resource | / | 4 | / | / | ||
| Logistic support | / | 12 | / | / | |||
| Third sector involvement | / | 1 | / | / | |||
| Community-based self-management programs | / | / | / | / | |||
| Social support | / | / | / | / | |||
| Overall | / | 5 | / | 5 | |||
| Delivery system | Patient care planning/discharge planning | 3 | 19 | 4 | 26 | ||
| Telemedicine/remote monitoring | 13 | 54 | 6 | 73 | |||
| Multidisciplinary team | 6 | 21 | 3 | 30 | |||
| Advanced practitioner nurse involvement | 13 | 37 | 7 | 57 | |||
| Nurse-led/nurse case manager | 20 | 57 | 8 | 85 | |||
| Overall | 28 | 85 | 12 | 125 | |||
| Clinical information system | Disease registry | / | / | / | / | ||
| Monitoring indicators and feedback to the provider | 8 | 24 | 3 | 35 | |||
| Advising/reminding system for providers | 3 | 2 | 2 | 7 | |||
| Sharing information between providers | 3 | 15 | 2 | 20 | |||
| Overall | 11 | 35 | 15 | 61 | |||
| Setting | HF Severity (LVEF) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inpatient n = 56 | Outpatient n = 53 | Primary Care n = 13 | Home n = 118 | Overall | ≥50% N = 1 | 40–49% N = 4 | <40% N = 19 | ≥45% N = 4 | ≤45% N = 15 | ≤55% N = 3 | Overall | |
| Advance practice nurse | 19 | 22 | 3 | 44 | 88 | 1 | 2 | 7 | 3 | 7 | 1 | 21 |
| Nurse-led | 10 | 14 | 3 | 26 | 53 | / | 4 | 4 | 2 | 10 | ||
| Nurse | 29 | 19 | 6 | 50 | 104 | / | 1 | 6 | 1 | 7 | 1 | 16 |
| Cardiologist | 19 | 22 | 7 | 32 | 80 | / | 2 | 10 | / | 2 | 1 | 15 |
| Geriatrician | 2 | 1 | / | 1 | 4 | / | / | / | / | / | / | / |
| Pharmacist | 7 | 4 | 3 | 13 | 27 | / | / | 2 | / | 2 | 1 | 5 |
| Physician | / | 1 | / | 1 | 2 | / | / | 7 | / | 2 | 1 | 10 |
| Psychiatrist | 2 | / | 1 | 1 | 4 | / | / | 1 | / | 3 | / | 4 |
| Psychologist | 2 | 2 | 2 | 1 | 7 | / | / | / | / | / | / | / |
| Physiotherapist | 1 | / | / | / | 1 | / | / | / | / | 1 | / | 1 |
| Dietist/nutritionist | 12 | 6 | 2 | 12 | 32 | / | / | / | / | 2 | / | 2 |
| Social worker | 7 | 5 | 1 | 9 | 22 | / | / | 1 | / | / | / | 1 |
| Occupational therapist | / | / | 1 | / | 1 | / | / | / | / | / | 1 | 1 |
| Students pursuing premedical track | 1 | / | / | 1 | 2 | / | / | / | / | / | / | / |
| Patients | / | / | / | 1 | 1 | / | / | / | 1 | / | / | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroni, C.; Djuric, O.; Bassi, M.C.; Mione, L.; Caleffi, D.; Testa, G.; Prandi, C.; Navazio, A.; Giorgi Rossi, P. Elements Characterising Multicomponent Interventions Used to Improve Disease Management Models and Clinical Pathways in Acute and Chronic Heart Failure: A Scoping Review. Healthcare 2023, 11, 1227. https://doi.org/10.3390/healthcare11091227
Pedroni C, Djuric O, Bassi MC, Mione L, Caleffi D, Testa G, Prandi C, Navazio A, Giorgi Rossi P. Elements Characterising Multicomponent Interventions Used to Improve Disease Management Models and Clinical Pathways in Acute and Chronic Heart Failure: A Scoping Review. Healthcare. 2023; 11(9):1227. https://doi.org/10.3390/healthcare11091227
Chicago/Turabian StylePedroni, Cristina, Olivera Djuric, Maria Chiara Bassi, Lorenzo Mione, Dalia Caleffi, Giacomo Testa, Cesarina Prandi, Alessandro Navazio, and Paolo Giorgi Rossi. 2023. "Elements Characterising Multicomponent Interventions Used to Improve Disease Management Models and Clinical Pathways in Acute and Chronic Heart Failure: A Scoping Review" Healthcare 11, no. 9: 1227. https://doi.org/10.3390/healthcare11091227
APA StylePedroni, C., Djuric, O., Bassi, M. C., Mione, L., Caleffi, D., Testa, G., Prandi, C., Navazio, A., & Giorgi Rossi, P. (2023). Elements Characterising Multicomponent Interventions Used to Improve Disease Management Models and Clinical Pathways in Acute and Chronic Heart Failure: A Scoping Review. Healthcare, 11(9), 1227. https://doi.org/10.3390/healthcare11091227








