Abstract
South Asians (SAs) are among the fastest-growing ethnic groups in the U.S. Metabolic syndrome (MetS) is a condition that is characterized by multiple health factors that increase the risk for chronic diseases, such as cardiovascular disease (CVD) and diabetes. MetS prevalence among SA immigrants ranges from 27–47% in multiple cross-sectional studies using different diagnostic criteria, which is generally higher compared to other populations in the receiving country. Both genetic and environmental factors are attributed to this increased prevalence. Limited intervention studies have shown effective management of MetS conditions within the SA population. This review reports MetS prevalence in SAs residing in non-native countries, identifies contributing factors, and discusses ways to develop effective community-based strategies for health promotion targeting MetS among SA immigrants. There is a need for more consistently evaluated longitudinal studies to facilitate the development of directed public health policy and education to address chronic diseases in the SA immigrant community.
1. Introduction
Metabolic syndrome (MetS) is a cluster of chronic disease risk factors, including abdominal obesity, hypertension, dyslipidemia, and impaired glucose tolerance [1]. Because MetS is directly related to risk for both cardiovascular disease (CVD) and type 2 diabetes mellitus [2,3], it can incur a high cost to both individuals and society. Reports from the International Diabetes Federation (IDF) have estimated that, in 2021 alone, the global healthcare costs associated with diabetes were USD 966 billion and are expected to grow to a projected rate of USD 1054 billion by 2045 [4]. As such, MetS and its related chronic conditions are public health issues of global significance. While the prevalence of MetS across different ethnic groups varies widely [5], the IDF estimates that, from 2011–2016, the overall prevalence of MetS in the United States was 36.9% [6].
Currently, there are distinct criteria for MetS that have been established by several institutions (Table 1). The most widely used definitions have been developed by the World Health Organization (WHO); the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III); the American Health Association (AHA), in conjunction with the National Heart, Lung, and Blood Institute (NHLBI); and the IDF [7].

Table 1.
Major definitions/criteria for metabolic syndrome.
Recently, the IDF and the AHA/NHLBI released a joint statement unifying several definitions into one consensus set of criteria [8]. All of these definitions incorporate cutoff points for body mass index (BMI) and waist circumference (WC), blood glucose and lipid levels, and blood pressure. Additionally, the consensus definition uses ethnic-specific criteria for WC, including distinct cutoff points for the Asian population. The term “South Asian” (SA) is typically used to refer to individuals with ethnic origins in India, Pakistan, Bangladesh, Nepal, Bhutan, Maldives, or Sri Lanka. Over the last two centuries, individuals of South Asian (SA) origin, particularly those originating from India and Pakistan, have immigrated to different countries, including the U.S. According to census data, there were over 4.5 million SA immigrants living in the U.S. in 2022, making this the fastest growing ethnic group in the country [9].
Studies have reported disparities in chronic disease between SA immigrants and the general U.S. population. Despite a lower average BMI [10], SA immigrants have higher prevalence rates of both diabetes [11,12,13,14] and CVD [15,16] compared to the majority population. Individuals of SA descent show higher amounts of abdominal fat and stronger insulin resistance when compared to individuals of European descent [17,18,19], suggesting an increased risk for metabolic syndrome. However, there are few large-scale studies examining MetS in the SA immigrant population. The purpose of this review is to compile and summarize studies focusing on MetS in SAs residing in non-native countries and to explore the factors that may influence the high burden of chronic disease observed in this population. Additionally, this review seeks to identify existing interventions and make suggestions for further research and practice to more effectively address the chronic disease burden in this burgeoning, yet underserved community.
2. Materials and Methods
For the determination of MetS prevalence, factors, and interventions among SA immigrants, a literature search was performed with the PubMed (National Library of Medicine, Bethesda, MD, USA) search engine. Multiple PubMed literature searches were performed using the following search terms and combinations contained in all fields of publications: (“Metabolic Syndrome”), (“Metabolic Syndrome” AND “South Asians”), (“Metabolic Syndrome” AND “South Asian Immigrants”), (“Metabolic Syndrome” AND “India”), (“Metabolic Syndrome” AND “Pakistan”), (“Metabolic Syndrome” AND “Bangladesh”), (“Metabolic Syndrome” AND “Sri Lanka”), (“Metabolic Syndrome” AND “Nepal”), (“Metabolic Syndrome” AND “Bhutan”), and (“Metabolic Syndrome” AND “Maldives”). Additional searches were performed to obtain articles pertaining to factors associated with MetS, such as acculturation, diet, physical activity, genetic/biochemical, and other psychosocial and environmental factors (e.g., food access and food availability). Finally, searches were also performed to identify interventions and lifestyle approaches to treat and manage MetS in these groups. This review method allowed for an in-depth examination of articles (Figure 1). As the focus of this review was SA immigrants, exclusion criteria were studies that did not relate to any of the aforementioned countries of origin, or the prevalence, contributing factors, or interventions targeting MetS in this population.
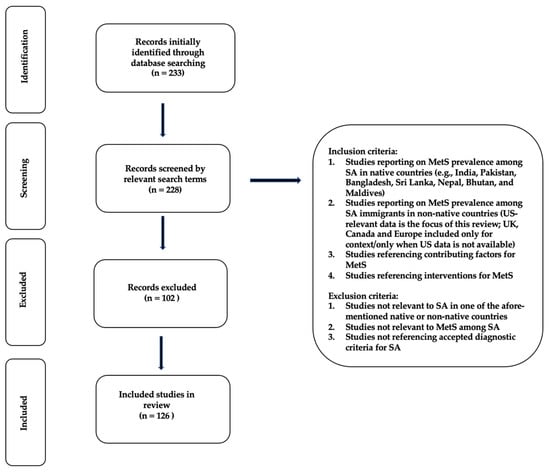
Figure 1.
Prisma-flow diagram for literature review.
3. Results
The Prevalence of MetS in SAs residing in non-native countries: Studies examining the prevalence of MetS in SAs residing in non-native countries have reported rates ranging from 20–51% (Table 2). A large 2005 study (n = 1603) conducted in the U.K. reported a prevalence of 31–46% among SA immigrants, which was significantly higher than that among European subjects (9–18% prevalence, p < 0.001) [20]. A subset of that population, consisting of only males (n = 1420), showed a MetS prevalence of 44.6%; a higher prevalence compared to Europeans was still observed [21]. Another U.K. study found that MetS prevalence in SAs (n = 245) was nearly double what was observed among white subjects (39% vs. 20%, p < 0.001) [22]. Smaller studies in the U.S. [23] and the Netherlands [24] reported similar findings. In contrast, one study in Canada found that there were no significant differences in MetS prevalence between SAs (25.9%, n = 342) and whites (22%, n = 326) [25]. The specific countries of origin were not described in this study.

Table 2.
Prevalence of MetS in SA in non-native countries.
Among the large-scale studies conducted in the U.S. (n = 1403), one study showed an overall prevalence of 47% in a population of Sas residing in the country [26]. An earlier study observing Sas in the U.S. (n = 1445) found the prevalence of MetS to be 27% [27], while another report found that, among a population of 997 SAs living in the U.S., the prevalence of MetS was 37.6% [28]. These, and other large-scale studies in the U.S., have been comprised of SAs mostly of Indian descent [29,30]. One study involving Bangladeshi men living in Texas, USA (n = 91) reported MetS prevalence at 38% using NHLBI/AHA guidelines [31], while another showed that the highest prevalence of MetS was among Bangladeshi participants [26]. This suggests that the degree of variability in prevalence reported in these studies may be related to the ethnicity or country of origin of the participants.
Prevalence of MetS in SAs residing in native countries: A few studies have examined the prevalence of MetS among SAs residing in their native countries [32,33,34,35,36,37,38] (Table 3). Researchers have suggested that the rising prevalence can be attributed to the tremendous economic growth brought on by rapid transitions in nutrition, culture, and the environment in their native countries, especially over the past three decades. For example, some studies show that increased access to processed and refined foods [39,40,41,42], a decline in the availability and accessibility of healthier food options [43], along with a concurrent increase in sedentary behavior [44], are the results of globalization and industrialization [45,46]. Ultimately, these factors have led to an imbalance in energy intake and expenditure among its citizens [47]. Other environmental factors, such as an increase in air pollution, which is described as having a negative impact on the pathways regulating macronutrient metabolism [48], and psychosocial factors, such as depression, anxiety, and a lack of social support [49], have also been implicated in the development of chronic disease risks in these groups. More research examining these factors is necessary to get a clearer picture of whether or not disparities in the prevalence of MetS are due to differences in ethnicity.

Table 3.
Prevalence of MetS in SAs in native countries.
Differences in MetS in SAs between native and non-native countries: Few studies have compared differences in risks for chronic disease among SA immigrants and their contemporaries residing in their native countries. These studies have consistently reported a higher BMI and waist circumference among SA immigrants compared to their native counterparts [50,51,52,53,54]. A 2011 meta-analysis of 10 studies reported that indices of obesity were greater in migrant Indian populations compared to native Indians [55]. Other studies have noted a higher risk for insulin resistance, diabetes, CVD, dyslipidemia, and hypertension among SA immigrants compared to the non-native country’s majority population [56,57].
In South Asians, polymorphisms for the gene encoding Apolipoprotein A-I, a protein component of high-density lipoprotein (HDL) particles, were significantly associated with MetS as well as low HDL levels, suggesting that the racial disparity of MetS between SAs and other races may be due to genetic differences [58,59,60,61,62,63]. While genetic predisposition is noteworthy, it does not provide a complete picture of the factors that contribute to greater risk for MetS in SA immigrants and their descendants. The sections below describe these factors.
Acculturation: The migration process has been linked to the increased prevalence of chronic diseases among SAs in non-native countries, suggesting the strong role of acculturation [64]. Acculturation is defined as the process by which a particular culture adopts the tenets and behaviors of a new culture, typically due to immigration [65]. Studies of immigrant cultures in the U.S. show that acculturation and its multiple dimensions might play a significant role in the development of chronic disease [66,67,68,69,70,71].
Acculturation and diet: Dietary acculturation refers to the process by which immigrants adopt the food habits of their host culture over a period of time [65]. Dietary behavior and the duration of residence in the U.S. have been identified as strong predictors of MetS in some SA immigrants [72]. For instance, several studies show that the adoption of a predominantly western diet, which is typically positively associated with increased duration of residence, contributes to the increased chronic disease burden in this group [73,74,75,76,77,78,79,80,81]. In one study, a high protein intake was related to an increased risk for diabetes, increased BMI, and higher waist circumference among SA immigrants living in America [82]. Of the total 146 participants in this study, 85 reported eating meat, while the rest adhered to a lacto-vegetarian diet (n = 29), or a lacto-ovo-vegetarian diet (n = 32). Among the meat eaters, the source of protein intake (vegetable, fish, or animal) was not associated with diabetes status. Compared to the meat eaters, however, both of the vegetarian diets were associated with lower insulin resistance. Also, those maintaining one of the two vegetarian diets had resided in the U.S. for a shorter duration than those who ate meat. An earlier study among Sri Lankan and Pakistani immigrants living in Norway found similar results, although a better understanding of the Norwegian language was associated with lower fat consumption [83].
It is worth noting here that using BMI as a proxy for obesity has several limitations. Unlike bioelectrical impedance, which measures both body fat and muscle mass, BMI is based solely on height and weight. Therefore, it is considered an indirect measure of body fat [84]. Additionally, it fails to reflect the differences and changes in these two components that may occur with gender, age, or physical activity level [85]. Among athletes, for instance, a higher weight may be due to higher muscle mass, thus resulting in overestimation [86]. Finally, two people may have considerably different BMIs, despite having identical or nearly identical percentages of body fat [87]. For these reasons, the use of BMI as a measure of obesity can misclassify, resulting in bias when estimating effects related to obesity. Nevertheless, these findings warrant further research, as they suggest that a transition away from a plant-based diet may contribute to increased chronic disease risk.
Acculturation and physical activity: Within a small population of Indian immigrants in California, U.S., even a moderate amount of physical activity was correlated with a lower prevalence of MetS in men [29]. Another study reported that duration of exercise was a statistically accurate predictor of MetS in SA immigrant women, but not in men [88]. The decrease in physical activity was associated with increases in both anthropometric (BMI and waist circumference) and biochemical (glucose and triglycerides) measures of MetS risk in this study. Increased acculturation was significantly associated with vigorous and moderate leisure-time physical activity, but not with light physical activity among Indian immigrants living in Canada [89]. A study of Indian immigrants in New Zealand that used pedometer measurements also reported decreased steps with increased acculturation [90]. These studies emphasize the role of physical activity in possibly mitigating the risk of MetS. However, the conflicting results with respect to gender and exercise intensity warrant further investigation.
Interventions for MetS: Several researchers have demonstrated the effectiveness of diet- and/or physical activity-based interventions in reducing the prevalence of MetS and chronic disease risks among various SA immigrant groups [91,92,93,94,95,96]. A randomized, controlled trial involving Pakistani immigrant men living in Norway (n = 150) found that after a 5-month intervention promoting physical activity (both cardiorespiratory and strength training), there was a slight decrease in the prevalence of MetS, and a significantly greater reduction in waist circumference and serum insulin concentration in the intervention group compared to the control group [97]. Another randomized, controlled trial involving Pakistani immigrant women living in Norway (n = 198) found that a 7-month intervention promoting both diet and physical activity resulted in a significant reduction in risk for Type 2 diabetes and MetS [98]. A smaller study of Pakistani immigrant women living in Australia (n = 40) also found that a 24-week intervention promoting healthy dietary behaviors and regular physical activity significantly decreased the participants’ BMI, blood pressure, cholesterol, and glucose levels compared to baseline [99].
Studies involving Indian immigrants have utilized a wide range of intervention strategies to address MetS, including nutrition education sessions on incorporating more plant-based protein sources, such as nuts [100,101]; exercise [102]; or a combination of diet- and exercise-based approaches [103]. Some of these interventions were delivered in a variety of formats, including one-on-one counseling sessions; group education workshops involving friends, peers, or family members; offered in the privacy of a participant’s home; or offered in a faith-based setting, such as a Hindu temple. Some educational components were also tailored to be more culture- and/or gender-specific, while others were tailored for audiences with low levels of literacy. Regardless of the approach, there were improvements noted across a range of parameters, including BMI, blood pressure, blood glucose, and waist circumference [104,105,106,107,108,109].
A review of randomized control trials from India showed that, in addition to diet, incorporating regular physical activity resulted in marked changes in risk factors for MetS, including a decreased BMI, waist circumference, and serum triglycerides and increased HDL-c levels [110,111,112,113]. Literature examining the impact of such interventions on MetS among other SA groups in their native countries is limited. Nevertheless, these data show that lifestyle interventions have the potential to positively impact MetS among SAs regardless of their country of residence.
4. Discussion
Gaps in prevalence: The harmonized consensus definition for MetS has recommended that waist circumference cutoff in Asian Americans be set at ≥90 cm for men, and ≥80 cm for women as a risk factor for MetS, which is in contrast to the values established by the WHO, NCEP ATP III, and the AHA/NHLBI, which are set to be higher at ≥102 cm for men and ≥88 cm for women, regardless of ethnicity [114]. These differences in criteria make it difficult to compare the prevalence of MetS across studies using different MetS definitions; this may explain the variability in these reports among SAs. In studies that describe MetS in SAs across multiple MetS definitions, disparate prevalence proportions have been reported within the same population [26,104,115]. Therefore, the discrepancies between MetS definitions need to be considered when comparing the prevalence of MetS in SAs across different studies. Additionally, most studies covered in this review are cross-sectional, suggesting a need for more longitudinal, prospective studies of MetS in SAs to develop more ethnic-specific criteria and apply these criteria in the formulation of effective treatment and prevention strategies for chronic disease in SAs. Future studies on MetS in the SA population warrant the application of a consistent MetS definition that takes into consideration ethnic-specific criteria, such as that of waist circumference in the IDF and harmonized consensus definitions.
Gaps in interventions: The studies reviewed thus far demonstrate that lifestyle interventions focusing on healthy eating and/or physical activity may be effective in preventing and treating MetS, in general. A scoping review comparing physical activity levels and their impact on MetS among Indian, Pakistani, and Bangladeshi immigrants settled in the UK describes these differences [116]. While levels of physical activity were generally lower compared to the majority population among all three groups, Bangladeshis had the lowest levels of physical activity, while Indians had the highest. In all three groups, women were less physically active than men, and older adults were the least physically active. Another review on cardio-metabolic risk factors among SA labor migrants who were hired for semi-skilled or unskilled jobs in the Middle East reported a high prevalence of being overweight/obese and related chronic diseases, such as diabetes and hypertension. Despite the high burden, there was a lack of focus on screening and inadequate provisions for health care [117].
These data show that educational components within interventions that are targeted toward reducing the rising burden of MetS in SAs need to vary depending on the group’s unique contextual needs; universal approaches that fail to consider ethnic and other societal inequities may fail in their compliance and overall effectiveness, likely widening the racial disparities in MetS prevalence. Most studies have grouped all SA immigrants together, even though there may be considerable differences in each ethnic group’s nutrition and lifestyle behaviors. Furthermore, the majority of the participants in these studies have been Indians. This group tends to be over-represented in South Asian chronic disease research.
Some studies have alluded to an inverse relationship between socioeconomic status and chronic disease in SAs living in non-native countries [26,31,70,76,85]. Studies on the relationship between mental health and chronic disease in this population have been inconclusive [30,70]. The influence of factors, such as health care access and differences in chronic disease risks due to occupational category or ethnicity, have not been studied sufficiently in this group either. Published literature that recognizes these differences and has reported the results separately is either limited or outdated [118,119].
Implications: Adopting a lower BMI cut-off for SA immigrants would serve to increase opportunities for improved diagnosis and intervention. However, a cross-sectional survey of primary care physicians practicing in a major southern city in the U.S. found only 9% of physicians reported measuring waist circumference, and only 21% of physicians were aware of ethnicity-specific guidelines. Most lacked the knowledge and training to appropriately assess overweight/obesity and related chronic disease risks in SA immigrants [120]. These data highlight the need for more culturally sensitive clinical strategies to reduce the burden of MetS in SA immigrant communities across the U.S. [121,122,123].
The socio-ecological model has been recognized as a systematic and coordinated approach to understanding and reducing disease risks, particularly among underserved and vulnerable population groups [124]. The model assumes that individuals are more likely to sustain disease treatment and management requirements within a comprehensive network that considers their individual (demographic characteristics, knowledge, attitudes, and beliefs), interpersonal (social support and size of social networks), environmental (availability of healthy food options and other resources), and institutional (public health policies) needs [125]. There appears to be a scientific gap, however, in developing and testing relevant policies and programs for SA immigrants that are designed around the constructs of the socio-ecological model. While most healthcare organizations provide basic services, they are largely based on models that focus on a single disease risk and do not address the personal, familial, or social needs of the various ethnic groups within this community. Few programs have addressed cultural relevancy in screening, diagnostic, and treatment tools. As summarized in Figure 2, translational and prospective research that provides a clear evaluation of the importance of ethnic-specific guidelines for diet and physical activity may ultimately help reduce the chronic disease burden in SA immigrants.
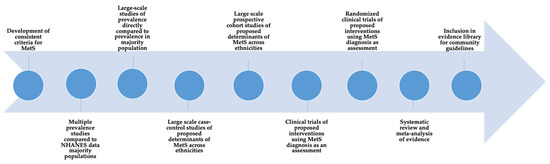
Figure 2.
Conceptual model for data gathering for MetS and chronic disease risks in South Asian immigrants.
An optimal case management model for SA immigrants might be one that includes components that are not only culturally competent but ones that also strengthen and facilitate an individual’s environmental, psychological, and social networks [126]. For example, economic and zoning policies that ensure the availability of healthy foods and affordable preventive services in the neighborhoods in which these immigrant communities live and congregate may decrease risks for chronic diseases in the long term. Components that focus on personal control and self-esteem can help an individual regain better control over his or her health and attain weight management goals more effectively. Service facilities consisting of support staff that are more vigilant, especially to immigrant clients’ needs, may be critical to removing institutional barriers and building on assets to reduce the prevalence of MetS in this vulnerable population.
5. Conclusions
The toll of MetS and related chronic disease risks among individuals of South Asian origin is a rising public health problem. There is a need for an innovative treatment and management approach that balances ethnic-specific guidelines and recommendations with cultural and social norms and preferences, disease severity, disparities in access to neighborhood resources, and social support networks. Such an approach might affect SA immigrants to better engage in healthy behaviors.
Author Contributions
Conceptualization, M.M. and M.B.; methodology, M.B. and K.M.G.; formal analysis, M.B. and K.M.G.; investigation, M.B. and K.M.G.; resources, M.M., M.B. and R.B.; data curation, M.B. and K.M.G.; writing—M.B. and M.M.; writing—review and editing, M.M.; visualization, M.B. and M.M.; supervision, M.M. and M.B.; project administration, M.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to profusely thank Valerie Kwong for her assistance. Without her commitment and dedication, this manuscript would not be in its current form. The authors would also like to thank Matthew Lock, Rory K. Koleman, Heidi Harris, and Eugenie Verdel for their assistance.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Grundy, S.M. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2243–2244. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-A.; Lee, J.-H.; Lim, S.-Y.; Ha, H.-S.; Kwon, H.-S.; Park, Y.-M.; Lee, W.-C.; Kang, M.-I.; Yim, H.-W.; Yoon, K.-H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.; Eisenberg, M. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Salpea, K.D.; Mikhailidis, D.P. The prevalence of metabolic syndrome in various populations. Am. J. Med. Sci. 2007, 333, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- U.S. Census Bureau. Asian-American and Pacific Islander Heritage Month: May 2022. Census.gov. Available online: https://www.census.gov/newsroom/facts-for-features/2022/asian-american-pacific-islander.html (accessed on 1 February 2023).
- Bajaj, H.S.; Pereira, M.A.; Anjana, R.M.; Deepa, R.; Mohan, V.; Mueller, N.T.; Rao, G.H.; Gross, M.D. Comparison of relative waist circumference between Asian Indian and US adults. J. Obes. 2014, 2014, 461956. [Google Scholar] [CrossRef]
- Mohanty, S.A.; Woolhandler, S.; Himmelstein, D.U.; Bor, D.H. Diabetes and cardiovascular disease among Asian Indians in the United States. J. Gen. Intern. Med. 2005, 20, 474–478. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Ewing, S.K.; Liu, K.; Blaha, M.J.; Dave, S.S.; Qureshi, F.; Kandula, N.R. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care 2014, 37, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Karter, A.J.; Schillinger, D.; Adams, A.S.; Moffet, H.H.; Liu, J.; Adler, N.E.; Kanaya, A.M. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013, 36, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.R.; Brancati, F.L.; Yeh, H.C. Trends in the prevalence of type 2 diabetes in Asians versus Whites: Results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 2011, 34, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hajra, A.; Li, Y.; Siu, S.; Udaltsova, N.; Armstrong, M.A.; Friedman, G.D.; Klatsky, A.L. Risk of coronary disease in the South Asian American population. J. Am. Coll. Cardiol. 2013, 62, 644–645. [Google Scholar] [CrossRef]
- Palaniappan, L.; Wang, Y.; Fortmann, S.P. Coronary heart disease mortality for six ethnic groups in California, 1990–2000. Ann. Epidemiol. 2004, 14, 499–506. [Google Scholar] [CrossRef]
- Anand, S.S.; Tarnopolsky, M.A.; Rashid, S.; Schulze, K.M.; Desai, D.; Mente, A.; Rao, S.; Yusuf, S.; Gerstein, H.C.; Sharma, A.M. Adipocyte hypertrophy, fatty liver, and metabolic risk factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS ONE 2011, 6, e22112. [Google Scholar] [CrossRef]
- Chandalia, M.; Lin, P.; Seenivasan, T.; Livingston, E.H.; Snell, P.G.; Grundy, S.M.; Abate, N. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE 2007, 2, e812. [Google Scholar] [CrossRef]
- Raji, A.; Seely, E.W.; Arky, R.A.; Simonson, D.C. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J. Clin. Endocrinol. Metab. 2001, 86, 5366–5371. [Google Scholar] [CrossRef]
- Tillin, T.; Forouhi, N.; Johnston, D.G.; McKeigue, P.M.; Chaturvedi, N.; Godsland, I.F. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: A UK population-based cross-sectional study. Diabetologia 2005, 48, 649–656. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Sattar, N.; Tillin, T.; McKeigue, P.M.; Chaturvedi, N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia 2006, 49, 2580–2588. [Google Scholar] [CrossRef]
- Ajjan, R.; Carter, A.M.; Somani, R.; Kain, K.; Grant, P.J. Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J. Thromb. Haemost. 2007, 5, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Gupta, L.S.; Waddell, E.N.; Upadhyay, U.D.; Wildman, R.P.; Kaplan, R.; Wassertheil-Smoller, S.; Wylie-Rosett, J. Elevated risk of type 2 diabetes and metabolic syndrome among Asians and South Asians: Results from the 2004 New York City HANES. Ethn. Dis. 2010, 20, 225–230. [Google Scholar] [PubMed]
- Geragotou, T.; Jainandunsing, S.; Özcan, B.; de Rooij, F.W.M.; Kokkinos, A.; Tentolouris, N.; Sijbrands, E.J.G. The relationship of metabolic syndrome traits with beta-cell function and insulin sensitivity by oral minimal model assessment in South Asian and European families residing in the Netherlands. J. Diabetes Res. 2016, 2016, 9286303. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Yi, Q.; Gerstein, H.; Lonn, E.; Jacobs, R.; Vuksan, V.; Teo, K.; Davis, B.; Montague, P.; Yusuf, S. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation 2003, 108, 420–425. [Google Scholar] [CrossRef] [PubMed]
- A Khan, S.; Jackson, R.T. The prevalence of metabolic syndrome among low-income South Asian Americans. Public Health Nutr. 2016, 19, 418–428. [Google Scholar] [CrossRef]
- Flowers, E.; Molina, C.; Mathur, A.; Prasad, M.; Abrams, L.; Sathe, A.; Malhotra, D.; Basra, R.; Malgesini, N.; Ratnam, G.; et al. Prevalence of metabolic syndrome in South Asians residing in the United States. Metab. Syndr. Relat. Disord. 2010, 8, 417–423. [Google Scholar] [CrossRef]
- Misra, R.; Patel, T.; Kotha, P.; Raji, A.; Ganda, O.; Banerji, M.; Shah, V.; Vijay, K.; Mudaliar, S.; Iyer, D.; et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: Results from a national study. J. Diabetes Its Complicat. 2010, 24, 145–153. [Google Scholar] [CrossRef]
- Misra, K.B.; Endemann, S.W.; Ayer, M. Leisure time physical activity and metabolic syndrome in Asian Indian immigrants residing in northern California. Ethn. Dis. 2005, 15, 627–634. [Google Scholar]
- Balasubramanyam, A.; Rao, S.; Misra, R.; Sekhar, R.V.; Ballantyne, C.M. Prevalence of metabolic syndrome and associated risk factors in Asian Indians. J. Immigr. Minor. Health 2008, 10, 313–323. [Google Scholar] [CrossRef]
- Rianon, N.J.; Rasu, R.S. Metabolic syndrome and its risk factors in Bangladeshi immigrant men in the USA. J. Immigr. Minor. Health 2009, 12, 781–787. [Google Scholar] [CrossRef]
- Adil, S.O.; Islam, M.A.; Musa, K.I.; Shafique, K. Prevalence of Metabolic Syndrome among Apparently Healthy Adult Population in Pakistan: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Sundarakumar, J.S.; Stezin, A.; Menesgere, A.L.; Ravindranath, V. Rural-urban and gender differences in metabolic syndrome in the aging population from southern India: Two parallel, prospective cohort studies. EClinicalMedicine 2022, 47, 101395. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Das Trisha, A.; Hasan, A.; Islam, F. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020, 10, 7841. [Google Scholar] [CrossRef] [PubMed]
- Subramani, S.K.; Mahajan, S.; Chauhan, P.; Yadav, D.; Mishra, M.; Pakkirisamy, U.; Prasad, G. Prevalence of metabolic syndrome in Gwalior region of Central India: A comparative study using NCEP ATP III, IDF and Harmonized criteria. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 816–821. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.T.; Niriella, M.A.; Ediriweera, D.S.; Kottahachchi, D.; Kasturiratne, A.; de Silva, A.P.; Dassanayaka, A.S.; Pathmeswaran, A.; Wickramasinghe, R.; Kato, N.; et al. Incidence and risk factors for metabolic syndrome among urban, adult Sri Lankans: A prospective, 7-year community cohort, follow-up study. Diabetol. Metab. Syndr. 2019, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Mehata, S.; Shrestha, N.; Mehta, R.K.; Bista, B.; Pandey, A.R.; Mishra, S.R. Prevalence of the metabolic syndrome and its determinants among Nepalese adults: Findings from a nationally representative cross-sectional study. Sci. Rep. 2018, 8, 14995. [Google Scholar] [CrossRef]
- Sinha, S.; Misra, P.; Kant, S.; Krishnan, A.; Nongkynrih, B.; Vikram, N.K. Prevalence of metabolic syndrome and its selected determinants among urban adult women in South Delhi, India. Postgrad. Med. J. 2013, 89, 68–72. [Google Scholar] [CrossRef]
- Mehta, A.; Singh, S.; Saeed, A.; Mahtta, D.; Bittner, V.A.; Sperling, L.S.; Virani, S.S. Pathophysiological Mechanisms Underlying Excess Risk for Diabetes and Cardiovascular Disease in South Asians: The Perfect Storm. Curr. Diabetes Rev. 2021, 17, e070320183447. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B.; Bowen, L.; Bharathi, A.V.; Vaz, M.; Prabhakaran, D.; Reddy, K.S.; Ben-Shlomo, Y.; Smith, G.D.; Kinra, S.; et al. Dietary patterns in India and their association with obesity and central obesity. Public Health Nut. 2015, 18, 3031–3041. [Google Scholar] [CrossRef]
- Safdar, N.F.; Bertone-Johnson, E.; Cordeiro, L.; Jafar, T.H.; Cohen, N.L. Dietary patterns of Pakistani adults and their associations with sociodemographic, anthropometric and life-style factors. J. Nutr. Sci. 2014, 2, e42. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Khurana, L.; Isharwal, S.; Bhardwaj, S. South Asian diets and insulin resistance. Br. J. Nutr. 2009, 101, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Radhika, G.; Sathya, R.M.; Tamil, S.R.; Ganesan, A.; Sudha, V. Dietary carbohydrates, glycemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br. J. Nutr. 2009, 102, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.D.; Ranasinghe, P.; Jayawardena, R.; Misra, A. Physical activity patterns among South-Asian adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- AAnand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Work-shop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Arena, R.; Khunti, K.; Yajnik, C.S.; Jayawardena, R.; Henry, C.J.; Street, S.J.; Soares, M.J.; Misra, A. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018, 26, 966–978. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.D.; Kooner, I.; Steptoe, A.; Kooner, J.S. Psychosocial factors related to cardiovascular disease risk in UK South Asian men: A preliminary study. Br. J. Health Psychol. 2007, 12, 559–570. [Google Scholar] [CrossRef]
- Patel, D.; Winterbotham, M.; Britt, R.; Sutton, G.; Bhatnagar, D.; Mackness, M.; Creed, F.; Tomenson, B.; Durrington, P.; Anand, I.; et al. Coronary risk factors in people from the Indian subcontinent living in west London and their siblings in India. Lancet 1995, 345, 405–409. [Google Scholar] [CrossRef]
- Patel, J.; Vyas, A.; Cruickshank, J.; Prabhakaran, D.; Hughes, E.; Reddy, K.; Mackness, M.; Bhatnagar, D.; Durrington, P. Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 2006, 185, 297–306. [Google Scholar] [CrossRef]
- Zahid, N.; Meyer, H.E.; Kumar, B.N.; Claussen, B.; Hussain, A. High levels of cardiovascular risk factors among Pakistanis in Norway compared to Pakistanis in Pakistan. J. Obes. 2011, 2011, 163749. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, S.U.; Kumar, B.N.; Nugegoda, D.B.; Meyer, H.E. Comparison of cardiovascular risk factors between Sri Lankans living in Kandy and Oslo. BMC Public Health 2010, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Gujral, U.P.; Narayan, K.V.; Pradeepa, R.G.; Deepa, M.; Ali, M.K.; Anjana, R.M.; Kandula, N.R.; Mohan, V.; Kanaya, A.M. Comparing Type 2 Diabetes, Prediabetes, and Their Associated Risk Factors in Asian Indians in India and in the U.S.: The CARRS and MASALA Studies. Diabetes Care 2015, 38, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Miranda, C.; Everett, B. Prevalence of obesity among migrant Asian Indians: A systematic review and meta-analysis. Int. J. Evid.-Based Health 2011, 9, 420–428. [Google Scholar] [CrossRef]
- Gujral, U.P.; Pradeepa, R.; Weber, M.B.; Narayan, K.V.; Mohan, V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013, 1281, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Khurana, L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int. J. Obes. 2010, 35, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Guettier, J.-M.; Georgopoulos, A.; Tsai, M.Y.; Radha, V.; Shanthirani, S.; Deepa, R.; Gross, M.; Rao, G.H.; Mohan, V. Polymorphisms in the Fatty Acid-Binding Protein 2 and Apolipoprotein C-III Genes Are Associated with the Metabolic Syndrome and Dyslipidemia in a South Indian Population. J. Clin. Endocrinol. Metab. 2005, 90, 1705–1711. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Radha, V.; Mohan, V. Thr54 allele carriers of the Ala54Thr variant of FABP2 gene have associations with metabolic syndrome and hypertriglyceridemia in urban South Indians. Metabolism 2006, 55, 1222–1226. [Google Scholar] [CrossRef]
- Naran, N.H.; Chetty, N.; Crowther, N.J. The influence of metabolic syndrome components on plasma PAI-1 concentrations is modified by the PAI-1 4G/5G genotype and ethnicity. Atherosclerosis 2008, 196, 155–163. [Google Scholar] [CrossRef]
- Bajaj, H.; Pereira, M.; Anjana, R.M.; Deepa, R.; Mohan, V.; Mueller, N.; Rao, G.; Gross, M. Uncoupling protein 2 promoter polymorphism -866G/A, central adiposity, and metabolic syndrome in Asians. Obesity 2006, 14, 656–661. [Google Scholar]
- Zabaneh, D.; Balding, D.J. A Genome-Wide Association Study of the Metabolic Syndrome in Indian Asian Men. PLoS ONE 2010, 5, e11961. [Google Scholar] [CrossRef]
- Dodani, S.; Henkhaus, R.; Dong, L.; Butler, M.G. Apo lipoprotein A1 gene polymorphisms predict cardio-metabolic risk in South Asian immigrants. Dis. Markers 2012, 32, 9–19. [Google Scholar] [CrossRef]
- Lear, S.A.; Humphries, K.H.; Hage-Moussa, S.; Chockalingam, A.; Mancini, G.B.J. Immigration presents a potential increased risk for atherosclerosis. Atherosclerosis 2009, 205, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Class, M.; Castro, F.G.; Ramirez, A.G. Conceptions of acculturation: A review and statement of critical issues. Soc. Sci. Med. 2011, 72, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escamilla, R.; Putnik, P. The Role of Acculturation in Nutrition, Lifestyle, and Incidence of Type 2 Diabetes among Latinos. J. Nutr. 2007, 137, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Hazuda, H.P.; Haffner, S.M.; Stern, M.P.; Eifler, C.W. Effects of Acculturation and Socioeconomic Status on Obesity and Diabetes in Mexican Americans: The San Antonio Heart Study. Am. J. Epidemiol. 1988, 128, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Kandula, N.R.; Diez-Roux, A.V.; Chan, C.; Daviglus, M.L.; Jackson, S.A.; Ni, H.; Schreiner, P.J. Association of Acculturation Levels and Prevalence of Diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008, 31, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Roux, A.V.D.; Jacobs, D.R.; Burke, G.L.; Harman, J.; Shea, S.; Folsom, A.R. Associations of Acculturation and Socioeconomic Status With Subclinical Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis. Am. J. Public Health 2008, 98, 1963–1970. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Alos, V.A.; Davey, A.; Bueno, A.; Whitaker, R.C. Acculturation and the Prevalence of Diabetes in US Latino Adults, National Health and Nutrition Examination Survey 2007–2010. Prev. Chronic Dis. 2014, 11, E176. [Google Scholar] [CrossRef]
- Satia-Abouta, J.; Patterson, R.E.; Neuhouser, M.L.; Elder, J. Dietary acculturation: Applications to nutrition research and dietetics. J. Am. Diet. Assoc. 2002, 102, 1105–1118. [Google Scholar] [CrossRef]
- Lesser, I.A.; Gasevic, D.; Lear, S.A. The Association between Acculturation and Dietary Patterns of South Asian Immigrants. PLoS ONE 2014, 9, e88495. [Google Scholar] [CrossRef] [PubMed]
- Talegawkar, S.A.; Kandula, N.R.; Gadgil, M.D.; Desai, D.; Kanaya, A.M. Dietary intakes among South Asian adults differ by length of residence in the USA. Public Health Nutr. 2015, 19, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Guasch-Ferré, M.; Gadgil, M.D.; Newgard, C.B.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; Scholtens, D.M.; Hu, F.B.; Kanaya, A.M.; et al. Dietary Patterns among Asian Indians Living in the United States Have Distinct Metabolomic Profiles That Are Associated with Cardiometabolic Risk. J. Nutr. 2018, 148, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Garduno-Diaz, S.D.; Khokhar, S. South Asian dietary patterns and their association with risk factors for the metabolic syndrome. J. Hum. Nutr. Diet. 2013, 26, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Anderson, C.A.; Kandula, N.R.; Kanaya, A.M. Dietary Patterns Are Associated with Metabolic Risk Factors in South Asians Living in the United States. J. Nutr. 2015, 145, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, M.D.; Anderson, C.A.; Kandula, N.R.; Kanaya, A.M. Dietary patterns in Asian Indians in the United States: An analysis of the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study. J. Acad. Nutr. Diet. 2014, 114, 238–243. [Google Scholar] [CrossRef]
- Shah, A.D.; Vittinghoff, E.; Kandula, N.R.; Srivastava, S.; Kanaya, A.M. Correlates of prediabetes and type II diabetes in US South Asians: Findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Ann. Epidemiol. 2015, 25, 77–83. [Google Scholar] [CrossRef]
- Khan, S.A.; Jackson, R.T. Polyunsaturated fatty acids, inflammation, and metabolic syndrome in South Asian Americans in Maryland. Food Sci. Nutr. 2018, 6, 1575–1581. [Google Scholar] [CrossRef]
- Goel, M.S.; McCarthy, E.P.; Phillips, R.S.; Wee, C.C. Obesity among US immigrant subgroups by duration of residence. JAMA 2004, 292, 2860–2867. [Google Scholar] [CrossRef]
- Bharmal, N.; Kaplan, R.M.; Shapiro, M.F.; Mangione, C.M.; Kagawa-Singer, M.; Wong, M.D.; McCarthy, W.J. The Association of Duration of Residence in the United States with Cardiovascular Disease Risk Factors Among South Asian Immigrants. J. Immigr. Minor. Health 2014, 17, 781–790. [Google Scholar] [CrossRef]
- Wang, E.T.; De Koning, L.; Kanaya, A.M. Higher protein intake is associated with diabetes risk in South Asian Indians: The Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study. J. Am. Coll. Nutr. 2010, 29, 130–135. [Google Scholar] [CrossRef]
- Wandel, M.; Råberg, M.; Kumar, B.; Holmboe-Ottesen, G. Changes in food habits after migration among South Asians settled in Oslo: The effect of demographic, socio-economic and integration factors. Appetite 2008, 50, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Stanforth, P.; Gagnon, J.; Rankinen, T.; Leon, A.; Rao, D.; Skinner, J.; Bouchard, C.; Wilmore, J. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. 2002, 26, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Pinto, A.; Giusti, A.M.; Lenzi, A.; Poggiogalle, E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020, 7, 53. [Google Scholar] [CrossRef]
- Deurenberg, P.; Andreoli, A.; Borg, P.; Kukkonen-Harjula, K.; de Lorenzo, A.; Lichtenbelt, W.V.M.; Testolin, G.; Vigano, R.; Vollaard, N. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur. J. Clin. Nutr. 2001, 55, 973–979. [Google Scholar] [CrossRef]
- Daniel, M.; Wilbur, J. Physical Activity Among South Asian Indian Immigrants: An Integrative Review. Public Health Nurs. 2011, 28, 389–401. [Google Scholar] [CrossRef]
- Walker, G.J.; Caperchione, C.M.; Mummery, W.K.; Chau, S. Examining the role of acculturation in the leisure-time physical activity of South Asians living in Canada. J. Sci. Med. Sport 2015, 18, 156–160. [Google Scholar] [CrossRef]
- Kolt, G.S.; Schofield, G.M.; Rush, E.C.; Oliver, M.; Chadha, N.K. Body fatness, physical activity, and nutritional behaviors in Asian Indian immigrants to New Zealand. Asia Pacific J. Clin. Nutr. 2007, 16, 663–670. [Google Scholar]
- Misra, A.; Alappan, N.K.; Vikram, N.K.; Goel, K.; Gupta, N.; Mittal, K.; Bhatt, S.; Luthra, K. Effect of Supervised Progressive Resistance-Exercise Training Protocol on Insulin Sensitivity, Glycemia, Lipids, and Body Composition in Asian Indians With Type 2 Diabetes. Diabetes Care 2008, 31, 1282–1287. [Google Scholar] [CrossRef]
- Fischbacher, C.M.; Hunt, S.; Alexander, L. How physically active are South Asians in the United Kingdom? A literature review. J. Public Health 2004, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Chandu, V.; Plank, L.D. Reduction of abdominal fat and chronic disease factors by lifestyle change in migrant Asian Indians older than 50 years. Asia Pac. J. Clin. Nutr. 2007, 16, 671–676. [Google Scholar] [PubMed]
- Brown, T.; Smith, S.; Bhopal, R.; Kasim, A.; Summerbell, C. Diet and Physical Activity Interventions to Prevent or Treat Obesity in South Asian Children and Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2015, 12, 566–594. [Google Scholar] [CrossRef]
- Martin, C.A.; Gowda, U.; Smith, B.J.; Renzaho, A.M.N. Systematic Review of the Effect of Lifestyle Interventions on the Components of the Metabolic Syndrome in South Asian Migrants. J. Immigr. Minor. Health 2016, 20, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Araneta, M.R.G.; Pawlowsky, S.B.; Barrett-Connor, E.; Grady, D.; Vittinghoff, E.; Schembri, M.; Chang, A.; Carrion-Petersen, M.L.; Coggins, T.; et al. Restorative yoga and metabolic risk factors: The Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. J. Diabetes Complicat. 2013, 28, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.; Høstmark, A.T.; Anderssen, S.A. Effect of a Physical Activity Intervention on the Metabolic Syndrome in Pakistani Immigrant Men: A Randomized Controlled Trial. J. Immigr. Minor. Health 2012, 14, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hjellset, V.; Kjøllesdal MK, R.; Bjørge, B.; Holmboe-Ottesen, G.; Wandel, M.; Birkeland, K.I.; Eriksen, H.; Høstmark, A.T. The InnvaDiab-DE-PLAN study: A randomized controlled trial with a culturally adapted education program for improving the risk profile for type 2 diabetes in Pakistani immigrant women. Br. J. Nutr. 2013, 109, 529–538. [Google Scholar] [CrossRef]
- Kousar, R.; Burns, C.; Lewandowski, P. A culturally appropriate diet and lifestyle intervention can successfully treat the components of metabolic syndrome in female Pakistani immigrants residing in Melbourne, Australia. Metabolism 2008, 57, 1502–1508. [Google Scholar] [CrossRef]
- Mohan, V.; Gayathri, R.; Jaacks, L.M.; Lakshmipriya, N.; Anjana, R.M.; Spiegelman, D.; Jeevan, R.G.; Balasubramaniam, K.K.; Shobana, S.; Jayanthan, M.; et al. Cashew Nut Consumption Increases HDL Cholesterol and Reduces Systolic Blood Pressure in Asian Indians with Type 2 Diabetes: A 12-Week Randomized Controlled Trial. J. Nutr. 2018, 148, 63–69. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 158. [Google Scholar] [CrossRef]
- Volgman, A.S.; Palaniappan, L.S.; Aggarwal, N.T.; Gupta, M.; Khandelwal, A.; Krishnan, A.V.; Lichtman, J.H.; Mehta, L.S.; Patel, H.N.; Shah, K.S.; et al. Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement From the American Heart Association. Circulation 2018, 138, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Ali, M.K.; Ajay, V.S.; Shivashankar, R.; Mohan, V.; Pradeepa, R.; Deepa, M.; Khan, H.M.; Kadir, M.M.; Fatmi, Z.A.; et al. CARRS Surveillance study: Design and methods to assess burdens from multiple perspectives. BMC Public Health 2012, 12, 701. [Google Scholar] [CrossRef]
- Gujral, U.P.; Kanaya, A.M. Epidemiology of diabetes among South Asians in the United States: Lessons from the MASALA study. Ann. N. Y. Acad. Sci. 2020, 1495, 24–39. [Google Scholar] [CrossRef]
- Bajaj, M.; Banerji, M.A. Type 2 diabetes in South Asians: A pathophysiologic focus on the Asian-Indian epidemic. Curr. Diabetes Rep. 2004, 4, 213–218. [Google Scholar] [CrossRef]
- Enas, E.A.; Mohan, V.; Deepa, M.; Farooq, S.; Pazhoor, S.; Chennikkara, H. The Metabolic Syndrome and Dyslipidemia Among Asian Indians: A Population With High Rates of Diabetes and Premature Coronary Artery Disease. J. Cardiometab. Syndr. 2007, 2, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Ashcraft, A. Type 2 Diabetes Risk among Asian Indians in the US: A Pilot Study. Nurs. Res. Pr. 2013, 2013, 492893. [Google Scholar] [CrossRef] [PubMed]
- Savadatti, S.S.; Bell, E.M.; Gates, M.A.; Hosler, A.; Yucel, R.M.; Misra, R. Metabolic Syndrome Among Asian Indians in the United States. J. Public Health Manag. Pr. 2019, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, U.; Fatma, M.; Mohan, S.; Singh, P.; Misra, A. Randomized Control Trial for Reduction of Body Weight, Body Fat Patterning, and Cardiometabolic Risk Factors in Overweight Worksite Employees in Delhi, India. J. Diabetes Res. 2017, 2017, 7254174. [Google Scholar] [CrossRef]
- Anjana, R.M.; Pradeepa, R.; Das, A.K.; Deepa, M.; Bhansali, A.; Joshi, S.R.; Joshi, P.P.; Dhandhania, V.K.; Rao, P.V.; Sudha, V.; et al. Physical activity and inactivity patterns in India—results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5]. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 26. [Google Scholar] [CrossRef]
- Manchanda, S.C.; Mehrotra, U.C.; Makhija, A.; Mohanty, A.; Dhawan, S.; Sawhney, J.P.S. Reversal of early atherosclerosis in metabolic syndrome by yoga-a randomized controlled trial. J. Yoga Phys. Ther. 2013, 3, 1. [Google Scholar]
- Dodani, S.; Henkhaus, R.; Wick, J.; Vacek, J.; Gupta, K.; Dong, L.; Butler, M.G. Metabolic syndrome in South Asian immigrants: More than low HDL requiring aggressive management. Lipids Health Dis. 2011, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Araneta, M.R.G.; Kanaya, A.M.; Chiang, J.L.; Fujimoto, W. BMI Cut Points to Identify At-Risk Asian Americans for Type 2 Diabetes Screening. Diabetes Care 2015, 38, 150–158. [Google Scholar] [CrossRef]
- Khunti, K.; Taub, N.; Tringham, J.; Jarvis, J.; Farooqi, A.; Skinner, T.C.; Davies, M.J. Screening for the metabolic syndrome using simple anthropometric measurements in south Asian and white Europeans: A population-based screening study. The Leicester Ethnic Atherosclerosis and Diabetes Risk (LEADER) Study. Prim. Care Diabetes 2010, 4, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Cross-Bardell, L.; George, T.; Bhoday, M.; Tuomainen, H.; Qureshi, N.; Kai, J. Perspectives on enhancing physical activity and diet for health promotion among at-risk urban UK South Asian communities: A qualitative study. BMJ Open 2015, 5, e007317. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R.; Ghimire, S.; Joshi, C.; Gyawali, B.; Shrestha, A.; Neupane, D.; Sharma, S.R.; Pokharel, Y.; Virani, S.S. Cardio-metabolic disease risk factors among South Asian labour migrants to the Middle East: A scoping review and policy analysis. Glob. Health 2019, 15, 33. [Google Scholar] [CrossRef]
- Health Development Agency. Black and Minority Ethnic Groups in England: The Second Health and Lifestyles Survey; Health Education Authority: London, UK, 2000. [Google Scholar]
- Stanner, S. Health Survey for England 1999: The health of minority ethnic groups. Nutr. Bull. 2001, 26, 227–230. [Google Scholar] [CrossRef]
- Vasudevan, D.; Stotts, A.; Anabor, O.L.; Mandayam, S. Primary Care Physician’s Knowledge of Ethnicity-Specific Guidelines for Obesity Diagnosis and Readiness for Obesity Intervention Among South Asian Indians. J. Immigr. Minor. Health 2011, 14, 759–766. [Google Scholar] [CrossRef]
- Bodicoat, D.H.; Gray, L.J.; Henson, J.; Webb, D.; Guru, A.; Misra, A.; Gupta, R.; Vikram, N.; Sattar, N.; Davies, M.; et al. Body Mass Index and Waist Circumference Cut-Points in Multi-Ethnic Populations from the UK and India: The ADDITION-Leicester, Jaipur Heart Watch and New Delhi Cross-Sectional Studies. PLoS ONE 2014, 9, e90813. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Ghouri, N.; Bailey, M.E.; Sattar, N.; Gill, J.M. Should physical activity recommendations be ethnicity-specific?: Evidence from a cross-sectional study of South Asian and European men. PLoS ONE 2013, 8, e82568. [Google Scholar] [CrossRef]
- Song, J.; Hochberg, M.C.; Chang, R.W.; Hootman, J.M.; Manheim, L.M.; Lee, J.; Semanik, P.A.; Sharma, L.; Dunlop, R.D. Osteoarthritis Initiative Investigators Racial and ethnic differences in physical activity guidelines attainment among people at high risk of or having knee osteoarthritis. Arthritis Care Res. 2012, 65, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gregson, J.; Foerster, S.B.; Orr, R.; Jones, L.; Benedict, J.; Clarke, B.; Hersey, J.; Lewis, J.; Zotz, K. System, Environmental, and Policy Changes: Using the Social-Ecological Model as a Framework for Evaluating Nutrition Education and Social Marketing Programs with Low-Income Audiences. J. Nutr. Educ. 2001, 33, S4–S15. [Google Scholar] [CrossRef] [PubMed]
- Kumanyika, S.K.; Morssink, C.B. Bridging domains in efforts to reduce disparities in health and health care. Health Educ. Behav. 2006, 33, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Christakis, N.A. Social networks and health. Annu. Rev. Sociol. 2008, 34, 405–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).