New Hydronephrosis in the Native Kidney Is Associated with the Development of De Novo Urinary Bladder Urothelial Carcinoma in Patients with Post-Kidney Transplantation
Abstract
1. Introduction
2. Materials and Methods
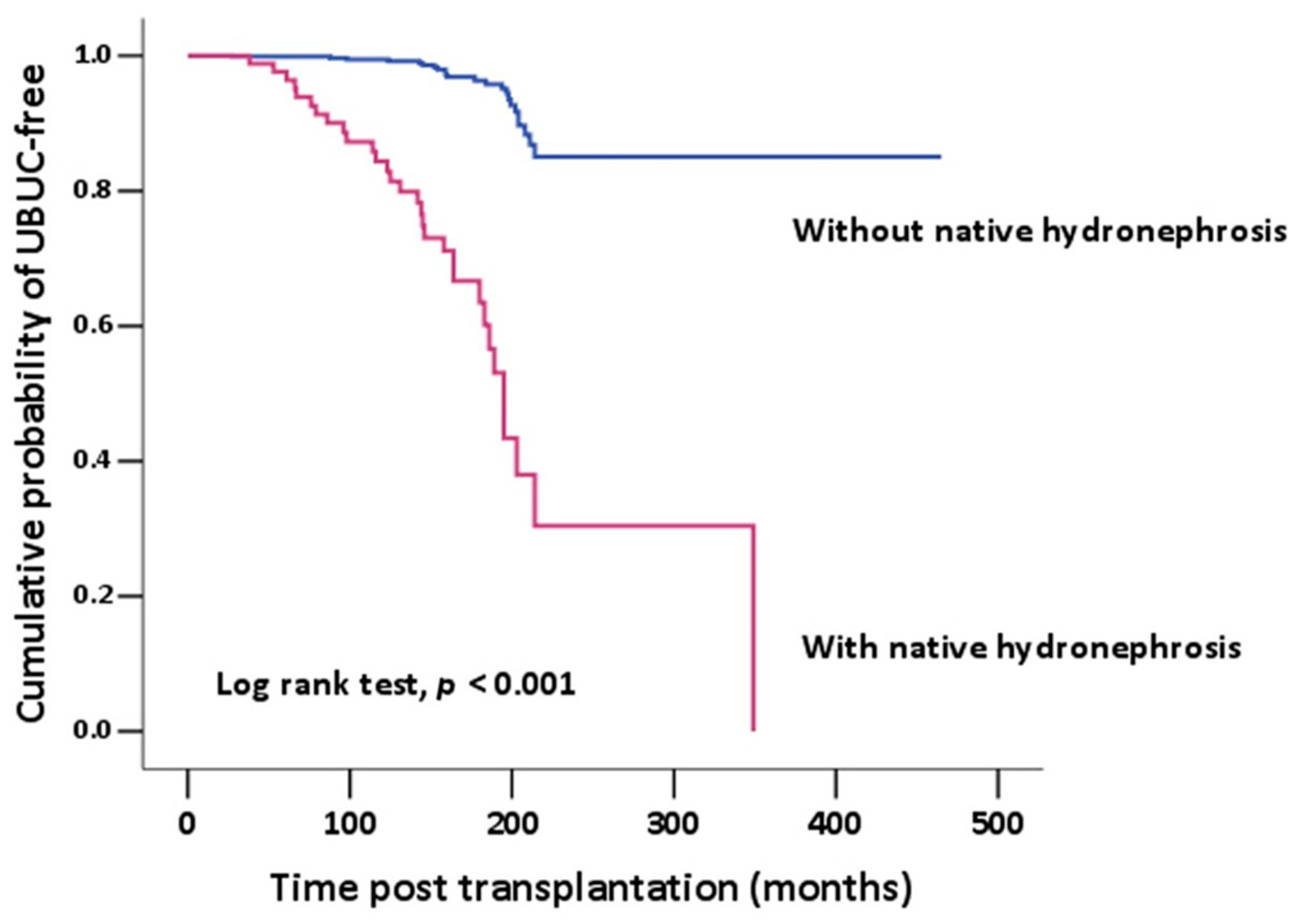
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, L.; Chen, P.; Chen, E.Z.; Gu, A.; Jiang, Z.Y. Risk of bladder cancer in renal transplant recipients: A meta-analysis. Br. J. Cancer 2014, 110, 1871–1877. [Google Scholar] [CrossRef]
- Li, W.H.; Chen, Y.J.; Tseng, W.C.; Lin, M.W.; Chen, T.J.; Chu, S.Y.; Hwang, C.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; et al. Malignancies after renal transplantation in Taiwan: A nationwide population-based study. Nephrol. Dial. Transpl. 2012, 27, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kyllonen, L.; Salmela, K.; Pukkala, E. Cancer incidence in a kidney-transplanted population. Transpl. Int. 2000, 13 (Suppl. S1), S394–S398. [Google Scholar] [CrossRef]
- Krynitz, B.; Edgren, G.; Lindelof, B.; Baecklund, E.; Brattstrom, C.; Wilczek, H.; Smedby, K.E. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—A Swedish population-based study. Int. J. Cancer 2013, 132, 1429–1438. [Google Scholar] [CrossRef]
- Kao, Y.L.; Ou, Y.C.; Yang, C.R.; Ho, H.C.; Su, C.K.; Shu, K.H. Transitional cell carcinoma in renal transplant recipients. World J. Surg. 2003, 27, 912–916. [Google Scholar] [CrossRef]
- Wu, M.J.; Lian, J.D.; Yang, C.R.; Cheng, C.H.; Chen, C.H.; Lee, W.C.; Shu, K.H.; Tang, M.J. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan. Am. J. Kidney Dis. 2004, 43, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- D’Costa, J.J.; Goldsmith, J.C.; Wilson, J.S.; Bryan, R.T.; Ward, D.G. A Systematic Review of the Diagnostic and Prognostic Value of Urinary Protein Biomarkers in Urothelial Bladder Cancer. Bladder Cancer 2016, 2, 301–317. [Google Scholar] [CrossRef]
- Yeh, C.F.; Chan, T.C.; Ke, H.L.; Chen, T.J.; Wu, L.C.; Lee, H.Y.; Wei, Y.C.; Wu, W.J.; Li, C.F.; Li, W.M. Prognostic Significance of ROR2 Expression in Patients with Urothelial Carcinoma. Biomedicines 2021, 9, 1054. [Google Scholar] [CrossRef]
- Liu, S.; Chaudhry, M.R.; Berrebi, A.A.; Papadimitriou, J.C.; Drachenberg, C.B.; Haririan, A.; Alexiev, B.A. Polyomavirus Replication and Smoking Are Independent Risk Factors for Bladder Cancer After Renal Transplantation. Transplantation 2017, 101, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Zhang, Y.; Zhao, P.; Zheng, T.; Dai, M. Human papillomavirus infection and bladder cancer risk: A meta-analysis. J. Infect. Dis. 2011, 204, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.J.; Hsieh, P.F.; Chang, C.H.; Wu, H.C.; Yang, C.R.; Huang, C.P. Higher risk of urothelial carcinoma in the upper urinary tract than in the urinary bladder in hemodialysis patients. Ren. Fail. 2016, 38, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.J.; Huang, Y.H.; Hsieh, T.Y.; Yang, M.H.; Wang, S.C.; Chen, W.J.; Lee, T.H.; Sung, W.W.; Chen, S.L. Native Kidney Hydronephrosis Is Associated with Upper Urinary Tract Urothelial Carcinoma in Post-Kidney Transplantation Patients. J. Clin. Med. 2021, 10, 4474. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.H.; Pan, C.C.; Lin, J.S.; Tzai, T.S.; Yang, W.H.; Chang, C.C.; Cheng, H.L.; Lin, Y.M.; Tong, Y.C. Transitional cell carcinoma in dialysis patients. Eur. Urol. 2000, 37, 90–94. [Google Scholar] [CrossRef]
- Ehdaie, B.; Stukenborg, G.J.; Theodorescu, D. Renal transplant recipients and patients with end stage renal disease present with more advanced bladder cancer. J. Urol. 2009, 182, 1482–1487. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Knowles, W.; Dickenmann, M.; Passweg, J.; Klimkait, T.; Mihatsch, M.J.; Steiger, J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 2002, 347, 488–496. [Google Scholar] [CrossRef]
- Schwarz, A.; Linnenweber-Held, S.; Heim, A.; Framke, T.; Haller, H.; Schmitt, C. Viral Origin, Clinical Course, and Renal Outcomes in Patients With BK Virus Infection After Living-Donor Renal Transplantation. Transplantation 2016, 100, 844–853. [Google Scholar] [CrossRef]
- Rogers, R.; Gohh, R.; Noska, A. Urothelial cell carcinoma after BK polyomavirus infection in kidney transplant recipients: A cohort study of veterans. Transpl. Infect. Dis. 2017, 19, e12752. [Google Scholar] [CrossRef]
- Chen, C.H.; Wen, M.C.; Wang, M.; Lian, J.D.; Cheng, C.H.; Wu, M.J.; Yu, T.M.; Chuang, Y.W.; Chang, D.; Shu, K.H. High incidence of malignancy in polyomavirus-associated nephropathy in renal transplant recipients. Transpl. Proc. 2010, 42, 817–818. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Drachenberg, C.B.; Papadimitriou, J.C. Polyomavirus-cystitis associated with in situ and invasive urothelial carcinoma in a heart transplant recipient: Evidence suggesting sequential progression/evolution from infection to carcinoma. Transplantation 2015, 99, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Husain, E.; Prowse, D.M.; Ktori, E.; Shaikh, T.; Yaqoob, M.; Junaid, I.; Baithun, S. Human papillomavirus is detected in transitional cell carcinoma arising in renal transplant recipients. Pathology 2009, 41, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Badawi, H.; Ahmed, H.; Ismail, A.; Diab, M.; Moubarak, M.; Badawy, A.; Saber, M. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J. Med. 2008, 10, 232. [Google Scholar] [PubMed]
- Khatami, A.; Salavatiha, Z.; Razizadeh, M.H. Bladder cancer and human papillomavirus association: A systematic review and meta-analysis. Infect. Agent Cancer 2022, 17, 3. [Google Scholar] [CrossRef]
- Buxeda, A.; Redondo-Pachon, D.; Perez-Saez, M.J.; Crespo, M.; Pascual, J. Sex differences in cancer risk and outcomes after kidney transplantation. Transpl. Rev. 2021, 35, 100625. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Chiang, Y.J.; Yang, P.S.; Wang, H.H.; Lin, K.J.; Liu, K.L.; Chu, S.H.; Hsieh, C.Y. Urothelial cancer after renal transplantation: An update. Transpl. Proc. 2012, 44, 744–745. [Google Scholar] [CrossRef]
- Tsai, H.L.; Chang, J.W.; Wu, T.H.; King, K.L.; Yang, L.Y.; Chan, Y.J.; Yang, A.H.; Chang, F.P.; Pan, C.C.; Yang, W.C.; et al. Outcomes of kidney transplant tourism and risk factors for de novo urothelial carcinoma. Transplantation 2014, 98, 79–87. [Google Scholar] [CrossRef]
- Shen, C.H.; Chiou, H.Y.; Tung, M.C.; Wu, C.C.; Kao, W.T.; Wang, Y.H.; Juang, G.D. Clinical and demographic characteristics among patients with urothelial carcinomas of the upper urinary tract and bladder in Taiwan. J. Chin. Med. Assoc. 2017, 80, 563–568. [Google Scholar] [CrossRef]
- Lin, S.H.; Luo, H.L.; Chen, Y.T.; Cheng, Y.T. Using Hematuria as Detection of Post-kidney Transplantation Upper Urinary Tract Urothelial Carcinoma Is Associated With Delayed Diagnosis of Cancer Occurrence. Transpl. Proc. 2017, 49, 1061–1063. [Google Scholar] [CrossRef]
- Vajdic, C.M.; McDonald, S.P.; McCredie, M.R.; van Leeuwen, M.T.; Stewart, J.H.; Law, M.; Chapman, J.R.; Webster, A.C.; Kaldor, J.M.; Grulich, A.E. Cancer incidence before and after kidney transplantation. JAMA 2006, 296, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, S.A.; Lokkegaard, H.; Storm, H.H. Cancer risk in patients on dialysis and after renal transplantation. Lancet 2000, 355, 1886–1887. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Laukhtina, E.; Shim, S.R.; Mori, K.; D’Andrea, D.; Soria, F.; Rajwa, P.; Mostafaei, H.; Comperat, E.; Cimadamore, A.; Moschini, M.; et al. Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. Oncol. 2021, 4, 927–942. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.W.; Hsia, K.T.; Liao, J.B.; Yeh, C.C.; Kuo, W.T.; Yang, Y.F. SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma. Diagnostics 2021, 11, 1928. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Chan, T.C.; Li, W.S.; Li, C.F.; Ke, H.L.; Wei, Y.C.; Wu, W.J.; Li, W.M. Utility of EFEMP1 in the Prediction of Oncologic Outcomes of Urothelial Carcinoma. Genes 2021, 12, 872. [Google Scholar] [CrossRef]
- Leibovitch, I.; Ben-Chaim, J.; Ramon, J.; Madjar, I.; Engelberg, I.S.; Goldwasser, B. The significance of ureteral obstruction in invasive transitional cell carcinoma of the urinary bladder. J. Surg. Oncol. 1993, 52, 31–35. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Messer, J.C.; Terrell, J.D.; Herman, M.P.; Ng, C.K.; Scherr, D.S.; Scoll, B.; Boorjian, S.A.; Uzzo, R.G.; Wille, M.; Eggener, S.E.; et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol. Oncol. 2013, 31, 904–908. [Google Scholar] [CrossRef]
- Stimson, C.J.; Cookson, M.S.; Barocas, D.A.; Clark, P.E.; Humphrey, J.E.; Patel, S.G.; Smith, J.A., Jr.; Chang, S.S. Preoperative hydronephrosis predicts extravesical and node positive disease in patients undergoing cystectomy for bladder cancer. J. Urol. 2010, 183, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total Population n = 1005 |
|---|---|
| Transplantation age, y/o | 48.2 ± 12.0 |
| Gender | |
| Male | 612 (60.9%) |
| Female | 393 (39.1%) |
| Smoking history | 168 (22.7%) |
| Hypertension | 648 (64.5%) |
| Diabetes mellitus | 541 (53.8%) |
| Dyslipidemia | 820 (82.0%) |
| Hyperuricemia | 780 (78.0%) |
| Hepatitis B virus infection | 172 (31.3%) |
| Hepatitis C virus infection | 94 (19.1%) |
| BK virus infection, | 281 (39.0%) |
| Native Hydronephrosis | 88 (9.4%) |
| Unilateral | 74 (84.1%) |
| Bilateral | 14 (15.9%) |
| Graft Hydronephrosis | 217 (23.1%) |
| Average time from KT to UBUC, years | 7.0 ± 5.1 |
| Characteristics | With UBUC n = 58 | Without UBUC n = 947 | p Value |
|---|---|---|---|
| Transplantation age, y/o | 51.6 ± 9.5 | 48.0 ± 12.0 | 0.029 |
| Gender | <0.001 | ||
| Male | 21 (36.2%) | 591 (62.4%) | |
| Female | 37 (63.8%) | 356 (37.6%) | |
| Smoking history | 6 (12.2%) | 162 (23.5%) | 0.007 |
| Hypertension | 36 (62.1%) | 612 (64.6%) | 0.693 |
| Diabetes mellitus | 32 (55.2%) | 509 (53.7%) | 0.833 |
| Dyslipidemia | 51 (87.9%) | 769 (81.6%) | 0.226 |
| Hyperuricemia | 44 (75.9%) | 736 (78.1) | 0.685 |
| Hepatitis B virus infection | 9 (28.1%) | 163 (31.5) | 0.687 |
| Hepatitis C virus infection | 4 (15.4%) | 90 (19.3%) | 0.620 |
| BK virus infection | 16 (45.7%) | 265 (38.6%) | 0.402 |
| Native Hydronephrosis | 31 (56.4%) | 57 (6.4%) | <0.001 |
| Unilateral | 29 (93.5%) | 45 (78.9%) | |
| Bilateral | 2 (6.5%) | 12 (21.1%) | |
| Graft Hydronephrosis | 12 (21.8%) | 205 (23.2%) | 0.811 |
| Clinical Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age > 65 | 1.029 | 0.361–2.939 | 0.957 | |||
| Gender (M/F) | 0.324 | 0.197–0.593 | <0.001 | 0.563 | 0.301–1.050 | 0.071 |
| Smoking history | 0.455 | 0.190–1.088 | 0.077 | |||
| Hypertension | 0.896 | 0.518–1.548 | 0.693 | |||
| Diabetes mellitus | 1.059 | 0.622–1.805 | 0.833 | |||
| Dyslipidemia | 1.639 | 0.731–3.674 | 0.230 | |||
| Hyperuricemia | 0.880 | 0.473–1.637 | 0.686 | |||
| HBV | 0.850 | 0.385–1.877 | 0.687 | |||
| HCV | 0.760 | 0.255–2.259 | 0.621 | |||
| BK virus | 1.338 | 0.676–2.648 | 0.403 | |||
| Native hydronephrosis | 18.740 | 10.319–34.034 | <0.001 | 16.033 | 8.660–29.683 | <0.001 |
| Graft hydronephrosis | 0.923 | 0.478–1.783 | 0.811 | |||
| Characteristics | With Native Hydronephrosis n = 23 | Without Native Hydronephrosis n = 19 | p Value |
|---|---|---|---|
| Histologic grade | |||
| Grade I, Low grade | 2 (8.7%) | 2 (10.5%) | 0.649 |
| Grade II, III, High grade | 21 (91.3%) | 13 (68.4%) | 0.649 |
| Unknown | 0 (0%) | 4 (21.1%) | |
| T stage | |||
| Ta | 4 (17.4%) | 2 (10.5%) | 0.815 |
| Tis | 1 (4.3%) | 1 (5.3%) | 0.706 |
| T1 | 12 (52.2%) | 5 (26.3%) | 0.314 |
| T2 | 3 (13.0%) | 4 (21.1%) | 0.225 |
| T3 | 0 | 0 | |
| T4 | 0 | 0 | |
| Unknown | 3 (13.0%) | 7 (36.8%) | |
| Multiple bladder tumors on initial | 11 (47.8%) | 3 (15.8%) | 0.028 |
| Bladder recurrence | 13 (56.5%) | 9 (47.4%) | 0.554 |
| Synchronous UTUC | 15 (65.2%) | 4 (21.1%) | 0.004 |
| UBUC first | 3 (13.0%) | 1 (5.3%) | 0.393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-J.; Huang, Y.-H.; Hsieh, T.-Y.; Yang, M.-H.; Wang, S.-C.; Chen, W.-J.; Sung, W.-W.; Chen, S.-L. New Hydronephrosis in the Native Kidney Is Associated with the Development of De Novo Urinary Bladder Urothelial Carcinoma in Patients with Post-Kidney Transplantation. Healthcare 2023, 11, 1209. https://doi.org/10.3390/healthcare11091209
Ho C-J, Huang Y-H, Hsieh T-Y, Yang M-H, Wang S-C, Chen W-J, Sung W-W, Chen S-L. New Hydronephrosis in the Native Kidney Is Associated with the Development of De Novo Urinary Bladder Urothelial Carcinoma in Patients with Post-Kidney Transplantation. Healthcare. 2023; 11(9):1209. https://doi.org/10.3390/healthcare11091209
Chicago/Turabian StyleHo, Cheng-Ju, Yu-Hui Huang, Tzuo-Yi Hsieh, Min-Hsin Yang, Shao-Chuan Wang, Wen-Jung Chen, Wen-Wei Sung, and Sung-Lang Chen. 2023. "New Hydronephrosis in the Native Kidney Is Associated with the Development of De Novo Urinary Bladder Urothelial Carcinoma in Patients with Post-Kidney Transplantation" Healthcare 11, no. 9: 1209. https://doi.org/10.3390/healthcare11091209
APA StyleHo, C.-J., Huang, Y.-H., Hsieh, T.-Y., Yang, M.-H., Wang, S.-C., Chen, W.-J., Sung, W.-W., & Chen, S.-L. (2023). New Hydronephrosis in the Native Kidney Is Associated with the Development of De Novo Urinary Bladder Urothelial Carcinoma in Patients with Post-Kidney Transplantation. Healthcare, 11(9), 1209. https://doi.org/10.3390/healthcare11091209








