Using Machine Learning for the Risk Factors Classification of Glycemic Control in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement
2.3. Data Reduction and Statistical Analysis
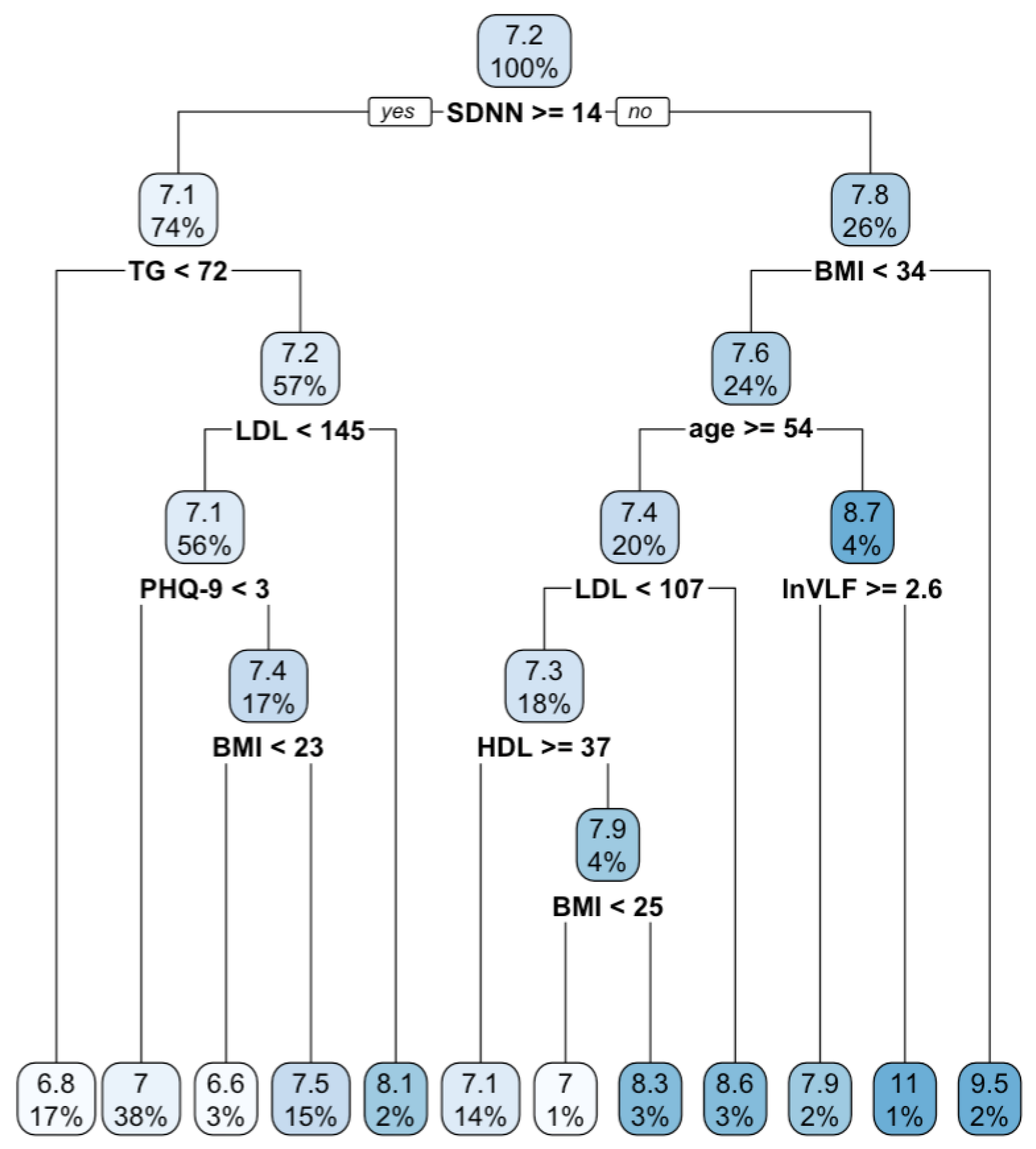
3. Results
3.1. Regression Tree
3.2. Comparisons of AI Machine Learning Classification Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. J. Diabetes Res. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ismail, L.; Materwala, H.; Al Kaabi, J. Association of risk factors with type 2 diabetes: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 1759–1785. [Google Scholar] [CrossRef] [PubMed]
- Haghighatpanah, M.; Nejad, A.S.M.; Haghighatpanah, M.; Thunga, G.; Mallayasamy, S. Factors that correlate with poor glycemic control in type 2 diabetes mellitus patients with complications. Osong Public Health Res. Perspect. 2018, 9, 167. [Google Scholar] [CrossRef]
- Kayar, Y.; Ilhan, A.; Kayar, N.B.; Unver, N.; Coban, G.; Ekinci, I.; Eroglu, H. Relationship between the poor glycemic control and risk factors, life style and complications. Biomed. Res. 2017, 28, 1581–1586. [Google Scholar]
- Artha, I.M.J.R.; Bhargah, A.; Dharmawan, N.K.; Pande, U.W.; Triyana, K.A.; Mahariski, P.A.; Yuwono, J.; Bhargah, V.; Prabawa, I.P.Y.; Manuaba, I.B.A.P.; et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc. Health Risk Manag. 2019, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Amiel, S.A.; Aschner, P.; Childs, B.; Cryer, P.E.; de Galan, B.E.; Frier, B.M.; Gonder-Frederick, L.; Heller, R.S.; Jones, T.; Khunti, K.; et al. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019, 7, 385–396. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chinatalapudi, N.; Amenta, F. Applications of machine learning predictive models in the chronic disease diagnosis. J. Pers. Med. 2020, 10, 21. [Google Scholar] [CrossRef]
- Mishra, S.; Mallick, P.K.; Tripathy, H.K.; Bhoi, A.K.; González-Briones, A. Performance evaluation of a proposed machine learning model for chronic disease datasets using an integrated attribute evaluator and an improved decision tree classifier. Appl. Sci. 2020, 10, 8137. [Google Scholar] [CrossRef]
- Daghistani, T.; Alshammari, R. Comparison of statistical logistic regression and random forest machine learning techniques in predicting diabetes. J. Inf. Technol. 2020, 11, 78–83. [Google Scholar] [CrossRef]
- Dritsas, E.; Trigka, M. Data-driven machine-learning methods for diabetes risk prediction. Sensors 2022, 22, 5304. [Google Scholar] [CrossRef]
- Kopitar, L.; Kocbek, P.; Cilar, L.; Sheikh, A.; Stiglic, G. Early detection of type 2 diabetes mellitus using machine learning-based prediction models. Sci. Rep. 2020, 10, 11981. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Huang, H.; Keshavjee, K.; Guergachi, A.; Gao, X. Predictive models for diabetes mellitus using machine learning techniques. BMC Endocr. Disord. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Laila, U.E.; Mahboob, K.; Khan, A.W.; Khan, F.; Taekeun, W. An ensemble approach to predict early-stage diabetes risk using machine learning: An empirical study. Sensors 2022, 22, 5247. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, M.; Wang, C.; Wang, Z. Machine learning for characterizing risk of type 2 diabetes mellitus in a rural Chinese population: The Henan Rural Cohort Study. Sci. Rep. 2020, 10, 4406. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Qu, K.; Luo, Y.; Yin, D.; Ju, Y.; Tang, H. Predicting diabetes mellitus with machine learning techniques. Front. Genet. 2018, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Nusinovici, S.; Tham, Y.C.; Yan, M.Y.C.; Ting, D.S.W.; Li, J.; Sabanayagam, C.; Wong, Y.T.; Cheng, C.Y. Logistic regression was as good as machine learning for predicting major chronic diseases. J. Clin. Epidemiol. 2020, 122, 56–69. [Google Scholar] [CrossRef]
- Dagliati, A.; Marini, S.; Sacchi, L.; Cogni, G.; Teliti, M.; Tibollo, V.; De Cata, P.; Chiovato, L.; Bellazzi, R. Machine learning methods to predict diabetes complications. J. Diabetes Sci. Technol. 2018, 12, 295–302. [Google Scholar] [CrossRef]
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–6731. [Google Scholar] [CrossRef]
- Bang, H.; Edwards, A.M.; Bomback, A.S.; Ballantyne, C.M.; Brillon, D.; Callahan, M.A.; Kern, L.M. A patient self-assessment diabetes screening score: Development, validation, and comparison to other diabetes risk assessment scores. Ann. Intern. Med. 2009, 151, 775. [Google Scholar] [CrossRef]
- Yang, H.; Luo, Y.; Ren, X.; Wu, M.; He, X.; Peng, B.; Deng, K.; Yan, D.; Tang, H.; Lin, H. Risk prediction of diabetes: Big data mining with fusion of multifarious physical examination indicators. Inf. Fusion 2021, 75, 140–149. [Google Scholar] [CrossRef]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine learning and data mining methods in diabetes research. Comput. Struct. Biotechnol. J. 2017, 15, 104–6116. [Google Scholar] [CrossRef]
- Abhari, S.; Kalhori, S.R.N.; Ebrahimi, M.; Hasannejadasl, H.; Garavand, A. Artificial intelligence applications in type 2 diabetes mellitus care: Focus on machine learning methods. Healthc. Inform. Res. 2019, 25, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, M.O.; Ogunsakin, R.E.; Ghai, M.; Adeleke, M.A. Accuracy of machine learning classification models for the prediction of type 2 diabetes mellitus: A systematic survey and meta-analysis approach. Int. J. Environ. Res. Public Health 2022, 19, 14280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Garg, S.; Garg, A. Assessment of anxiety, depression and stress using machine learning models. Procedia Comput. Sci. 2020, 171, 1989–1998. [Google Scholar] [CrossRef]
- Nemesure, M.D.; Heinz, M.V.; Huang, R.; Jacobson, N.C. Predictive modeling of depression and anxiety using electronic health records and a novel machine learning approach with artificial intelligence. Sci. Rep. 2021, 11, 1980. [Google Scholar] [CrossRef]
- Chu, H.; Chen, L.; Yang, X.; Qiu, X.; Qiao, Z.; Song, X.; Zhao, E.; Zhou, J.; Zhang, W.; Mehmood, A.; et al. Roles of anxiety and depression in predicting cardiovascular disease among patients with type 2 diabetes mellitus: A machine learning approach. Front. Psychol. 2021, 12, 645418. [Google Scholar] [CrossRef]
- Khalil, R.M.; Al-Jumaily, A. Machine learning based prediction of depression among type 2 diabetic patients. In Proceedings of the 2017 12th International Conference on Intelligent Systems and Knowledge Engineering, Nanjing, China, 24–26 November 2017. [Google Scholar]
- Rees, G.; Xie, J.; Fenwick, E.K.; Sturrock, B.A.; Finger, R.; Rogers, S.L.; Lim, L.; Lamoureux, E.L. Association between diabetes-related eye complications and symptoms of anxiety and depression. JAMA Ophthalmol. 2016, 134, 1007–1014. [Google Scholar] [CrossRef]
- Ducat, L.; Philipson, L.H.; Anderson, B.J. The mental health comorbidities of diabetes. JAMA 2014, 312, 691–692. [Google Scholar] [CrossRef]
- Grigsby, A.B.; Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. Prevalence of anxiety in adults with diabetes: A systematic review. J. Psychosom. Res. 2002, 53, 1053–1060. [Google Scholar] [CrossRef]
- Nouwen, A.; Adriaanse, M.C.; van Dam, K.; Iversen, M.M.; Viechtbauer, W.; Peyrot, M.; Caramlau, I.; Kokoszka, A.; Kanc, K.; de Groot, M.; et al. Longitudinal associations between depression and diabetes complications: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1562–1572. [Google Scholar] [CrossRef]
- Smith, K.J.; Deschênes, S.S.; Schmitz, N. Investigating the longitudinal association between diabetes and anxiety: A systematic review and meta-analysis. Diabet. Med. 2018, 35, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh-Khiabani, F.; Ramezankhani, A.; Azizi, F.; Hadaegh, F.; Steyerberg, E.W.; Khalili, D. A tutorial on variable selection for clinical prediction models: Feature selection methods in data mining could improve the results. J. Clin. Epidemiol. 2016, 71, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modeling. Fam. Med. Community Health 2020, 8, 4. [Google Scholar] [CrossRef]
- Lin, K.D.; Chang, L.H.; Wu, Y.R.; Hsu, W.H.; Kuo, C.H.; Tsai, J.R.; Yu, M.L.; Su, W.S.; Lin, I.M. Association of depression and parasympathetic activation with glycemic control in type 2 diabetes mellitus. J. Diabetes Complicat. 2022, 36, 108264. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Williams, J.B.; Kroenke, K.; Hornyak, R.; McMurray, J. Validity and utility of the PRIME-MD Patient Health Questionnaire in assessment of 3000 obstetric-gynecologic patients: The PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am. J. Obstet. Gynecol. 2000, 183, 759–769. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- King, M.W.; Resick, P.A. Data mining in psychological treatment research: A primer on classification and regression trees. J. Consult. Clin. Psychol. 2014, 82, 895. [Google Scholar] [CrossRef]
- Lemon, S.C.; Roy, J.; Clark, M.A.; Friedman, P.D.; Rakowski, W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann. Behav. Med. 2003, 26, 172–181. [Google Scholar] [CrossRef]
- Richardson, B.; Fuller-Tyszkiewicz, M.; O’Donnell, R.; Ling, M.; Staiger, P.K. Regression tree analysis of ecological momentary assessment data. Health Psychol. Rev. 2017, 11, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.C.; Hsu, C.C.; Chen, C.Y.; Chuang, S.C.; Cheng, C.W.; Hsieh, W.S.; Wu, M.S.; Liu, Y.T.; Liu, Y.H.; Tsai, T.L.; et al. Paradoxical relationship between glycated hemoglobin and longitudinal change in physical functioning in older adults: A prospective cohort study. J. Gerontol. A Biol. Sci. 2019, 74, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Japkowicz, N.; Stephen, S. The class imbalance problem: A systematic study. Intell. Data Anal. 2002, 6, 429–449. [Google Scholar] [CrossRef]
- Olsen, L.R. Groupdata2: Creating Groups from Data. 2019. Available online: https://cran.r-project.org/package=groupdata2 (accessed on 20 October 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://www.R-project.org/ (accessed on 20 October 2022).
- Therneau, T.; Atkinson, B.; Ripley, B.; Ripley, M.B. Package ‘Rpart’. Available online: https://cran.r-project.org/web/packages/rpart/rpart.pdf (accessed on 20 April 2016).
- JASP Team. JASP [Computer Software], Version 0.17.1; JASP Team: Washington, DC, USA, 2023.
- Darwish, L.; Beroncal, E.; Sison, M.V.; Swardfager, W. Depression in people with type 2 diabetes: Current perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, M.; Haghighatdoost, F.; Feizi, A.; Aminorroaya, A. The prevalence of comorbid depression in patients with type 2 diabetes: An updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019, 56, 631–650. [Google Scholar] [CrossRef]
- Rothenbacher, D.; Rüter, G.; Saam, S.; Brenner, H. Younger patients with type 2 diabetes need better glycaemic control: Results of a community-based study describing factors associated with a high HbA1c value. Br. J. Gen. Pract. 2003, 53, 389–391. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1314599/ (accessed on 20 October 2022).
- Raghavendra, S.; Santosh, K.J. Performance evaluation of random forest with feature selection methods in prediction of diabetes. Int. J. Electr. Comput. Eng. 2020, 10, 353–359. [Google Scholar] [CrossRef]
| Model | Testing Data | |||
|---|---|---|---|---|
| % | Sensitivity | Specificity | Area under Curves | Accuracy Rate |
| Boosting | 51% | 63% | 63% | 57% |
| Support vector machine | 52% | 65% | 58% | 58% |
| Classification tree | 59% | 80% | 69% | 69% |
| Neural network | 69% | 80% | 60% | 74% |
| K-nearest neighbors | 63% | 93% | 78% | 78% |
| Random forest | 77% | 91% | 95% | 84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-L.; Wu, Y.-R.; Lin, K.-D.; Lin, C.-H.R.; Lin, I.-M. Using Machine Learning for the Risk Factors Classification of Glycemic Control in Type 2 Diabetes Mellitus. Healthcare 2023, 11, 1141. https://doi.org/10.3390/healthcare11081141
Cheng Y-L, Wu Y-R, Lin K-D, Lin C-HR, Lin I-M. Using Machine Learning for the Risk Factors Classification of Glycemic Control in Type 2 Diabetes Mellitus. Healthcare. 2023; 11(8):1141. https://doi.org/10.3390/healthcare11081141
Chicago/Turabian StyleCheng, Yi-Ling, Ying-Ru Wu, Kun-Der Lin, Chun-Hung Richard Lin, and I-Mei Lin. 2023. "Using Machine Learning for the Risk Factors Classification of Glycemic Control in Type 2 Diabetes Mellitus" Healthcare 11, no. 8: 1141. https://doi.org/10.3390/healthcare11081141
APA StyleCheng, Y.-L., Wu, Y.-R., Lin, K.-D., Lin, C.-H. R., & Lin, I.-M. (2023). Using Machine Learning for the Risk Factors Classification of Glycemic Control in Type 2 Diabetes Mellitus. Healthcare, 11(8), 1141. https://doi.org/10.3390/healthcare11081141








