The Impact of Opioid Prescribing Limits on Drug Usage in South Carolina: A Novel Geospatial and Time Series Data Analysis
Abstract
1. Introduction
2. Methodology
2.1. Research Design
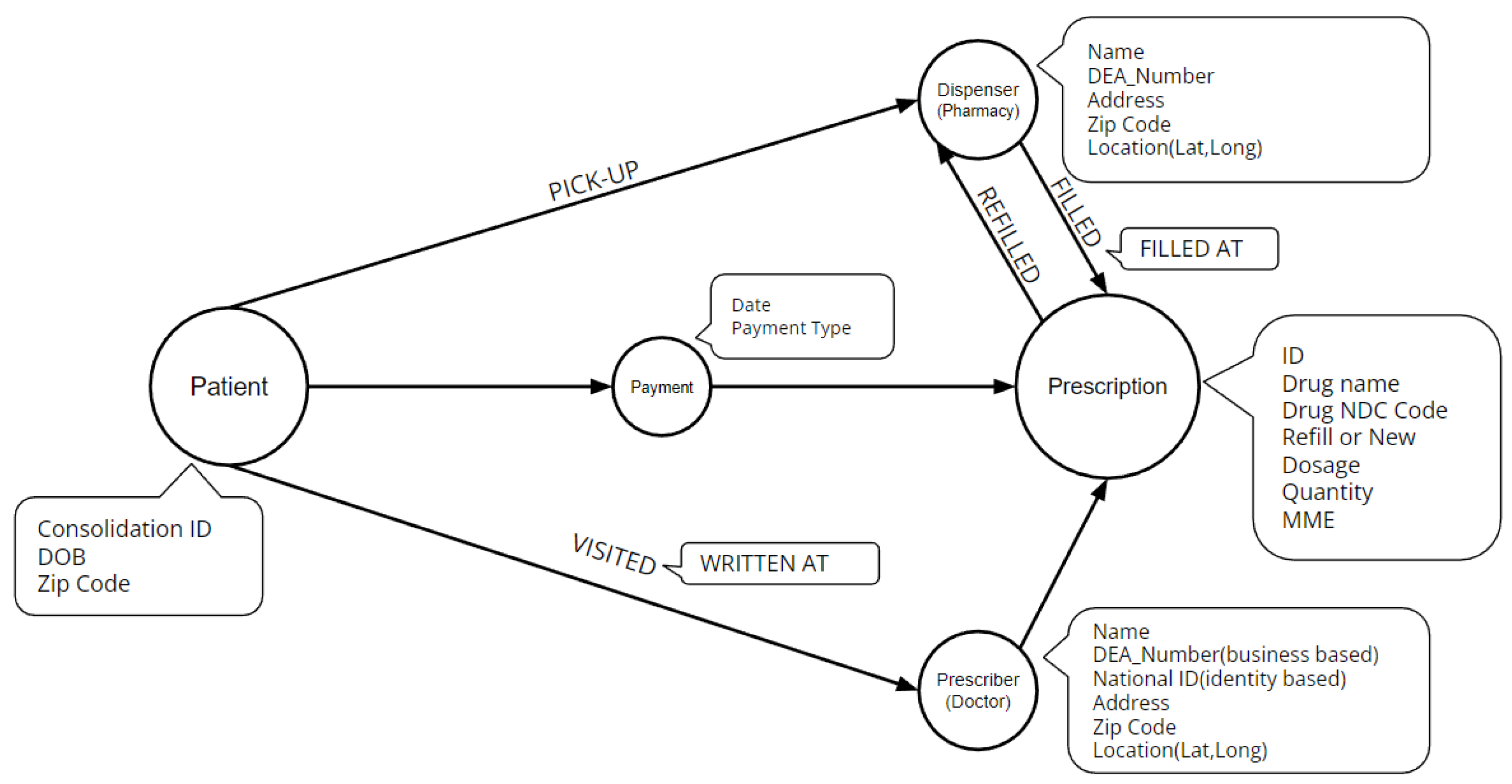
2.2. Data Description
- Drug Information
- –
- National Drug Code (NDC): which is a unique identifier given by the FDA to each medication sold in the United States. It is used to identify and track drugs through the supply chain from manufacturer to pharmacy. The NDC is a 11-digit number composed of three segments, which include the product code, package code, and labeler code. These segments respectively identify the active ingredients and strength of the medication, the package size and type, and the manufacturer or distributor of the medication.
- –
- Drug Name: which is used to classify each drug further. For the purpose of our study, we group drugs into benzodiazepine (16% share) and opioids (84% share). We also classify opioid drugs further into fentanyl (2% share), hydrocodone (50% share), hydromorphone (1% share), methadone (1% share), morphine (4% share), oxycodone (25% share), oxymorphone (less than 1% share), and tapentadol (1% share).
- –
- Drug Volume: This is quantified by the quantity of drugs and by using the Morphine Milligram Equivalent (MME) to standardize the measurement of opioid dosage across different medications. MME is a unit of measurement that expresses the total daily dose of opioids in terms of an equivalent dose of morphine. It is used to assess the risk of overdose and other adverse events, to monitor opioid prescribing practices, and to track trends in opioid use over time. The MME is calculated by converting the total daily dosage of each opioid to an equivalent dose of morphine using conversion factors determined by the FDA based on the relative potencies of the different opioids.
- Prescription Information
- –
- Days Supply: which refers to the number of days that a prescription for a specific medication will last, assuming average usage, which is calculated by dividing the total quantity of the medication prescribed by the average daily dosage. This measure is important in medication management, ensuring patients have enough medication and preventing overuse or misuse.
- –
- Refill Status: which refers to whether a prescription is for a new medication or a refill of a previously prescribed medication. A new medication prescription is written when a patient starts a new medication for the first time, while a refill prescription is a request to renew a previously filled prescription. It’s important to know the new or refill status because it can affect the dispensing process, patient’s insurance coverage and prescription regulations. Among all records 87% of them are new and only 13% are refilled.
- –
- Payment Type: which refers to the source of funds used to pay for the medication, the most common types being private insurance (36% share), Medicare (16% share), Medicaid (4% share) and other types with less than 2% share. It’s noteworthy to mention that 34% of the records have no available payment method.
- –
- Date: Each prescription has two important dates: the date the prescription was written by the prescriber, and the date the prescription was filled at the pharmacy.
- –
- Authorized Refill Count: which refers to the number of times a prescription can be refilled as authorized by the prescribing healthcare provider. It is specified on the prescription and can be used by pharmacies to track remaining refills.
- Prescriber Information
- –
- Location: which includes full address of the prescriber.
- –
- ID: For healthcare providers, there are two types of identification numbers: National Provider Identifier (NPI) and Drug Enforcement Administration (DEA) number. NPI is assigned by the Centers for Medicare and Medicaid Services (CMS) to identify healthcare providers in transactions such as submitting claims, ordering or prescribing medication, or referring patients to other providers. DEA number is assigned by the DEA to track and regulate the prescribing and dispensing of controlled substances, it is a requirement for healthcare providers to have a DEA number in order to prescribe controlled substances. In summary, NPI is used for administrative purposes and DEA number is used for regulatory purposes.
- Dispenser Information
- –
- Location: which includes full address of the dispenser.
- –
- ID: Dispensers have only one ID which is their DEA number.
- Patient Information
- –
- Consolidation ID: Pre-assigned id in order to identify each patient.
- –
- Age.
- –
- Location: Which includes patient’s zip code.
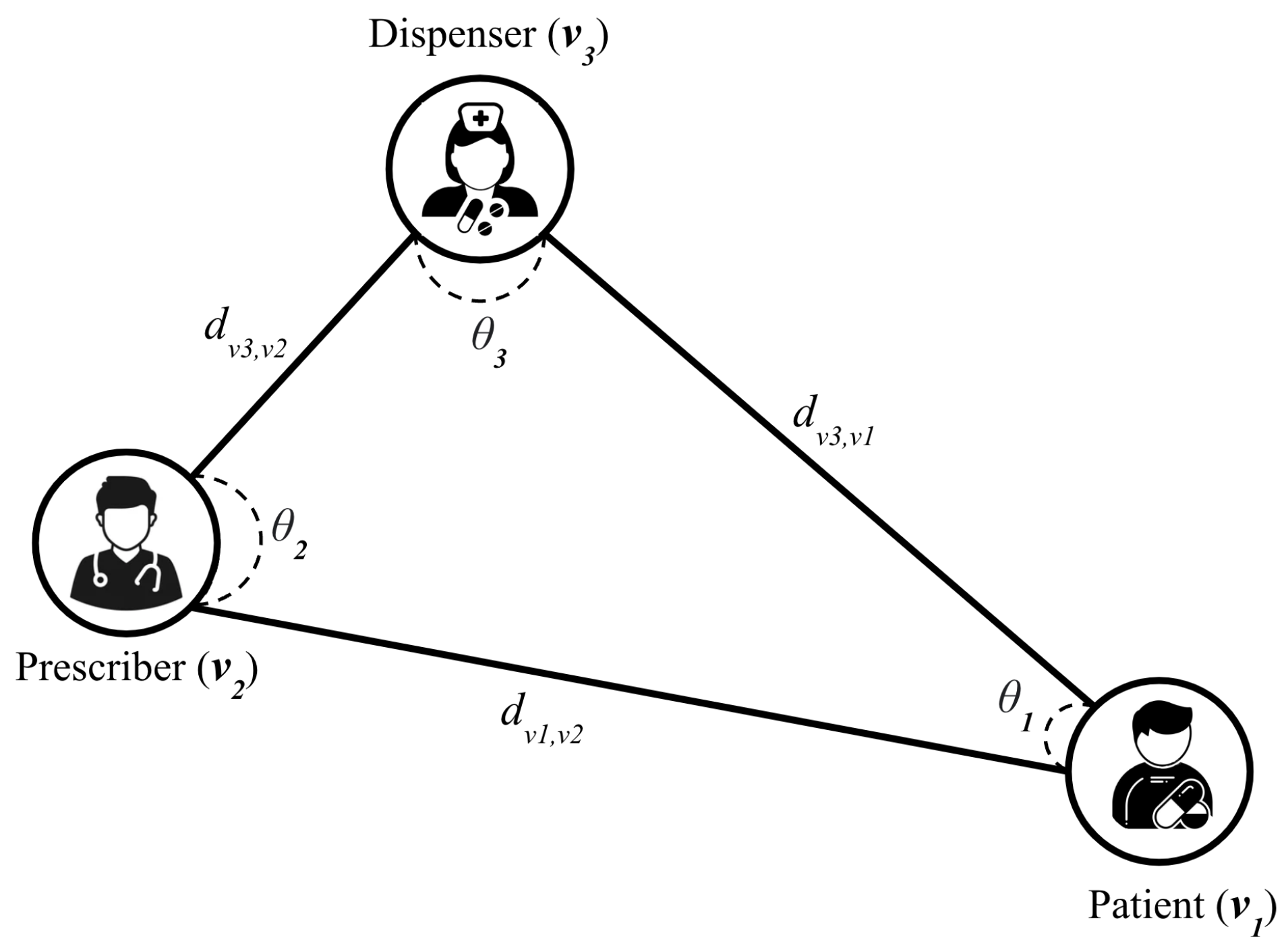
2.3. Classification System
2.3.1. Description
2.3.2. Graph Model Definition
2.3.3. Classification Algorithm
2.4. Statistical Analysis
2.4.1. Dependent Variable
| Algorithm 1 Two-digit coding classifier |
|
2.4.2. Independent Variables
2.4.3. One Way ANOVA
2.4.4. Interrupted Time Series Analysis
- An ARIMA model was fitted for each time series under study, specifically for the pre-intervention period. The pre-intervention period is considered the period before the policy enactment in May 2018.
- An autoregressive integrated moving average with exogenous variables (ARIMAX) model was estimated for the entire time series by using the best ARIMA orders found in the previous step, adding regressor variables corresponding to the announcement and implementation of the legislation.
- All previous steps were repeated for both subgroups proposed by the classification system and for the aggregated data without any subgroup.
3. Results
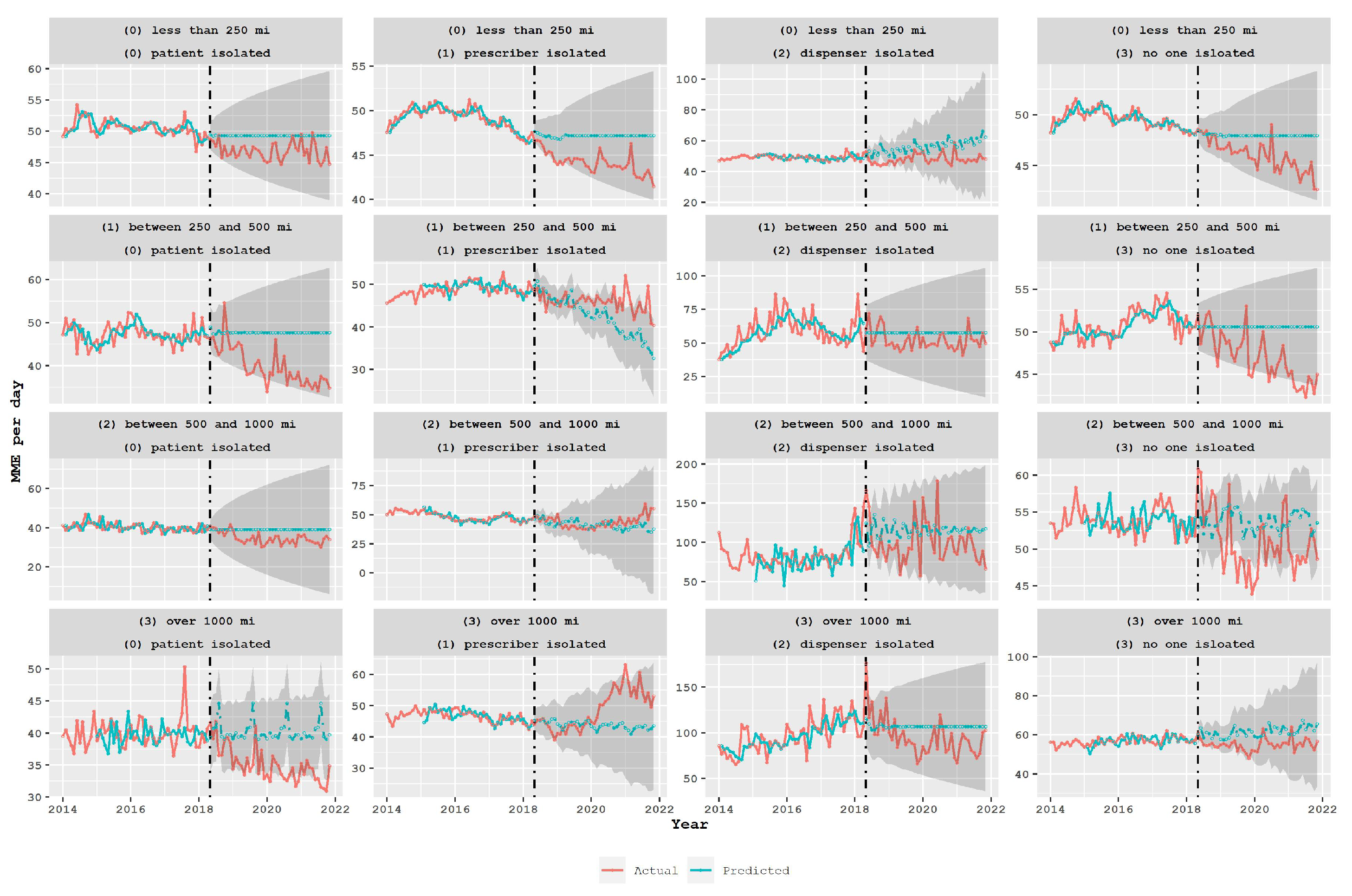
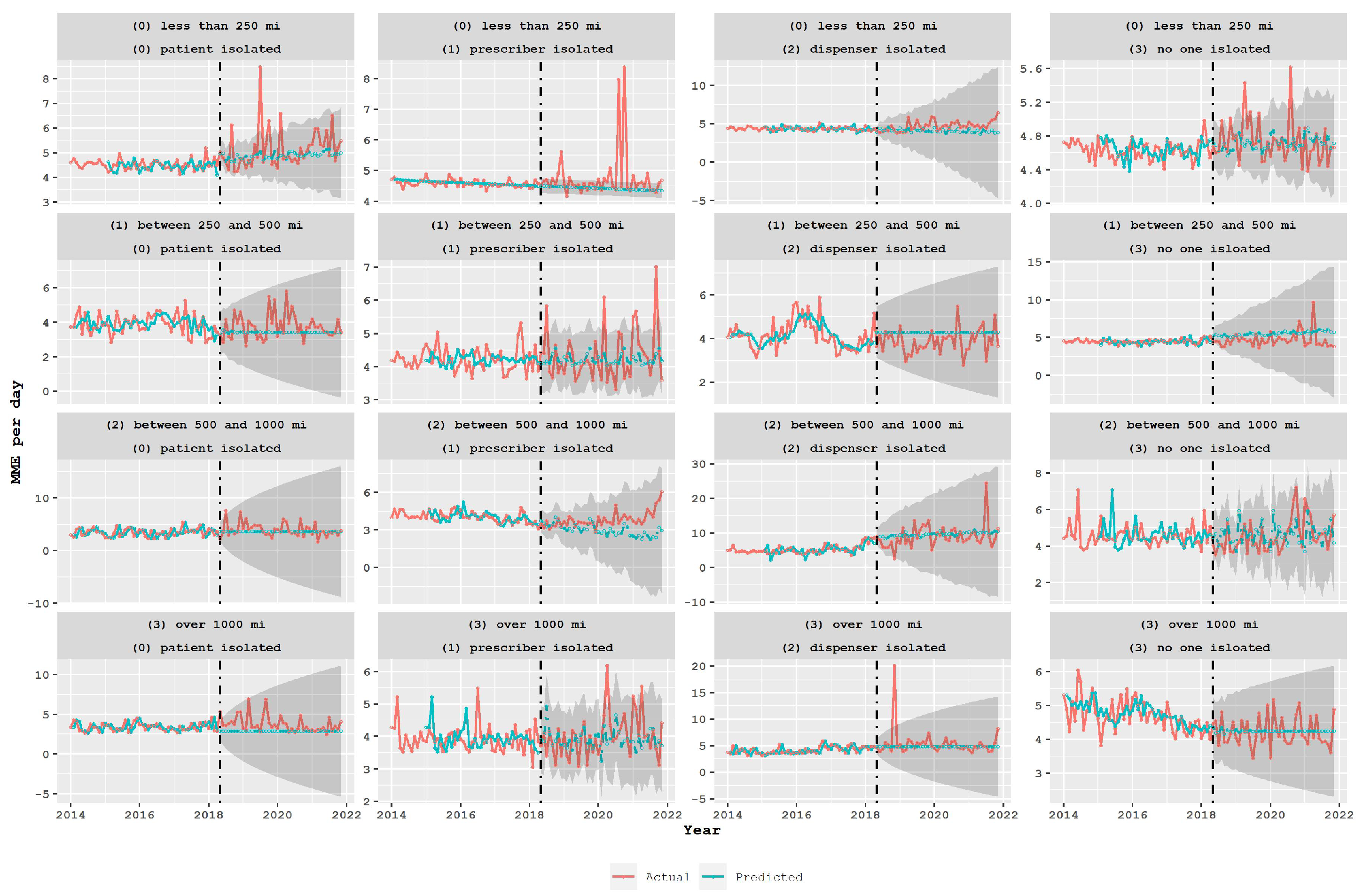
3.1. Opioid Usage in the Different Groups in the Spatial Classification
- Chain pharmacies are part of a larger corporation that operates multiple pharmacy locations. They offer a wide range of prescription and over-the-counter medications, health and wellness products, and services such as immunizations and health screenings.
- Retail pharmacies are typically located within supermarkets, department stores, or other retail establishments. They offer a selection of medications, health and wellness products, and over-the-counter medications.
- Mail-order pharmacies deliver medications directly to patients’ homes via mail or courier services. They are a convenient option for patients with chronic conditions who require regular medication refills.
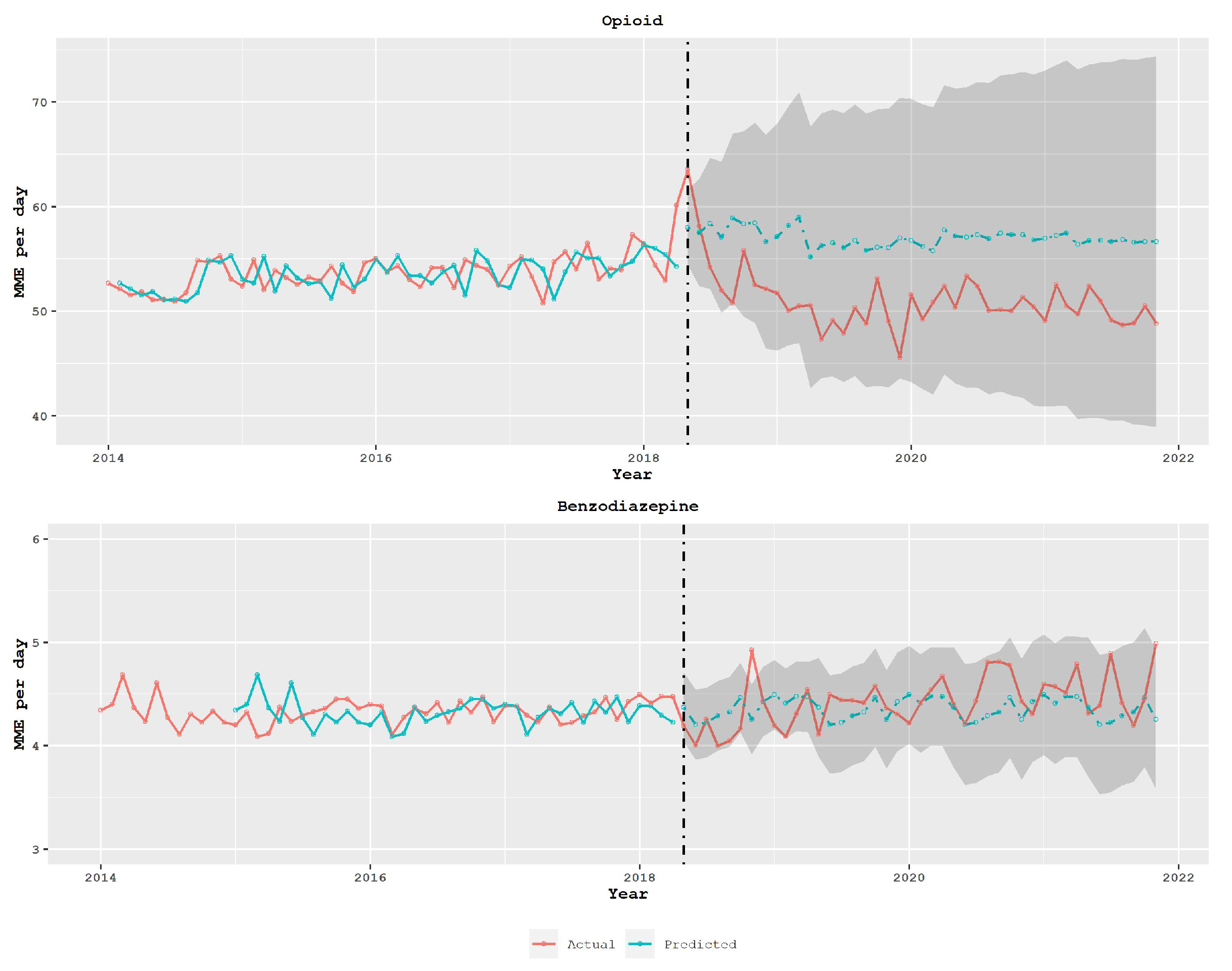
3.2. Policy Analysis Using Time Series Models
4. Discussion
- To avoid potential unintended negative outcomes such as patients seeking opioids from distant pharmacies or turning to harmful illicit drugs, policymakers should carefully assess the impact of opioid-limiting legislation. This requires evaluating the effectiveness of policies in achieving their intended goals, while also considering their impact on different groups of patients, prescribers, and dispensers.
- In particular, policymakers should examine the patterns of opioid consumption among different groups and explore the reasons for higher consumption levels among certain groups. Additionally, policymakers should take into account the characteristics of different types of pharmacies, such as independent retail or mail-order pharmacies, that may have a higher likelihood of being linked to drug diversion.
- Furthermore, policymakers should consider the impact of prescription limit policies on other potentially harmful drugs, such as benzodiazepines, and evaluate the effectiveness of the policy in preventing overdoses. This evaluation should also take into account the impact of the policy on different types of transactions, particularly those that occur across state borders.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Ciccarone, D. The triple wave epidemic: Supply and demand drivers of the US opioid overdose crisis. Int. J. Drug Policy 2019, 71, 183. [Google Scholar] [CrossRef] [PubMed]
- Babor, T.F.; Caulkins, J.P.; Edwards, G.; Fischer, B.; Foxcroft, D.R.; Humphreys, K.; Obot, I.S.; Rehm, J.; Reuter, P. Drug Policy and the Public Good; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Hedegaard, H.; Miniño, A.M.; Spencer, M.R.; Warner, M. Drug Overdose Deaths in the United States, 1999–2020; NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Ahmad, F.B.; Cisewski, J.A.; Anderson, R.N. Provisional Drug Overdose Death Counts; National Center for Health Statistics: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm (accessed on 7 March 2023).
- Mattson, C.L.; Tanz, L.J.; Quinn, K.; Kariisa, M.; Patel, P.; Davis, N.L. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. Morb. Mortal. Wkly. Rep. 2021, 70, 202. [Google Scholar] [CrossRef]
- Parker, A.M.; Strunk, D.; Fiellin, D.A. State responses to the opioid crisis. J. Law, Med. Ethics 2018, 46, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.S.; Guille, C.; McCauley, J.; Brady, K.T. Targeting practitioners: A review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017, 173, S22–S30. [Google Scholar] [CrossRef]
- Davis, C.S.; Carr, D. Physician continuing education to reduce opioid misuse, abuse, and overdose: Many opportunities, few requirements. Drug Alcohol Depend. 2016, 163, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Lieberman, A.J.; Hernandez-Delgado, H.; Suba, C. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug Alcohol Depend. 2019, 194, 166–172. [Google Scholar] [CrossRef]
- Jackson, J.R.; Harle, C.A.; Silverman, R.D.; Simon, K.; Menachemi, N. Characterizing variability in state-level regulations governing opioid treatment programs. J. Subst. Abus. Treat. 2020, 115, 108008. [Google Scholar] [CrossRef]
- Louca, C.; Michaely, R.; Petmezas, D. Spillover Effects of the Opioid Epidemic on Labor and Innovation; SSRN: Rochester, NY, USA, 2022. [Google Scholar]
- Karimian, S.; Rahimi, B. A systematic review of effects of exchanging and sharing medical images systems in a sociotechnical context: Evaluation perspectives. Informatics Med. Unlocked 2023, 38, 101212. [Google Scholar] [CrossRef]
- Lee, B.; Zhao, W.; Yang, K.C.; Ahn, Y.Y.; Perry, B.L. Systematic evaluation of state policy interventions targeting the US opioid epidemic, 2007–2018. JAMA Netw. Open 2021, 4, e2036687. [Google Scholar] [CrossRef]
- Buonora, M.J.; Hanna, D.B.; Zhang, C.; Bachhuber, M.A.; Moir, L.H.; Salvi, P.S.; Cunningham, C.O.; Starrels, J.L. US state policies on opioid prescribing during the peak of the prescription opioid crisis: Associations with opioid overdose mortality. Int. J. Drug Policy 2022, 110, 103888. [Google Scholar] [CrossRef]
- Mallatt, J. The Effect of Prescription Drug Monitoring Programs on Opioid Prescriptions and Heroin Crime Rates; SSRN: Rochester, NY, USA, 2018. [Google Scholar]
- Nam, Y.H.; Shea, D.G.; Shi, Y.; Moran, J.R. State prescription drug monitoring programs and fatal drug overdoses. Am. J. Manag. Care 2017, 23, 297–303. [Google Scholar] [PubMed]
- Sedney, C.L.; Khodaverdi, M.; Pollini, R.; Dekeseredy, P.; Wood, N.; Haggerty, T. Assessing the impact of a restrictive opioid prescribing law in West Virginia. Subst. Abus. Treat. Prev. Policy 2021, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maierhofer, C.N.; Ranapurwala, S.I.; DiPrete, B.L.; Fulcher, N.; Ringwalt, C.L.; Chelminski, P.R.; Ives, T.J.; Dasgupta, N.; Go, V.F.; Pence, B.W. Intended and unintended consequences: Changes in opioid prescribing practices for postsurgical, acute, and chronic pain indications following two policies in North Carolina, 2012–2018–Controlled and single-series interrupted time series analyses. Drug Alcohol Depend. 2023, 242, 109727. [Google Scholar] [CrossRef] [PubMed]
- Delcher, C.; Wagenaar, A.C.; Goldberger, B.A.; Cook, R.L.; Maldonado-Molina, M.M. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s Prescription Drug Monitoring Program. Drug Alcohol Depend. 2015, 150, 63–68. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Fife, D.; Berwaerts, J.; Friedman, A.; Yuan, Y.; Mastrogiovanni, G. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: Shoppers often pay in cash and cross state lines. Am. J. Drug Alcohol Abuse 2015, 41, 226–229. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Fife, D.; Yuan, Y.; Mastrogiovanni, G. Distance traveled and frequency of interstate opioid dispensing in opioid shoppers and nonshoppers. J. Pain 2013, 14, 1158–1161. [Google Scholar] [CrossRef]
- Sahebi-Fakhrabad, A.; Sadeghi, A.H.; Handfield, R. Evaluating State-Level Prescription Drug Monitoring Program (PDMP) and Pill Mill Effects on Opioid Consumption in Pharmaceutical Supply Chain. Healthcare 2023, 11, 437. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Jeffery, M.M.; Hooten, W.M.; Jena, A.B.; Ross, J.S.; Shah, N.D.; Karaca-Mandic, P. Rates of physician coprescribing of opioids and benzodiazepines after the release of the centers for disease control and prevention guidelines in 2016. JAMA Netw. Open 2019, 2, e198325. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2009; Volume 2. [Google Scholar]
- Fougère, D.; Jacquemet, N. Policy evaluation using causal inference methods. In Handbook of Research Methods and Applications in Empirical Microeconomics; Edward Elgar Publishing: Cheltenham, UK, 2021; pp. 294–324. [Google Scholar]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.L.; Dobbins, T.A.; Pearson, S.A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: A guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 2021, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.L.; Karahalios, A.; Forbes, A.B.; Taljaard, M.; Grimshaw, J.M.; McKenzie, J.E. Comparison of six statistical methods for interrupted time series studies: Empirical evaluation of 190 published series. BMC Med. Res. Methodol. 2021, 21, 134. [Google Scholar] [CrossRef]
- Branham, D.K. Time-series analysis of the impact of prescription drug monitoring programs on heroin treatment admissions. Subst. Use Misuse 2018, 53, 694–701. [Google Scholar] [CrossRef]
- Nguyen, T.; Meille, G.; Buchmueller, T. Mandatory prescription drug monitoring programs and overlapping prescriptions of opioids and benzodiazepines: Evidence from Kentucky. Drug Alcohol Depend. 2022, 243, 109759. [Google Scholar] [CrossRef] [PubMed]
- SAS® Visual Forecasting: Time Series Packages; SAS Institute Inc.: Cary, NC, USA, 2023.
- SAS/ETS® User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2021.
- Bicket, M.C.; Long, J.J.; Pronovost, P.J.; Alexander, G.C.; Wu, C.L. Prescription opioid analgesics commonly unused after surgery: A systematic review. JAMA Surg. 2017, 152, 1066–1071. [Google Scholar] [CrossRef]
- Donnelly, S.; Townsend, C.; Thomson, L. Prescription Drug Monitoring Program: South Carolina State Profile. 2021. Available online: https://scdhec.gov/sites/default/files/media/document/2021-pmp-annual_final_version_1.pdf (accessed on 10 February 2023).
- Maclean, J.C.; Mallatt, J.; Ruhm, C.J.; Simon, K. Economic Studies on the Opioid Crisis: A Review; National Bureau of Economic Research: Cambridge, MA, USA, 2020. [Google Scholar]
- Sansone, R.A.; Sansone, L.A. Doctor shopping: A phenomenon of many themes. Innov. Clin. Neurosci. 2012, 9, 42. [Google Scholar]
- Adan, A.; Torrens, M. Diagnosis and management of addiction and other mental disorders (Dual Disorders). J. Clin. Med. 2021, 10, 1307. [Google Scholar] [CrossRef]
- Gholami, M.H.; Abbaspour, A.; Tajik, F.; Zabolian, A.; Ma, Z. Long non-coding RNAs (lncRNAs) in cancer therapy response: Biological aspects. World Sci. 2023, 157–187. [Google Scholar]
- Mendoza, R.L. Continuity and change in the drug supply chain: Actors, actions, and aversions. J. Med. Econ. 2021, 24, 689–697. [Google Scholar] [CrossRef] [PubMed]
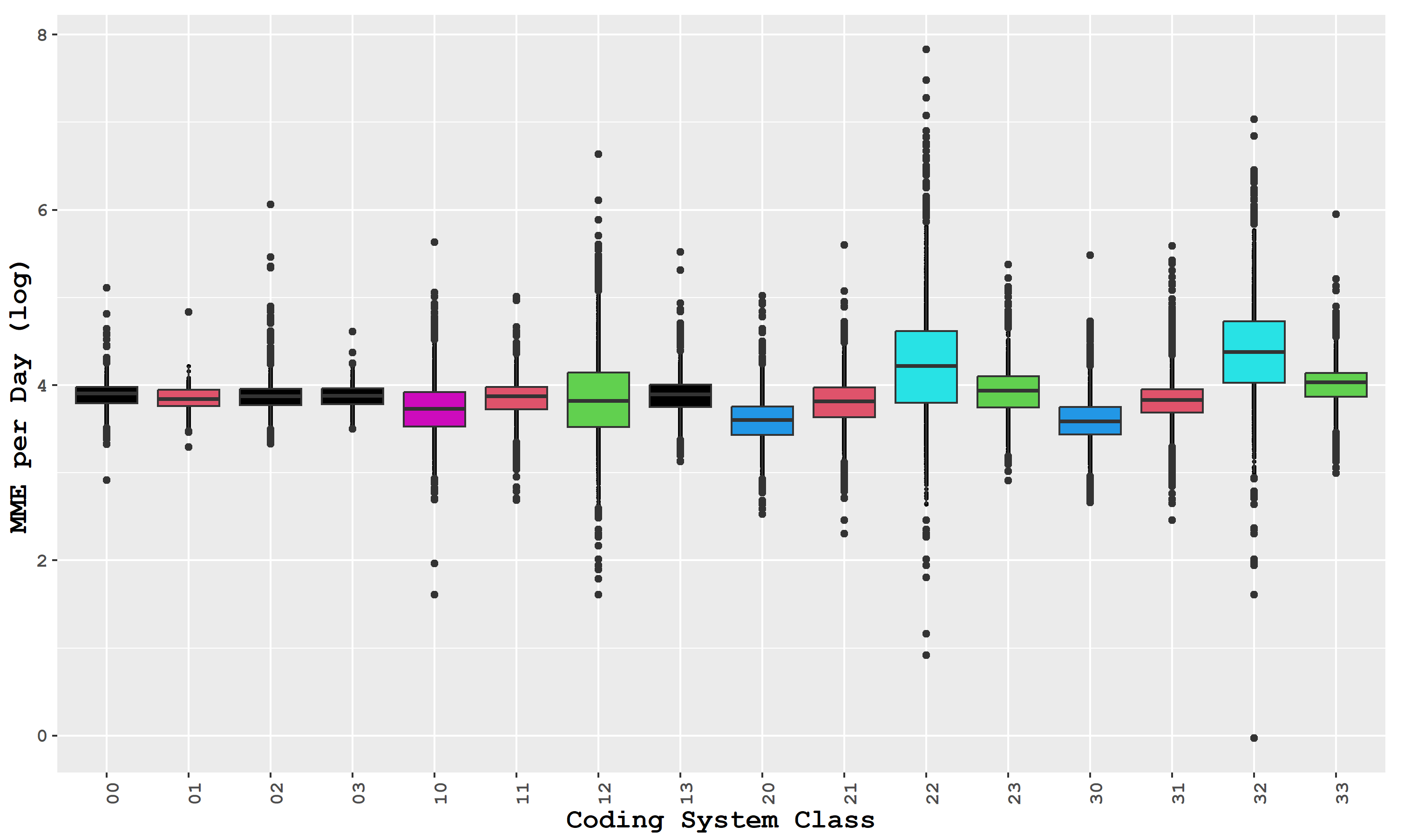

| Distance (Miles) | Disparity | Code | Distance (Miles) | Disparity | Code |
|---|---|---|---|---|---|
| Patient isolated | 00 | Patient isolated | 20 | ||
| Prescriber isolated | 01 | Prescriber isolated | 21 | ||
| Dispenser isolated | 02 | Dispenser isolated | 22 | ||
| Otherwise | 03 | Otherwise | 23 | ||
| Patient isolated | 10 | Patient isolated | 30 | ||
| Prescriber isolated | 11 | Prescriber isolated | 31 | ||
| Dispenser isolated | 12 | Dispenser isolated | 32 | ||
| Otherwise | 13 | Otherwise | 33 |
| Group Code | Mean (SD) Days Supply | Mean (SD) MME | MME per Day (95% CI) | Percentage of MME |
|---|---|---|---|---|
| 00 | 14.89 (4.87) | 802.32 (310.46) | 48.88 (48.61, 49.15) | 12.05 |
| 01 | 15.05 (5.09) | 771.95 (292.38) | 46.90 (46.70, 47.10) | 35.76 |
| 02 | 16.04 (4.91) | 809.49 (304.97) | 48.66 (48.20, 49.12) | 3.87 |
| 03 | 15.4 (5.15) | 800.95 (302.02) | 47.96 (47.75, 48.16) | 40.65 |
| 10 | 8.84 (3.74) | 478.36 (362.17) | 44.00 (43.43, 44.57) | 0.19 |
| 11 | 14.89 (5.95) | 815.39 (395.73) | 47.51 (47.15, 47.87) | 1.29 |
| 12 | 19.37 (7.62) | 1064.53 (994.58) | 55.26 (53.66, 56.86) | 0.08 |
| 13 | 14.06 (5.20) | 770.80 (357.95) | 49.08 (48.68, 49.47) | 1.66 |
| 20 | 7.56 (3.38) | 309.30 (219.66) | 37.84 (37.44, 38.24) | 0.16 |
| 21 | 13.42 (6.25) | 704.96 (411.89) | 46.40 (45.93, 46.93) | 0.80 |
| 22 | 18.93 (6.97) | 1110.24 (746.99) | 91.71 (87.20, 96.22) | 0.07 |
| 23 | 12.69 (5.09) | 721.36 (384.68) | 52.46 (51.91, 53.01) | 1.17 |
| 30 | 7.66 (3.15) | 322.93 (230.59) | 37.78 (37.38, 38.18) | 0.19 |
| 31 | 12.69 (5.03) | 723.15 (1336.44) | 47.50 (46.93, 48.07) | 1.22 |
| 32 | 20.06 (7.51) | 1374.09 (1153.52) | 96.50 (93.43, 99.57) | 0.14 |
| 33 | 12.81 (4.67) | 761.86 (375.38) | 56.00 (55.46, 56.54) | 1.87 |
| Distance Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code 0X: | Code 1X: | Code 2X: | Code 3X: | ||||||
| Mean | Mean | Mean | Mean | ||||||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||
| Pre-Policy | Post-Policy | Pre-Policy | Post-Policy | Pre-Policy | Post-Policy | Pre-Policy | Post-Policy | ||
| Disparity Level | Code X0: Patient isolated | 50.57 | 46.84 | 47.38 | 39.93 | 40.41 | 34.74 | 40.01 | 35.10 |
| (50.25, 50.89) | (46.42, 47.26) | (46.58, 48.18) | (39.18, 40.68) | (39.95, 40.87) | (34.11, 35.37) | (39.45, 40.57) | (34.55, 35.65) | ||
| Code X1: Prescriber isolated | 49.18 | 44.15 | 48.78 | 45.98 | 48.56 | 43.81 | 46.69 | 48.49 | |
| (48.91, 49.45) | (43.91, 44.39) | (48.38, 49.18) | (45.33, 46.63) | (48.03, 49.09) | (42.91, 44.71) | (46.35, 47.03) | (47.31, 49.67) | ||
| Code X2: Dispenser isolated | 49.14 | 48.08 | 58.43 | 51.92 | 85.07 | 99.76 | 97.10 | 95.78 | |
| (48.69, 49.59) | (47.21, 48.95) | (55.87, 60.99) | (50.06, 53.78) | (80.84, 89.30) | (91.22, 108.30) | (93.36, 100.84) | (90.70, 100.86) | ||
| Code X3: Otherwise | 49.57 | 46.01 | 50.78 | 47.02 | 53.77 | 50.89 | 56.81 | 55.02 | |
| (49.30, 49.84) | (45.73, 46.29) | (50.35, 51.21) | (46.34, 47.70) | (53.16, 54.38) | (49.93, 51.85) | (56.23, 57.39) | (54.05, 55.99) | ||
| Class | Model | Coefficient | Estimate | Standard Error | p Value | Significance Level |
|---|---|---|---|---|---|---|
| Overall | ARIMA (1,1,0) | AR (12) | (***) | |||
| Inverse Trend D(1) | (**) | |||||
| 11 | ARIMA (1,1,1) | Constant | (***) | |||
| AR (12) | (*) | |||||
| MA (1) | (***) | |||||
| Ramp | (**) | |||||
| Level Shift | (***) | |||||
| 31 | ARIMA (1,2,2) | Constant | (*) | |||
| MA (1) | (***) | |||||
| MA (12) | (***) | |||||
| Ramp | (***) | |||||
| Level Shift | (**) | |||||
| 03 | ARIMA (1,2,2) | MA (1) | (***) | |||
| MA (12) | (***) | |||||
| Level Shift | (**) | |||||
| 30 | ARIMA (1,2,1) | MA (12) | (***) | |||
| Level Shift | (***) | |||||
| 13 | ARIMA (1,1,1) | MA (1) | (***) | |||
| Level Shift | (***) | |||||
| 23 | ARIMA (2,1,0) | AR (1) | (***) | |||
| AR (12) | (***) | |||||
| Level Shift | (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahebi-Fakhrabad, A.; Sadeghi, A.H.; Kemahlioglu-Ziya, E.; Handfield, R.; Tohidi, H.; Vasheghani-Farahani, I. The Impact of Opioid Prescribing Limits on Drug Usage in South Carolina: A Novel Geospatial and Time Series Data Analysis. Healthcare 2023, 11, 1132. https://doi.org/10.3390/healthcare11081132
Sahebi-Fakhrabad A, Sadeghi AH, Kemahlioglu-Ziya E, Handfield R, Tohidi H, Vasheghani-Farahani I. The Impact of Opioid Prescribing Limits on Drug Usage in South Carolina: A Novel Geospatial and Time Series Data Analysis. Healthcare. 2023; 11(8):1132. https://doi.org/10.3390/healthcare11081132
Chicago/Turabian StyleSahebi-Fakhrabad, Amirreza, Amir Hossein Sadeghi, Eda Kemahlioglu-Ziya, Robert Handfield, Hossein Tohidi, and Iman Vasheghani-Farahani. 2023. "The Impact of Opioid Prescribing Limits on Drug Usage in South Carolina: A Novel Geospatial and Time Series Data Analysis" Healthcare 11, no. 8: 1132. https://doi.org/10.3390/healthcare11081132
APA StyleSahebi-Fakhrabad, A., Sadeghi, A. H., Kemahlioglu-Ziya, E., Handfield, R., Tohidi, H., & Vasheghani-Farahani, I. (2023). The Impact of Opioid Prescribing Limits on Drug Usage in South Carolina: A Novel Geospatial and Time Series Data Analysis. Healthcare, 11(8), 1132. https://doi.org/10.3390/healthcare11081132










