Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report
Abstract
1. Introduction
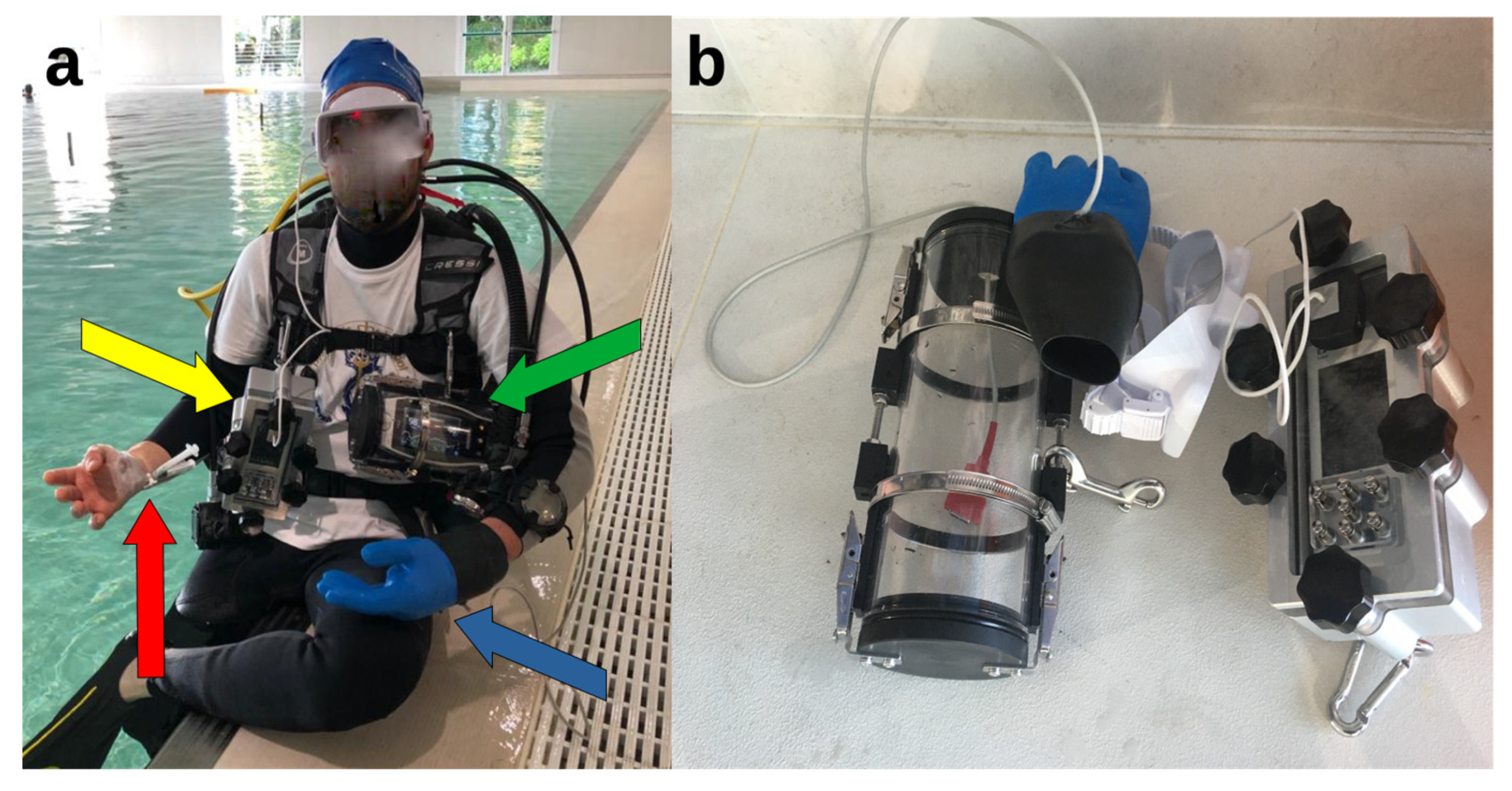
2. Materials and Methods
- At the surface, on the stretcher after the arterial cannula placement, with the diver breathing ambient air (dry conditions, rest);
- Underwater, at a depth of −15 m of free water (mfw), after 15 min spent on an underwater bicycle (OKEO, Genova, Italy) pedaling at a rate of 25 rpm to ensure no difference in ventilation and gas exchange in all dives, guided by the Borg category ratio 0–10 scale at an intensity of 3 [25], with the SCUBA diver breathing compressed air;
- Immediately after resurfacing from the dive, head out of the water, with the diver still breathing air from the SCUBA apparatus (Figure 2).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trout, B.M.; Caruso, J.L.; Nelson, C.; Denoble, P.J.; Nord, D.A.; Chimiak, J.; Martina, S.D.; Nochetto, M.; Pollock, N.W.; Lippmann, J.; et al. DAN Annual Diving Report 2012-2015 Edition: A Report on 2010-2013 Data on Diving Fatalities, Injuries, and Incidents; Buzzacott, P., Ed.; Divers Alert Network: Durham, NC, USA, 2015; ISBN 978-1-941027-52-3. [Google Scholar]
- Bosco, G.; Rizzato, A.; Moon, R.E.; Camporesi, E.M. Environmental Physiology and Diving Medicine. Front. Psychol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Rand, M.J. Nitrergic transmission: Nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin. Exp. Pharmacol. Physiol. 1992, 19, 147–169. [Google Scholar] [CrossRef]
- Balestra, C.; Theunissen, S.; Papadopoulou, V.; Le Mener, C.; Germonpré, P.; Guerrero, F.; Lafère, P. Pre-Dive Whole-Body Vibration Better Reduces Decompression-Induced Vascular Gas Emboli than Oxygenation or a Combination of Both. Front. Physiol. 2016, 7, 586. [Google Scholar] [CrossRef] [PubMed]
- Professional Association of Diving Instructors. PADI. Available online: https://www.padi.com/ (accessed on 7 March 2023).
- The Diving Equipment & Marketing Association. Available online: https://www.dema.org/ (accessed on 7 March 2023).
- Saunders. Bennett and Elliott’s Physiology and Medicine of Diving, 5th ed.; Brubakk, A.O., Bennett, P.B., Elliot, D.H., Elliott, D.H., Eds.; Saunders: London, UK, 2007; ISBN 978-0-7020-2571-6. [Google Scholar]
- Cialoni, D.; Pieri, M.; Balestra, C.; Marroni, A. Dive Risk Factors, Gas Bubble Formation, and Decompression Illness in Recreational SCUBA Diving: Analysis of DAN Europe DSL Data Base. Front. Psychol. 2017, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.J.; Willems, C.H.; van Amsterdam, K.; van den Berg, J.P.; Spanjersberg, R.; Struys, M.M.R.F.; Scheeren, T.W.L. Oxygen Reserve Index: Validation of a New Variable. Anesth. Analg. 2019, 129, 409–415. [Google Scholar] [CrossRef]
- Lance, R.M.; Natoli, M.J.; Di Pumpo, F.; Beck, T.P.; Gatrell, A.; Brown, G.J.; Schocken, D.; Moon, R.E. The Dewey Monitor: Pulse Oximetry Can Warn of Hypoxia in an Immersed Rebreather Diver in Multiple Scenarios. Ann. Biomed. Eng. 2022, 50, 222–232. [Google Scholar] [CrossRef]
- Lance, R.M.; Natoli, M.J.; Dunworth, S.A.S.; Freiberger, J.J.; Moon, R.E. The Dewey Monitor: Pulse Oximetry Can Independently Detect Hypoxia in a Rebreather Diver. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2017, 44, 569–580. [Google Scholar] [CrossRef]
- Di Pumpo, F.; Ruffino, G.; Malacarne, P. Pulse Oximeter to Detect Peripheral Oxygen Saturation in Underwater Rebreather ECCR Diver: A Preliminary Study. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc 2019, 46, 1. [Google Scholar]
- ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [CrossRef] [PubMed]
- Pourmand, A.; Robinson, C.; Dorwart, K.; O’Connell, F. Pre-Oxygenation: Implications in Emergency Airway Management. Am. J. Emerg. Med. 2017, 35, 1177–1183. [Google Scholar] [CrossRef]
- Chen, S.-T.; Min, S. Oxygen Reserve Index, a New Method of Monitoring Oxygenation Status: What Do We Need to Know? Chin. Med. J. (Engl.) 2020, 133, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Szmuk, P.; Steiner, J.W.; Olomu, P.N.; Ploski, R.P.; Sessler, D.I.; Ezri, T. Oxygen Reserve Index: A Novel Noninvasive Measure of Oxygen Reserve--A Pilot Study. Anesthesiology 2016, 124, 779–784. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Belda, F.J.; Perel, A. The Oxygen Reserve Index (ORI): A New Tool to Monitor Oxygen Therapy. J. Clin. Monit. Comput. 2018, 32, 379–389. [Google Scholar] [CrossRef]
- Lindholm, P.; Lundgren, C.E. The Physiology and Pathophysiology of Human Breath-Hold Diving. J. Appl. Physiol. 2009, 106, 284–292. [Google Scholar] [CrossRef]
- Scott, T.; van Waart, H.; Vrijdag, X.C.E.; Mullins, D.; Mesley, P.; Mitchell, S.J. Arterial Blood Gas Measurements during Deep Open-Water Breath-Hold Dives. J. Appl. Physiol. Bethesda Md. 1985 2021, 130, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Rizzato, A.; Martani, L.; Schiavo, S.; Talamonti, E.; Garetto, G.; Paganini, M.; Camporesi, E.M.; Moon, R.E. Arterial Blood Gas Analysis in Breath-Hold Divers at Depth. Front. Physiol. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Rizzato, A.; Martani, L.; Garetto, G.; Lion, J.; Camporesi, E.M.; Moon, R.E. Arterial Blood Gases in Divers at Surface after Prolonged Breath-Hold. Eur. J. Appl. Physiol. 2020, 120, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Linér, M.H.; Andersson, J.P.A. Pulmonary Edema after Competitive Breath-Hold Diving. J. Appl. Physiol. 2008, 104, 986–990. [Google Scholar] [CrossRef]
- Schagatay, E.; Lodin-Sundström, A.; Schagatay, F.; Engan, H. Can SaO2 Measurements during Recovery Be Used to Detect Lung Barotrauma in Freedivers? In Proceedings of the 41st Congress of the European Underwater & Baromedical Society (EUBS), Amsterdam, The Netherlands, 19–22 August 2015. [Google Scholar]
- Paganini, M.; Moon, R.E.; Boccalon, N.; Melloni, G.E.M.; Giacon, T.A.; Camporesi, E.M.; Bosco, G. Blood Gas Analyses in Hyperbaric and Underwater Environments: A Systematic Review. J. Appl. Physiol. 2022, 132, 283–293. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Vinetti, G.; Lopomo, N.F.; Taboni, A.; Fagoni, N.; Ferretti, G. The Current Use of Wearable Sensors to Enhance Safety and Performance in Breath-Hold Diving: A Systematic Review. Diving Hyperb. Med. J. 2020, 50, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Paoli, A.; Rizzato, A.; Marcolin, G.; Guagnano, M.T.; Doria, C.; Bhandari, S.; Pietrangelo, T.; Verratti, V. Body Composition and Endocrine Adaptations to High-Altitude Trekking in the Himalayas. Adv. Exp. Med. Biol. 2019, 1211, 61–68. [Google Scholar] [CrossRef]
- Verratti, V.; Bosco, G.; Zanon, V.; Pietrangelo, T.; Camporesi, E.; Bondi, D.; Pokorski, M. Pathophysiological Responses to a Record-Breaking Multi-Hour Underwater Endurance Performance: A Case Study. Adv. Exp. Med. Biol. 2020, 1289, 79–88. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers during 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Hyperbaric Oxygen: Its Mechanisms and Efficacy. Plast. Reconstr. Surg. 2011, 127, 131S–141S. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative Stress Assessment in Breath-Hold Diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, E.M.; Bosco, G. Mechanisms of Action of Hyperbaric Oxygen Therapy. Undersea Hyperb. Med. 2014, 41, 247–252. [Google Scholar] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-Induced Oxidative Stress: Past, Present and Future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production According to Duration of a Competitive Race. Oxid. Med. Cell Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Porcelli, S.; Pugliese, L.; Pavei, G.; Bellistri, G.; Montorsi, M.; Tacchini, P.; Vezzoli, A. Training Effects on ROS Production Determined by Electron Paramagnetic Resonance in Master Swimmers. Oxid. Med. Cell Longev. 2015, 2015, 804794. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Vezzoli, A.; Dellanoce, C.; Comassi, M.; Giardini, G.; Bruno, R.M.; Montorsi, M.; Corciu, A.; Greco, F.; et al. Acute Effects of Triathlon Race on Oxidative Stress Biomarkers. Oxid. Med. Cell Longev. 2020, 2020, 3062807. [Google Scholar] [CrossRef]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Bosco, G.; Paganini, M.; Pieri, M.; Lancellotti, V.; Marroni, A. Nitric Oxide and Oxidative Stress Changes at Depth in Breath-Hold Diving. Front. Physiol. 2021, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mrakic-Sposta, S.; Moretti, S.; Balestra, C.; Rubini, A. Spirometry and Oxidative Stress after Rebreather Diving in Warm Water. Undersea Hyperb. Med. J. Undersea Hyperb. Med. Soc. Inc. 2018, 45, 191–198. [Google Scholar] [CrossRef]
- Perovic, A.; Unic, A.; Dumic, J. Recreational Scuba Diving: Negative or Positive Effects of Oxidative and Cardiovascular Stress? Biochem. Med. 2014, 24, 235–247. [Google Scholar] [CrossRef]
- Zwart, S.R.; Jessup, J.M.; Ji, J.; Smith, S.M. Saturation Diving Alters Folate Status and Biomarkers of DNA Damage and Repair. PLoS ONE 2012, 7, e31058. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, R.; Segadal, K.; Svardal, A.M. Glutathione in Blood Cells Decreases without DNA Breaks after a Simulated Saturation Dive to 250 Msw. Aviat. Space Environ. Med. 2006, 77, 597–604. [Google Scholar] [PubMed]
- Ishida, Y.; Okada, T.; Kobayashi, T.; Uchino, H. ORiTM: A New Indicator of Oxygenation. J. Anesth. 2021, 35, 734–740. [Google Scholar] [CrossRef] [PubMed]

| Event | Time | ORi™ | SpO2 (%) | Heart Rate (bpm) | Dive Time | Depth (mfw) | Water Temperature | pH | PaCO2 (mmHg) | PaO2 (mmHg) | SaO2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABG at rest, out of water | 17:49:00 | 0.0 | 98.0 | 80.0 | - | - | (Out of water) | 7.398 | 38.7 | 103.3 | 98 |
| ABG at 15 mfw | 18:31:01 | 0.23 | 100.0 | 108.0 | 18:00 | 13.5 | Not available | 7.339 | 47.4 | 236.7 | 100 |
| ABG—end of the dive | 18:37:41 | 0.0 | 96.0 | 106.0 | 24:40 | 2.5 | 35° | 7.418 | 36.9 | 108.3 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pumpo, F.; Meloni, G.; Paganini, M.; Cialoni, D.; Garetto, G.; Cipriano, A.; Giacon, T.A.; Martani, L.; Camporesi, E.; Bosco, G. Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report. Healthcare 2023, 11, 1102. https://doi.org/10.3390/healthcare11081102
Di Pumpo F, Meloni G, Paganini M, Cialoni D, Garetto G, Cipriano A, Giacon TA, Martani L, Camporesi E, Bosco G. Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report. Healthcare. 2023; 11(8):1102. https://doi.org/10.3390/healthcare11081102
Chicago/Turabian StyleDi Pumpo, Fabio, Gualtiero Meloni, Matteo Paganini, Danilo Cialoni, Giacomo Garetto, Alessandro Cipriano, Tommaso Antonio Giacon, Luca Martani, Enrico Camporesi, and Gerardo Bosco. 2023. "Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report" Healthcare 11, no. 8: 1102. https://doi.org/10.3390/healthcare11081102
APA StyleDi Pumpo, F., Meloni, G., Paganini, M., Cialoni, D., Garetto, G., Cipriano, A., Giacon, T. A., Martani, L., Camporesi, E., & Bosco, G. (2023). Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report. Healthcare, 11(8), 1102. https://doi.org/10.3390/healthcare11081102












