Evaluating the Feasibility of Pro-Neurotensin and 25-Hydroxyvitamin D3 as Possible Indicators for Type 2 Diabetes Mellitus and Its Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Sample Collection
2.3. Ethics Approval
2.4. Statistical Analysis of Data
3. Results
3.1. Demographic and Clinical Features of the Study Population
3.2. Comparison in Pro-NT and 25-OH Vitamin D3 between Both Groups
3.3. Determination of the Prediction Value of Pro-NT and 25-OH Vitamin D3 for T2DM
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007, 30 Suppl. S1, S4–S41. [Google Scholar] [CrossRef] [PubMed]
- Baynest, H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015, 6, 541. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 9782930229874. Available online: https://www.diabetesatlas.org (accessed on 20 February 2023).
- Polak, J.M.; Sullivan, S.N.; Bloom, S.R.; Buchan, A.M.J.; Facer, P.; Brown, M.R.; Pearse, A.G.E. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature 1977, 270, 183–184. [Google Scholar] [CrossRef]
- Carraway, R.; Leeman, S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B.; et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef]
- Kim, E.R.; Mizuno, T.M. Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin-related peptides. Neurosci. Lett. 2010, 468, 64–67. [Google Scholar] [CrossRef]
- Auguet, T.; Aragonès, G.; Berlanga, A.; Martínez, S.; Sabench, F.; Aguilar, C.; Villar, B.; Sirvent, J.J.; Del Castillo, D.; Richart, C. Low Circulating Levels of Neurotensin in Women with Nonalcoholic Fatty Liver Disease Associated with Severe Obesity. Obesity 2018, 26, 274–278. [Google Scholar] [CrossRef]
- Ernst, A.; Hellmich, S.; Bergmann, A. Proneurotensin 1-117, a stable neurotensin precursor fragment identified in human circulation. Peptides 2006, 27, 1787–1793. [Google Scholar] [CrossRef]
- Melander, O.; Maisel, A.S.; Almgren, P.; Manjer, J.; Belting, M.; Hedblad, B.; Engström, G.; Kilger, U.; Nilsson, P.; Bergmann, A.; et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012, 308, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Fawad, A.; Nilsson, P.M.; Struck, J.; Bergmann, A.; Melander, O.; Bennet, L. The association between plasma proneurotensin and glucose regulation is modified by country of birth. Sci. Rep. 2019, 9, 13640. [Google Scholar] [CrossRef]
- Nicoli, C.D.; Carson, A.P.; Plante, T.B.; Leann Long, D.; McClure, L.A.; Schulte, J.; Cushman, M. Pro-Neurotensin/Neuromedin N and Risk of Incident Metabolic Syndrome and Diabetes Mellitus in the REGARDS Cohort. J. Clin. Endocrinol. Metab. 2021, 106, E3483–E3494. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Leonetti, F.; Capoccia, D.; Di Cristofano, C.; Silecchia, G.; Orho-Melander, M.; Melander, O.; Cavallo, M.G. Increased Plasma Proneurotensin Levels Identify NAFLD in Adults With and Without Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Ganeb, S.S.; Eltanawy, R.M.; Marei, Y.M.; Abdelazez, H.M.; Mahgoub, M.Y.; Ganeb, S. Implications of vitamin D Deficiency on Egyptian Adults’ Health. Benha Med. J. 2021, 38, 831–847. [Google Scholar] [CrossRef]
- Arabi, A.; El Rassi, R.; El-Hajj Fuleihan, G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat. Rev. Endocrinol. 2010, 6, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Nader, N.S.; Weaver, A.L.; Singh, R.; Kumar, S. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J. Pediatr. 2010, 156, 444–449.e1. [Google Scholar] [CrossRef] [PubMed]
- Shivaprakash, N.C.; Baby Joseph, R. Relationships between Serum 25-Hydroxy Vitamin D Levels and Plasma Glucose and Lipid Levels in Pediatric Patients in a Rural Hospital. Int. J. Sci. C Study 2014, 1, 24–31. [Google Scholar]
- Yılmaz, S.K.; Mertoğlu, C.; Ayaz, A. Assessment of Relationship Between Serum Vitamin D Levels and Metabolic Syndrome Components in Hemodialysis Patients. Galician Med. J. 2021, 28, E202113. [Google Scholar] [CrossRef]
- Sung, C.C.; Liao, M.T.; Lu, K.C.; Wu, C.C. Role of Vitamin D in Insulin Resistance. J. Biomed. Biotechnol. 2012, 2012, 11. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 2013, 59, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Mondal, S.A.; Choudhuri, S.; Maisnam, I.; Hasanoor Reza, A.H.; Bhattacharya, B.; Chowdhury, S.; Mukhopadhyay, S. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res. Clin. Pract. 2014, 103, e18–e23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Ogunkolade, B.W.; Boucher, B.J.; Prahl, J.M.; Bustin, S.A.; Burrin, J.M.; Noonan, K.; North, B.V.; Mannan, N.; McDermott, M.F.; Deluca, H.F.; et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002, 51, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- New Weight Standards for Men and Women; Metropolitan Life Insurance Company: New York, NY, USA, 1959; Volume 40, pp. 1–10.
- Salgado, A.L.F.D.A.; De Carvalho, L.; Oliveira, A.C.; Dos Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef]
- Khan, H.A.; Sobki, S.H.; Khan, S.A. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin. Exper. Med. 2007, 7, 24–29. [Google Scholar] [CrossRef]
- Cigolini, M.; Iagulli, M.P.; Miconi, V.; Galiotto, M.; Lombardi, S.; Targher, G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care 2006, 29, 722–724. [Google Scholar] [CrossRef]
- Hyppönen, E.; Power, C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: The role of obesity. Diabetes Care 2006, 29, 2244–2246. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Praveenkumar., R.; Varadarajan, S.; Rani, J.R.; James, J.; Porkodi, A. Effectiveness And Safety Of Low Dose Vitamin D As An Adjunct Therapy In Patients With Type 2 Diabetes Mellitus: A Double-Blind, Randomised, Controlled Study. J. Pharm. Negat. Results 2022, 13, 2076–2081. [Google Scholar] [CrossRef]
- Kawahara, T.; Suzuki, G.; Mizuno, S.; Inazu, T.; Kasagi, F.; Kawahara, C.; Okada, Y.; Tanaka, Y. Effect of active vitamin D treatment on development of type 2 diabetes: DPVD randomised controlled trial in Japanese population. BMJ 2022, 377, e066222. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South. Med. J. 2005, 98, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: Dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dȩbski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Tjønneland, A.; Olsen, A.; Halkjær, J.; Lind, B.; Heegaard, A.M.; Schwarz, P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J. Clin. Endocrinol. Metab. 2015, 100, 2339–2346. [Google Scholar] [CrossRef]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. The Impact of Vitamin D in Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Patients with Morbid Obesity. Diabetes. Metab. Syndr. Obes. 2021, 14, 487–495. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Sasso, B.; Lo Ciaccio, M. Non-Skeletal Activities of Vitamin D: From Physiology to Brain Pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef]
- Henry, H.L.; Bouillon, R.; Norman, A.W.; Gallagher, J.C.; Lips, P.; Heaney, R.P.; Vieth, R.; Pettifor, J.M.; Dawson-Hughes, B.; Lamberg-Allardt, C.J.; et al. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J. Steroid Biochem. Mol. Biol. 2010, 121, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Talarico, M.; Pascale, A.; Pascale, V.; Minici, R.; Boriani, G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics 2022, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Mosekilde, L.; Juhl, C.; Moller, N.; Christensen, B.; Rejnmark, L.; Wamberg, L.; Orskov, L. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency—A double-blind, randomized, placebo-controlled trial. Metabolism 2014, 63, 1115–1124. [Google Scholar] [CrossRef]
- Pang, Z.; Yi, Y.; Qu, T.; Gao, S.; Shi, A.; Zhao, Y.; Xu, S.; Yang, L.; Lin, Y.; Liu, Y.; et al. The beneficial cutoffs of vitamin D for metabolic syndrome varies by sex among the elderly Chinese population: A cross-sectional study. Nutr. Res. 2022, 104, 91–100. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abo-Elmatty, D.M.; Ezzat, O.; Mesbah, N.M.; Ali, N.S.; El Fatah, A.S.A.; Alsayed, E.; Hamada, M.; Hassnine, A.A.; Abd-Elsalam, S.; et al. Pro-Neurotensin as a Potential Novel Diagnostic Biomarker for Detection of Nonalcoholic Fatty Liver Disease. Diabetes. Metab. Syndr. Obes. 2022, 15, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Tönjes, A.; Kralisch, S.; Hoffmann, A.; Schleinitz, D.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Fasshauer, M.; Ebert, T. Circulating Pro-Neurotensin in gestational diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 23–29. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Bertoccini, L.; Ceccarelli, V.; Baroni, M.G.; Melander, O.; Cavallo, M.G. High pro-neurotensin levels in individuals with type 1 diabetes associate with the development of cardiovascular risk factors at follow-up. Acta Diabetol. 2022, 59, 49–56. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Lyass, A.; Liu, Y.; Gaggin, H.; Trebnick, A.; Maisel, A.S.; D’Agostino, R.B.; Wang, T.J.; Massaro, J.; Vasan, R.S. Circulating Proneurotensin Concentrations and Cardiovascular Disease Events in the Community: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1692–1697. [Google Scholar] [CrossRef]
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 1724–1734. [Google Scholar] [CrossRef]
- Osadchii, O.E. Emerging role of neurotensin in regulation of the cardiovascular system. Eur. J. Pharmacol. 2015, 762, 184–192. [Google Scholar] [CrossRef]

| Variable | Group I (n = 100) (Diabetic) | Group II (n = 100) (Control) | T | p | |||
|---|---|---|---|---|---|---|---|
| Age: (years) | Mean ± SD | 48.19 ± 12.24 | 44.66 ± 11.48 | 1.98 | 0.06 NS | ||
| Range | 23–67 | 25–67 | |||||
| Duration of T2DM: (years) | Mean ± SD | 7.62 ± 4.67 | |||||
| Range | 0.17–20 | ||||||
| BMI: (kg/m2) | Mean ± SD | 29.85 ± 4.38 | 28.77 ± 4.61 | 1.7 | 0.09 NS | ||
| Range | 20–40 | 20–38 | |||||
| SBP: (mmHg) | Mean ± SD | 122.2 ± 14.64 | 112.73 ± 9.97 | 5.35 | <0.001 ** | ||
| Range | 100–160 | 90–152 | |||||
| DBP: (mmHg) | Mean ± SD | 73.74 ± 10.72 | 69.52 ± 9.33 | 2.97 | 0.003 * | ||
| Range | 40–97 | 50–93 | |||||
| Waist circumference: (cm) | Mean ± SD | 112.88 ± 12.16 | 104.67 ± 13.09 | 4.60 | <0.001 ** | ||
| Range | 87–140 | 80–156 | |||||
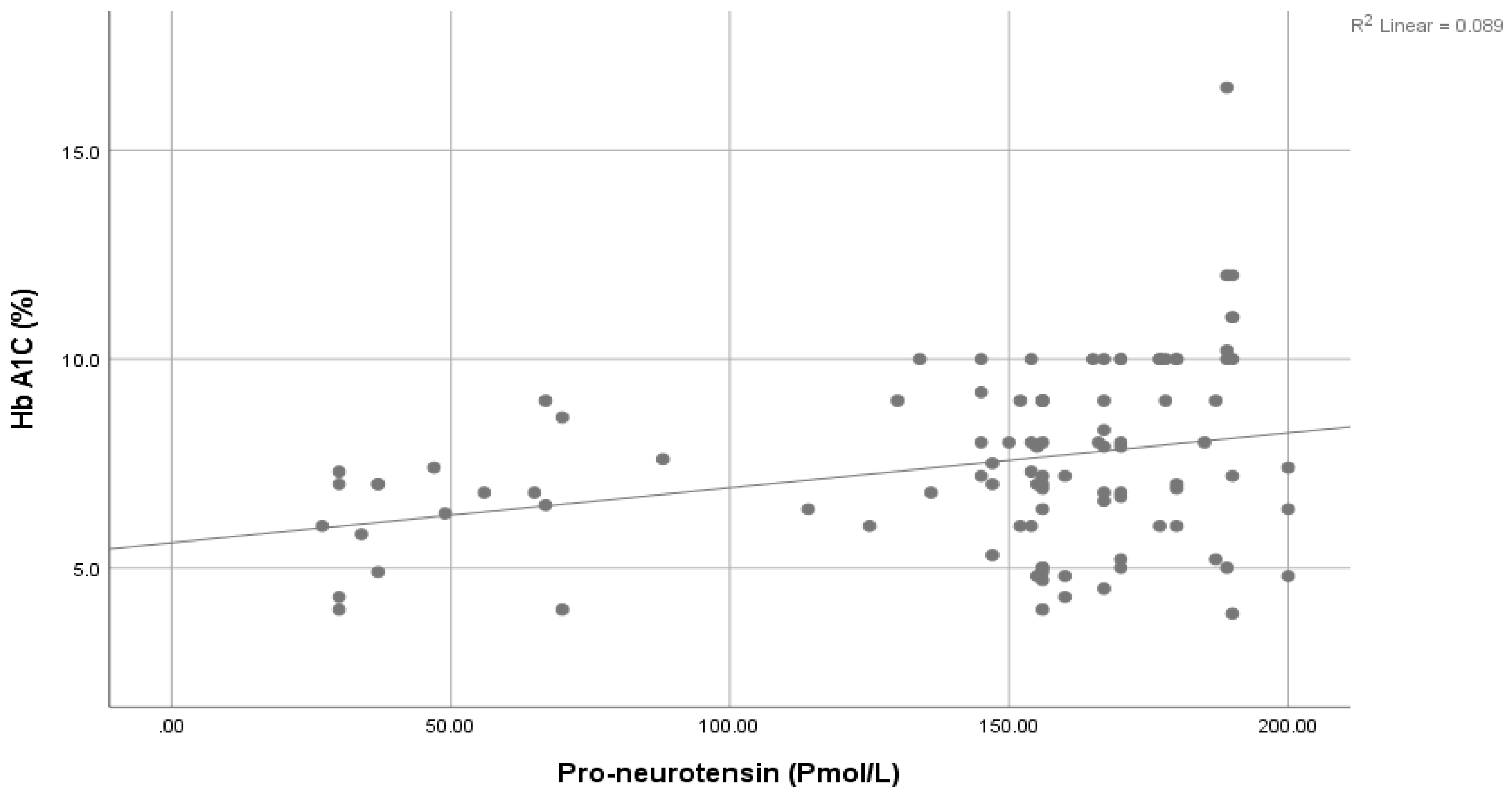
| HbA1c: (%) | Mean ± SD | 7.46 ± 2.13 | 3.33 ± 1.22 | 16.79 | <0.001 ** | ||
| Range | 3.9–16.5 | 1.2–5 | |||||
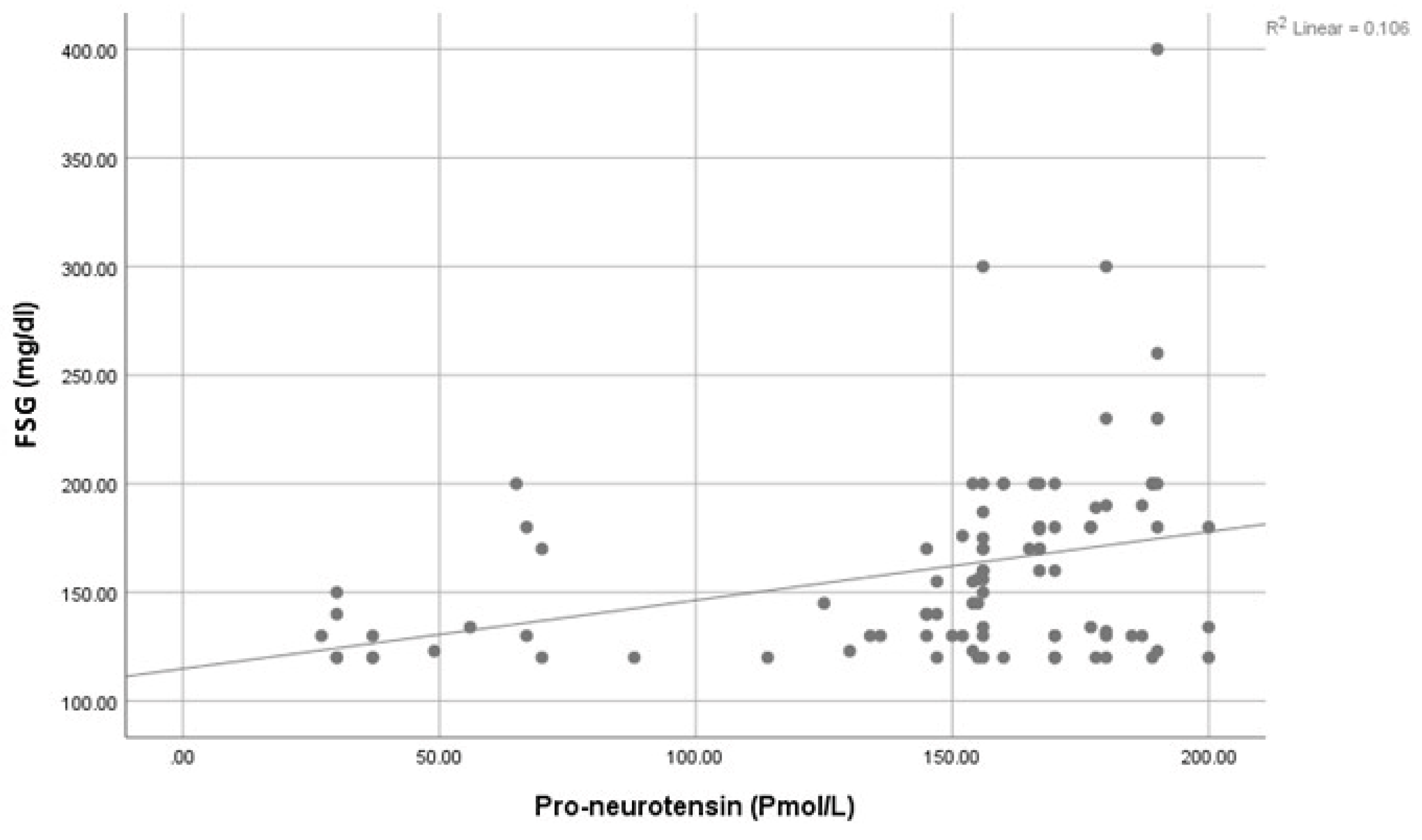
| FSG: (mg/dL) | Mean ± SD | 160.95 ± 45.22 | 85.23 ± 8.84 | 14.13 | <0.001 ** | ||
| Range | 120–400 | 67–102 | |||||
| Post prandial serum glucose: (mg/dL) | Mean ± SD | 251.4 ± 72.11 | 119.43 ± 14.9 | 17.92 | <0.001 ** | ||
| Range | 140–600 | 90–177 | |||||
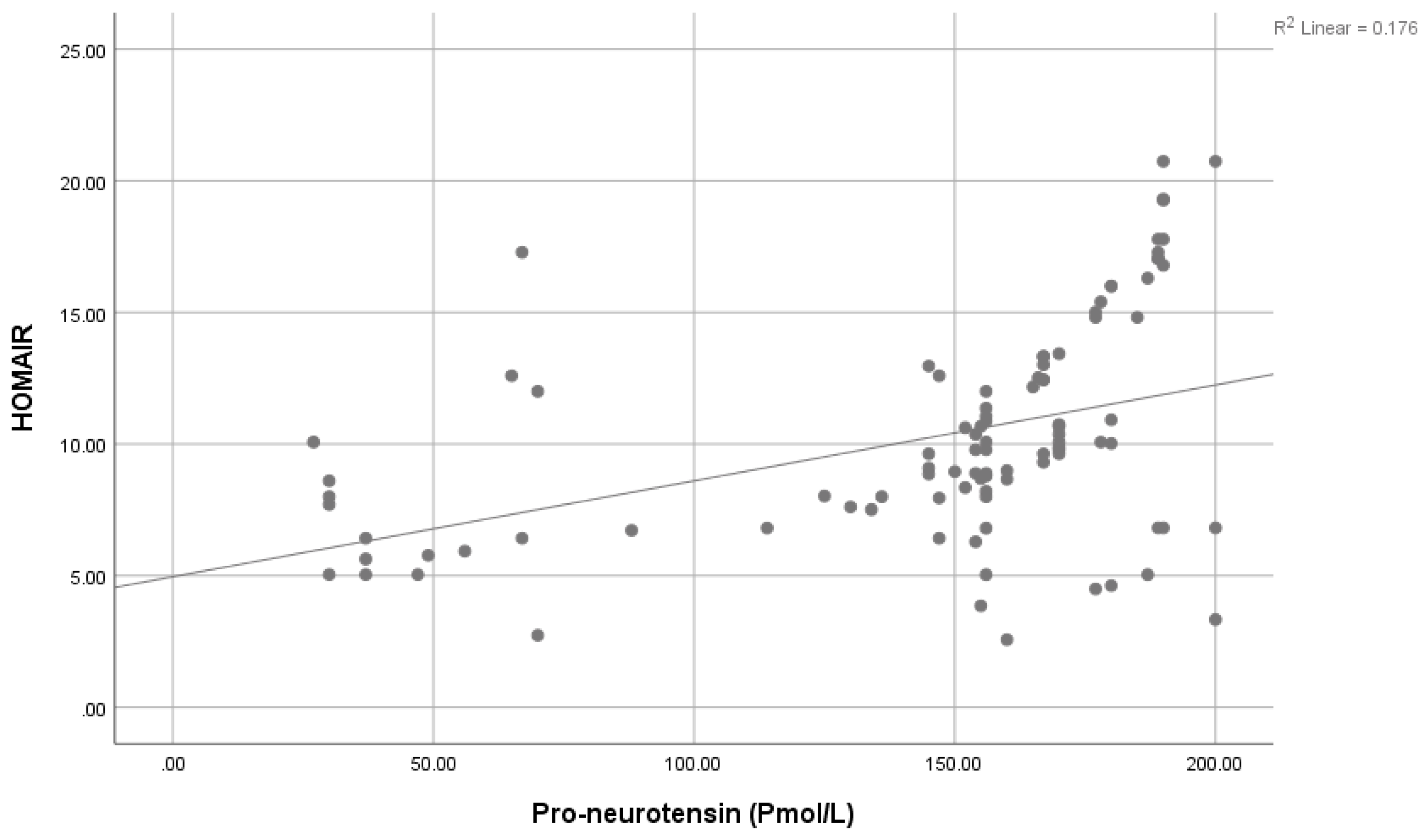
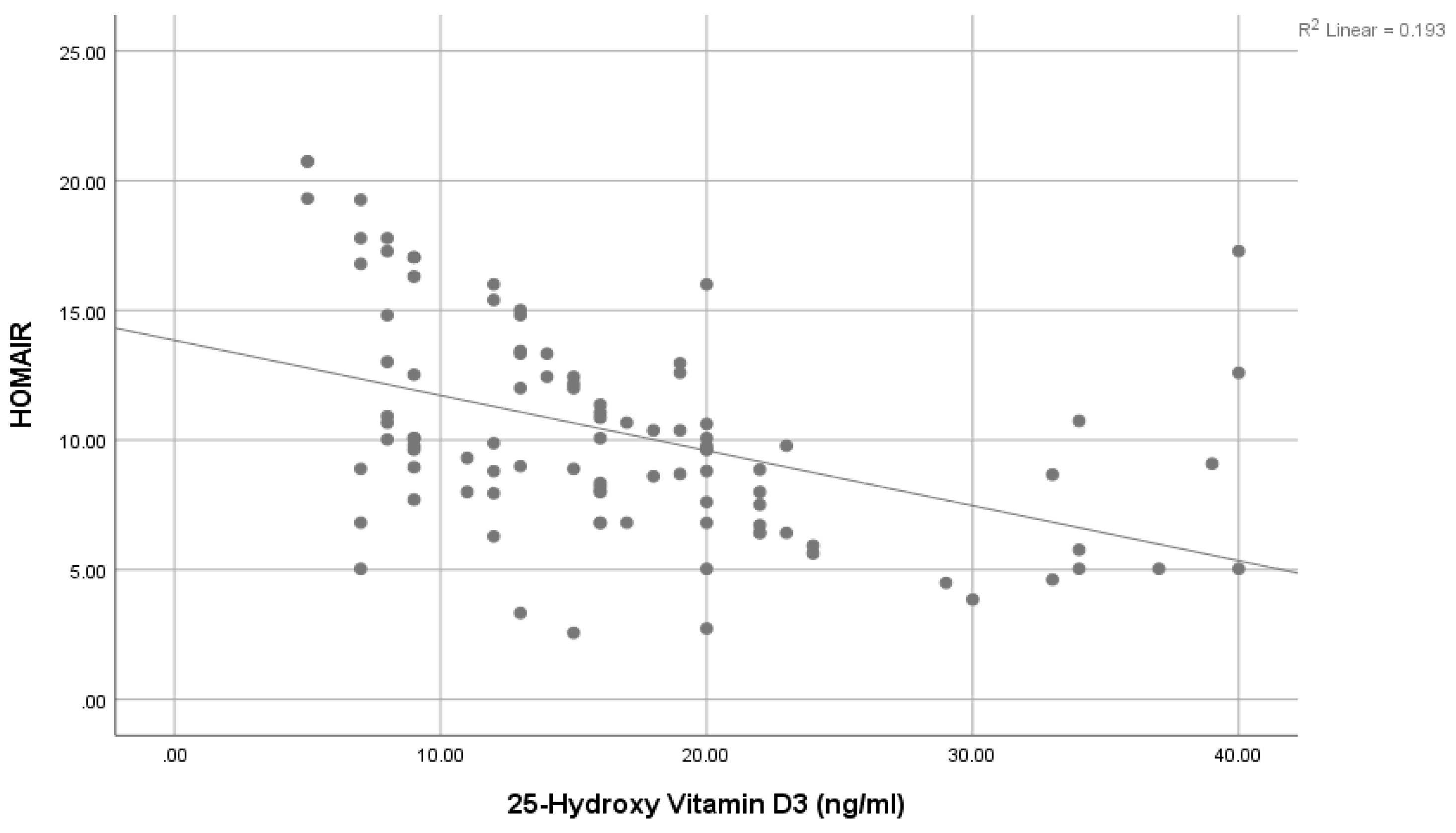
| HOMA IR: | Mean ± SD | 10.31 ± 3.15 | 4.44 ± 1.42 | 13.4 | <0.001 ** | ||
| Range | 2.57–20.74 | 1.91–6 | |||||
| INR: | Mean ± SD | 1.23 ± 0.18 | 1.07 ± 0.10 | 7.6 | <0.001 ** | ||
| Range | 1–1.73 | 1–1.4 | |||||
| Creatinine: (mg/dL) | Mean ± SD | 1.01 ± 0.19 | 0.95 ± 0.16 | 2.21 | 0.03 * | ||
| Range | 0.6–1.5 | 0.7–1.2 | |||||
| Urea: (mg/dL) | Mean ± SD | 34.79 ± 10.67 | 29.8 ± 8.34 | 3.68 | <0.001 ** | ||
| Range | 20–67 | 13–55 | |||||
| Cholesterol: (mg/dL) | Mean ± SD | 172.78 ± 41.01 | 148.12 ± 18.75 | 5.47 | <0.001 ** | ||
| Range | 87–280 | 115–203 | |||||
| Triglyceride: (mg/dL) | Mean ± SD | 189.77 ± 24.76 | 146.72 ± 22.84 | 12.78 | <0.001 ** | ||
| Range | 120–270 | 121–220 | |||||
| Chol-HDL: (mg/dL) | Mean ± SD | 32.88 ± 9.93 | 43.83 ± 8.70 | 8.29 | <0.001 ** | ||
| Range | 20–60 | 30–88 | |||||
| Chol-LDL: (mg/dL) | Mean ± SD | 126.75 ± 27.83 | 107.94 ± 10.99 | 6.29 | <0.001 ** | ||
| Range | 99–202 | 89–134 | |||||
| Variable | No | % | No | % | χ2 | p | |
| Sex: | Male | 63 | 63% | 53 | 53% | 2.05 | 0.15 |
| Female | 37 | 37% | 47 | 47% | NS | ||
| Smoking: | No | 84 | 84% | 79 | 79% | 0.83 | 0.36 |
| Yes | 16 | 16% | 21 | 21% | NS | ||
| Hypertension stage: | Normal | 50 | 50% | 84 | 84% | 27.12 | <0.001 ** |
| Pre-hypertensive | 30 | 30% | 12 | 12% | |||
| Stage 1 | 19 | 19% | 4 | 4% | |||
| Stage 2 | 1 | 1% | 0 | 0% | |||
| Variable | Group I (Diabetic) (n = 100) | Group II (Control) (n = 100) | Mann–Whitney Test | p | |
|---|---|---|---|---|---|
| Pro-neurotensin: (pmol/L) | Mean ± SD | 144.65 ± 48.76 | 85.43 ± 30.23 | 7.97 | <0.001 ** |
| Range | 27–200 | 34–180 | |||
| Median (IQR) | 156 (145–177) | 80(70–98) | |||
| 25-Hydroxy vitamin D3: (ng/mL) | Mean ± SD | 17.03 ± 8.77 | 39.32 ± 13.1 | 10.67 | <0.001 ** |
| Range | 5–40 | 12–98 | |||
| Median (IQR) | 16 (9–20) | 38 (30.75–44) | |||
| Complication | Group I (Diabetic) (n = 100) | |
|---|---|---|
| Number | % | |
| Retinopathy: | 15 | 15 |
| Neurological complications: | 12 | 12 |
| Coronary artery diseases: | 8 | 8 |
| Complication | Pro-Neurotensin | 25 (OH) Vitamin D3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | MW | p | N | Mean | SD | Median | MW | p | ||
| Retinopathy | No | 85 | 140.01 | 49.58 | 156 | 3.01 | 0.003 * | 85 | 18.09 | 8.9 | 16 | 3.12 | 0.002 * |
| Yes | 15 | 170.93 | 34.55 | 189 | 15 | 11 | 4.8 | 9 | |||||
| Neurological complications | No | 88 | 145.45 | 48.4 | 156 | 0.14 | 0.89 NS | 88 | 17.01 | 8.64 | 17 | 0.19 | 0.85 NS |
| Yes | 12 | 138.75 | 53.18 | 145 | 12 | 17.17 | 10.09 | 15 | |||||
| Coronary artery diseases | No | 92 | 143.15 | 48.82 | 156 | 1.69 | 0.09 NS | 92 | 17.61 | 8.84 | 16 | 2.48 | 0.01 * |
| Yes | 8 | 161.88 | 47.64 | 178 | 8 | 10.38 | 4.17 | 10 | |||||
| Standardized Coefficients | p | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|
| Beta | ||||
| Sex: (m/f) | −0.092 | 0.364 | −0.264 | 0.098 |
| Age: (year) | −0.124 | 0.240 | −0.012 | 0.003 |
| BMI: (kg/m2) | −0.041 | 0.699 | −0.025 | 0.017 |
| Disease duration: (year) | −0.081 | 0.436 | −0.027 | 0.012 |
| Waist circumference: (cm) | 0.011 | 0.918 | −0.007 | 0.008 |
| SBP: (mmHg) | 0.023 | 0.850 | −0.006 | 0.008 |
| DBP: (mmHg) | 0.016 | 0.892 | −0.009 | 0.010 |
| Fasting serum glucose: (mg/dL) | 0.614 | 0.002 * | 0.601 | 1.006 |
| Post prandial serum glucose: (mg/dL) | −0.033 | 0.937 | −0.053 | 0.048 |
| Fasting insulin: (mIU/L) | 0.529 | 0.257 | −0.004 | 0.014 |
| HbA1c: (%) | 0.640 | 0.041 * | 0.604 | 1.040 |
| HOMA-IR: | 0.228 | 0.037 * | 0.033 | 1.750 |
| INR: | 0.190 | 0.750 | −0.105 | 0.145 |
| Creatinine: (mg/dL) | 0.049 | 0.671 | −0.002 | 0.003 |
| Urea: (mg/dL) | 0.079 | 0.448 | −0.005 | 0.012 |
| Cholesterol: (mg/dL) | 0.055 | 0.629 | −0.002 | 0.003 |
| Triglyceride: (mg/dL) | 0.150 | 0.229 | −0.002 | 0.007 |
| Chol-HDL: (mg/dL) | 0.252 | 0.015 | 0.002 | 0.020 |
| Chol-LDL: (mg/dL) | −0.056 | 0.656 | −0.005 | 0.003 |
| Pro-neurotensin: (Pmol/L) | 0.351 | 0.002 * | 0.309 | 1.311 |
| 25-Hydroxy vitamin D3: (ng/mL) | −0.369 | 0.006 * | −0.013 | −0.010 |
| Variable | Cut Off | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | Accuracy | p |
|---|---|---|---|---|---|---|---|---|
| Pro-neurotensin: (Pmol/L) | >124 | 0.83 (0.76–0.89) | 81% | 88% | 87.1% | 82.2% | 84.5% | <0.001 ** |
| 25-Hydroxy vitamin D3: (ng/mL) | <29.5 | 0.94 (0.9–0.97) | 88% | 93% | 92.6% | 88.6% | 90.50% | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.A.; Abo El-Matty, D.M.; Abd ElSalam, E.A.; Hussein, M.A.; Hafez, W.; Ibrahim, S.A.; Shaheen, E.A.H.; Awad, E.A.; Osman, M.A.; Abd El-Raouf, M.S.; et al. Evaluating the Feasibility of Pro-Neurotensin and 25-Hydroxyvitamin D3 as Possible Indicators for Type 2 Diabetes Mellitus and Its Complications. Healthcare 2023, 11, 1088. https://doi.org/10.3390/healthcare11081088
Mohammed AA, Abo El-Matty DM, Abd ElSalam EA, Hussein MA, Hafez W, Ibrahim SA, Shaheen EAH, Awad EA, Osman MA, Abd El-Raouf MS, et al. Evaluating the Feasibility of Pro-Neurotensin and 25-Hydroxyvitamin D3 as Possible Indicators for Type 2 Diabetes Mellitus and Its Complications. Healthcare. 2023; 11(8):1088. https://doi.org/10.3390/healthcare11081088
Chicago/Turabian StyleMohammed, Amal A., Dina M. Abo El-Matty, Esraa A. Abd ElSalam, Mona A. Hussein, Wael Hafez, Sharehan A. Ibrahim, Eman A. H. Shaheen, Eman A. Awad, Marwa A. Osman, Marwa S. Abd El-Raouf, and et al. 2023. "Evaluating the Feasibility of Pro-Neurotensin and 25-Hydroxyvitamin D3 as Possible Indicators for Type 2 Diabetes Mellitus and Its Complications" Healthcare 11, no. 8: 1088. https://doi.org/10.3390/healthcare11081088
APA StyleMohammed, A. A., Abo El-Matty, D. M., Abd ElSalam, E. A., Hussein, M. A., Hafez, W., Ibrahim, S. A., Shaheen, E. A. H., Awad, E. A., Osman, M. A., Abd El-Raouf, M. S., Saed, S. M., El-Amir, R. Y., Ghaith, D., Al Anouti, F., & Wahba, A. S. (2023). Evaluating the Feasibility of Pro-Neurotensin and 25-Hydroxyvitamin D3 as Possible Indicators for Type 2 Diabetes Mellitus and Its Complications. Healthcare, 11(8), 1088. https://doi.org/10.3390/healthcare11081088









