Application of Artificial Intelligence in Assessing the Self-Management Practices of Patients with Type 2 Diabetes
Abstract
:1. Introduction
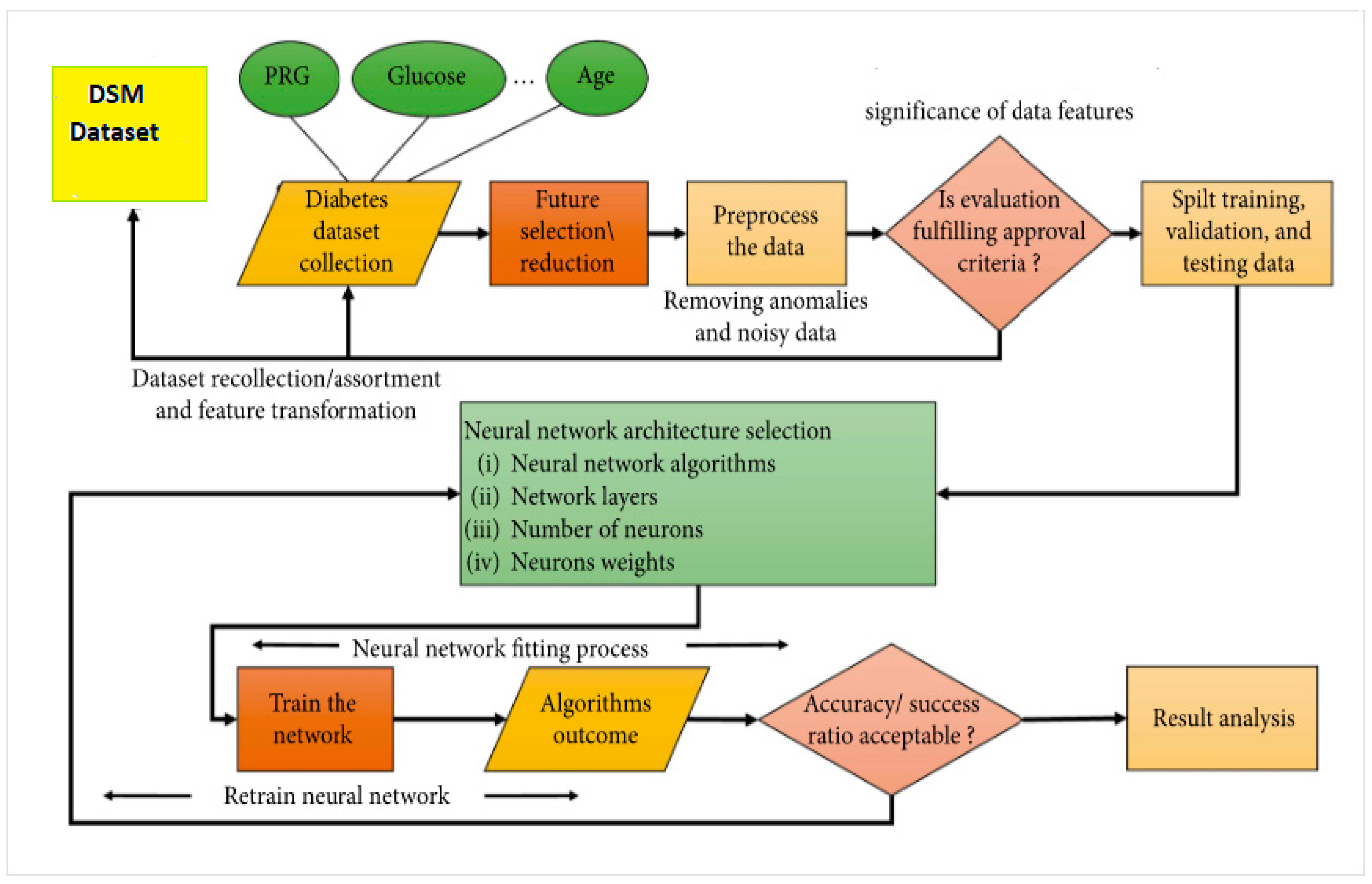
2. Material and Method
2.1. Dataset Description
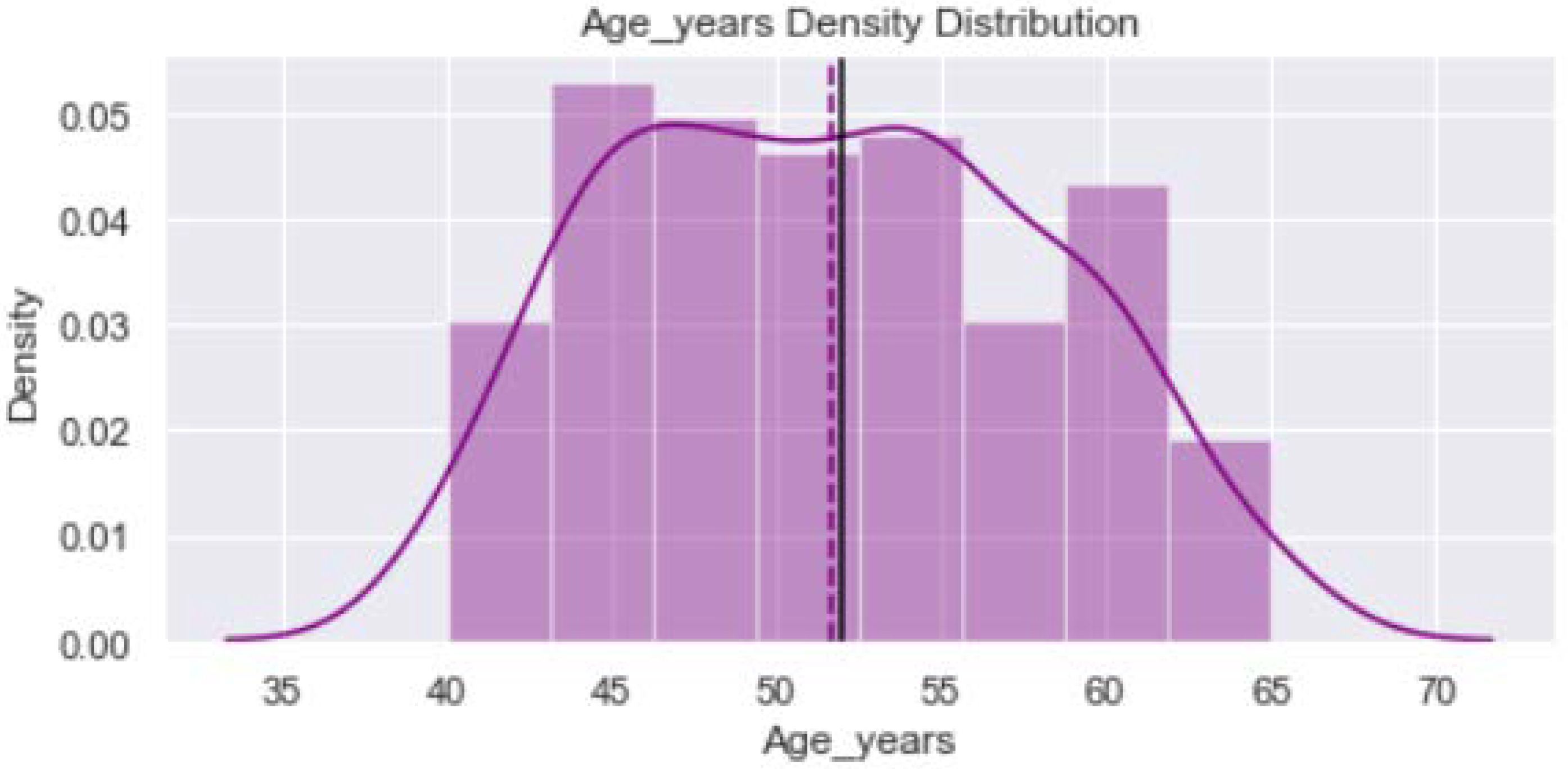
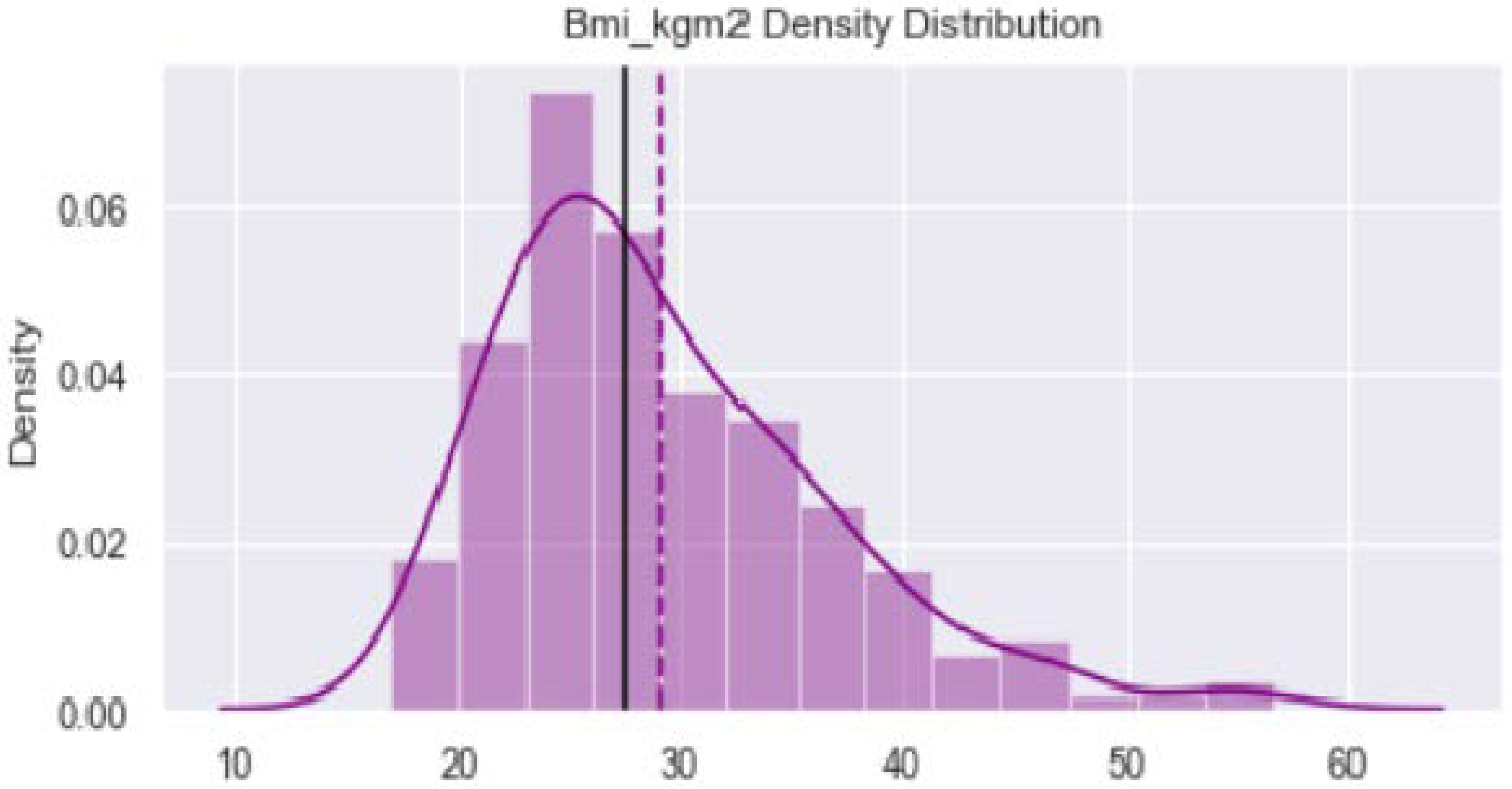
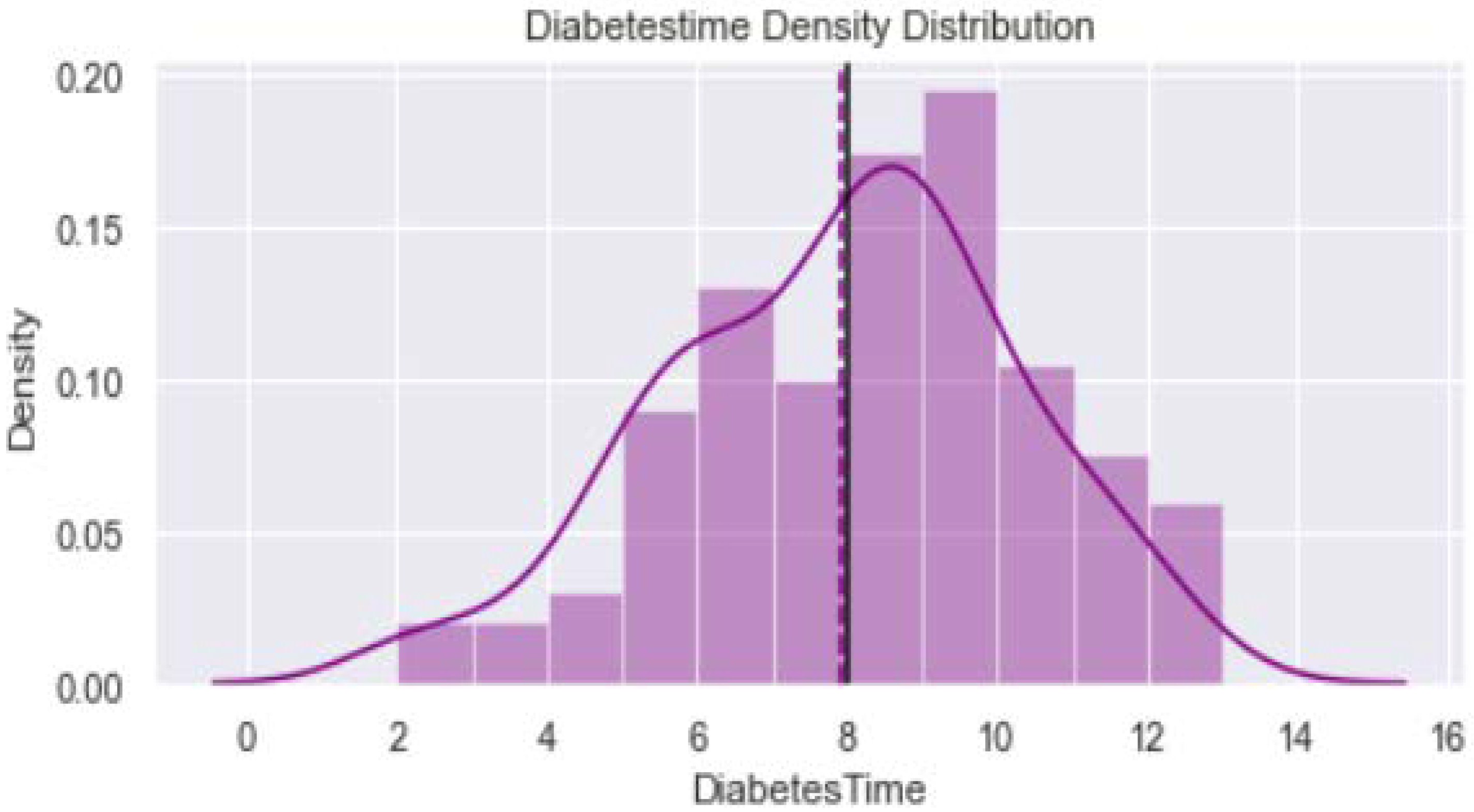
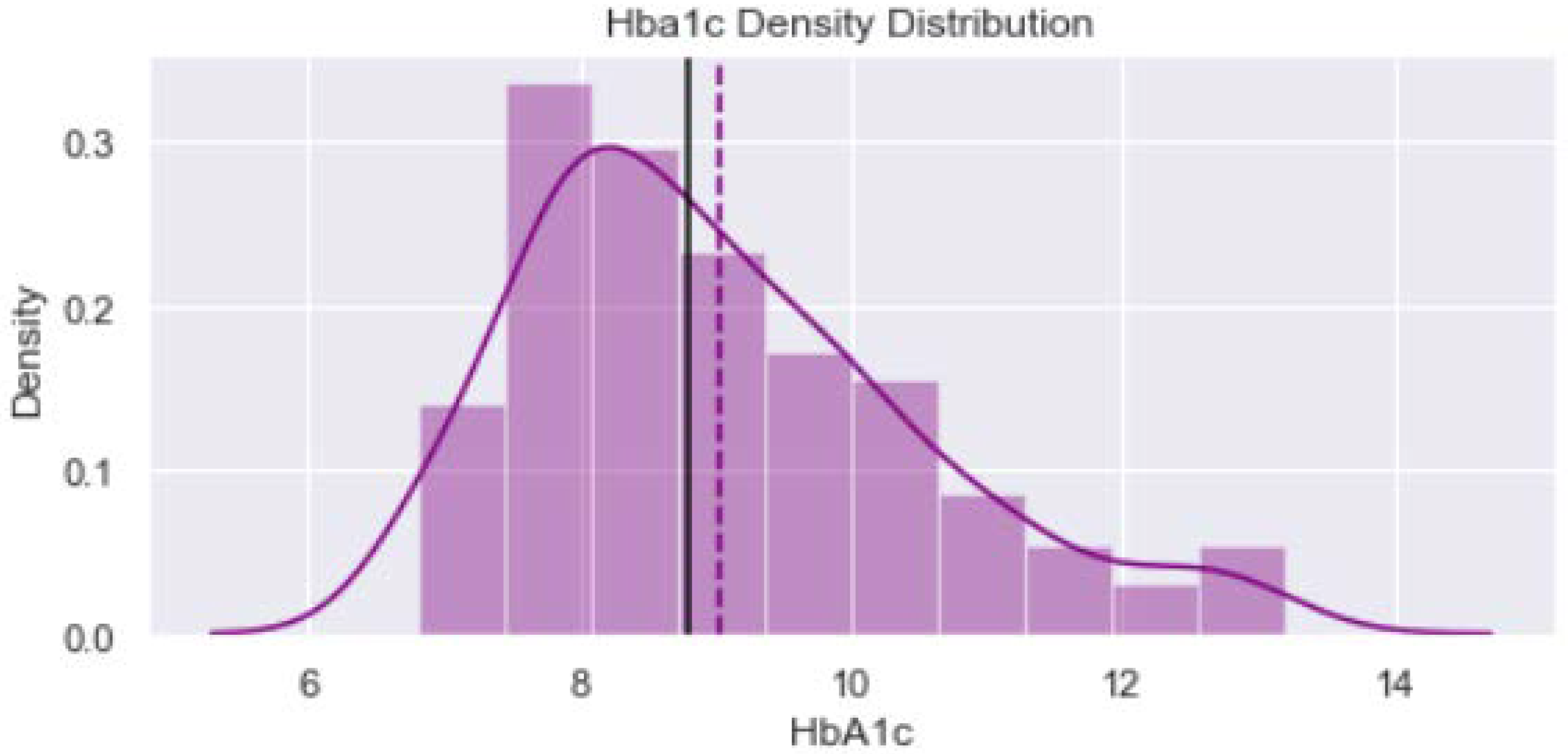
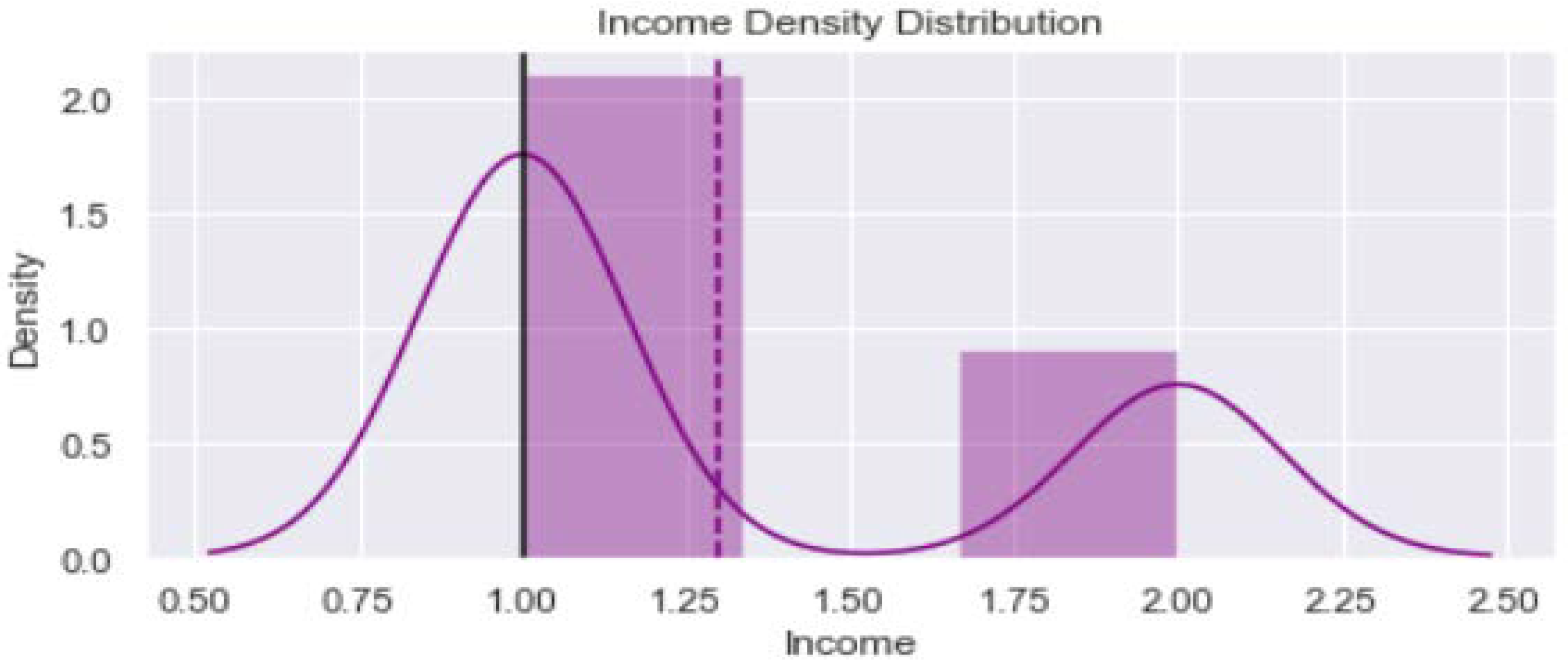
2.2. Data Visualization
2.3. Aims and Objective
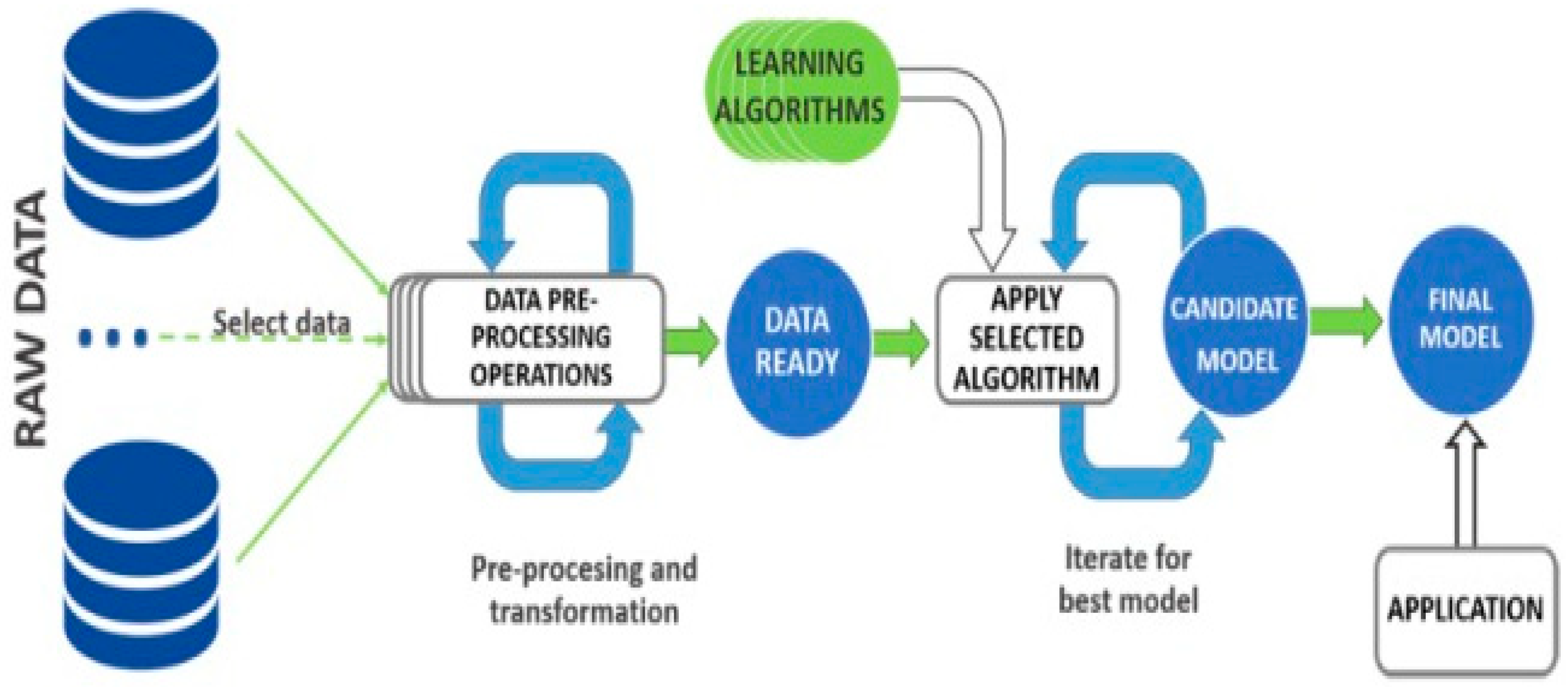
2.4. Data Pre-Processing
3. Results
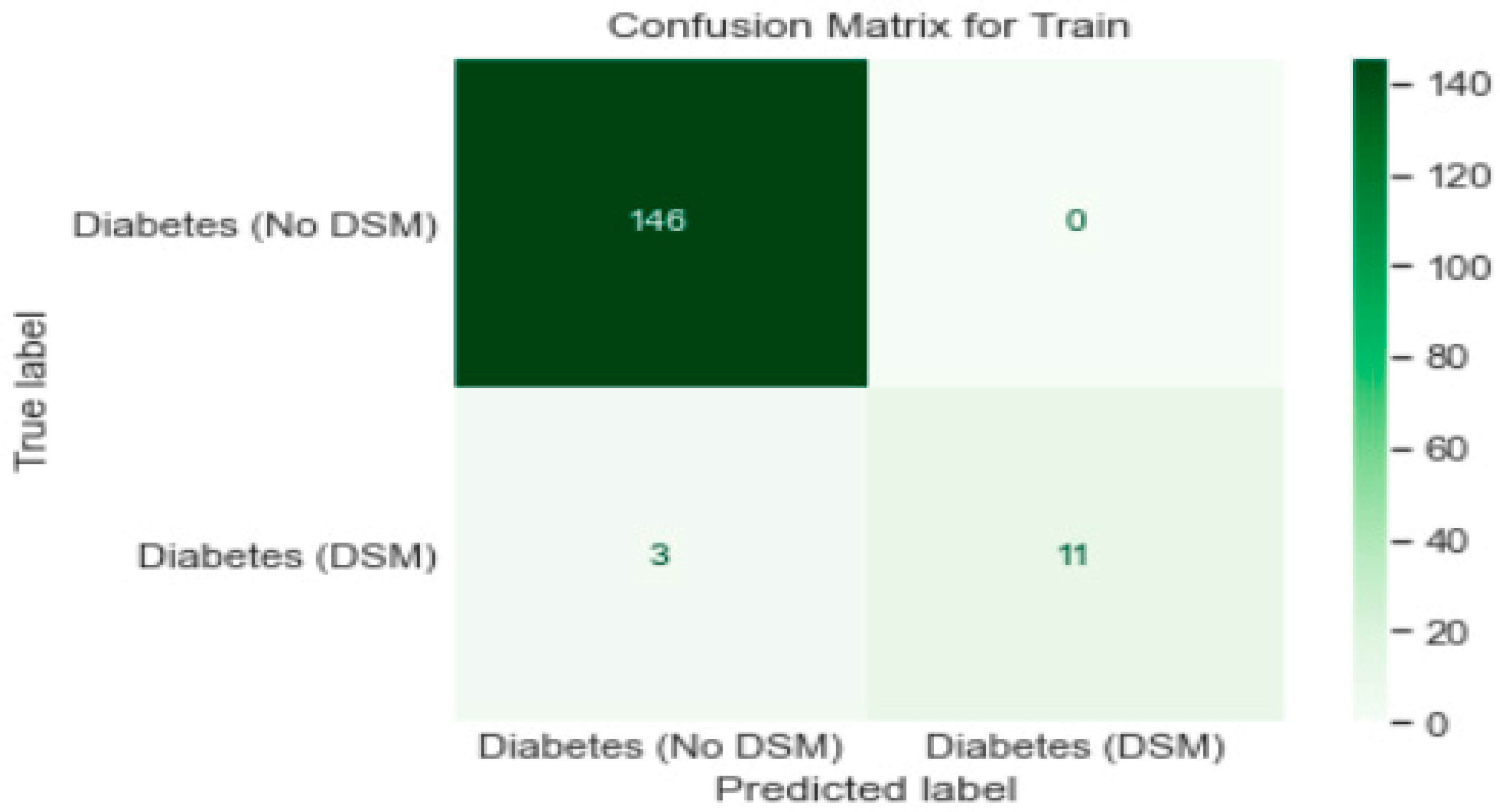
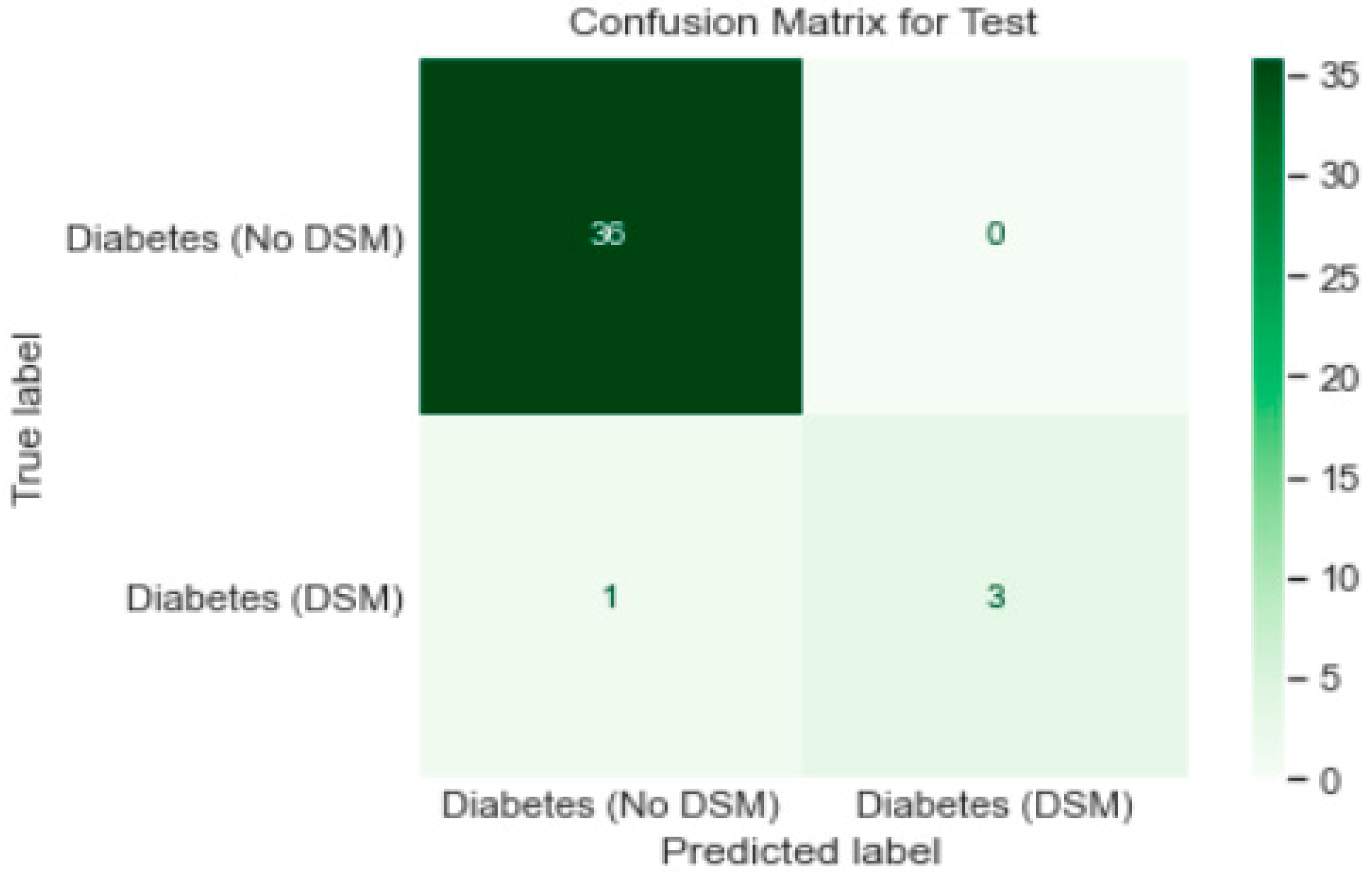
3.1. Algorithms Used for Classification
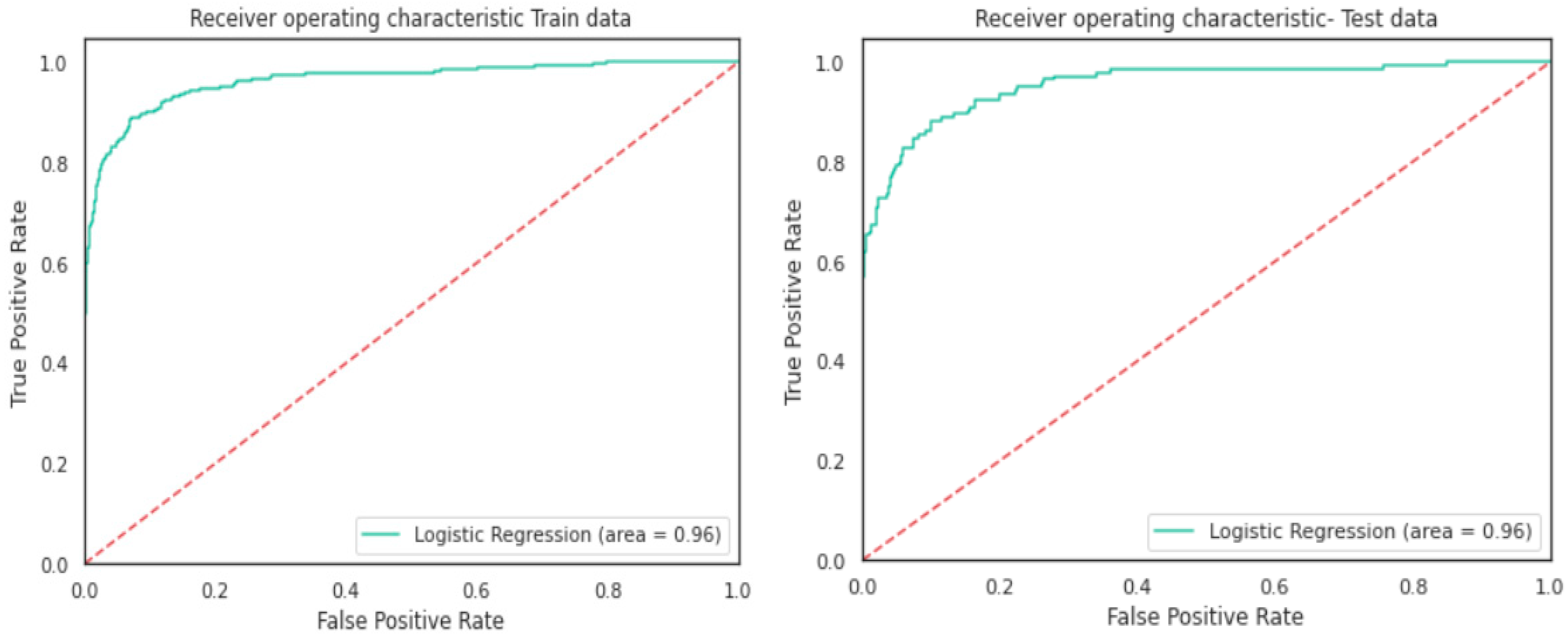
Logistic Regression Analysis
- π = Probability of response variable or dependent variable
- β1 = Log of without exposure variable
- X = Independent variables (x1, …….., xk, are different predictors)
- β2 = Change in each unit as an increase in the independent variable or exposure var
- α = the intercept alpha
- Log is a link function
- Logistic Regression model gave a generalised performance on training and test set.
- ROC-AUC score of 0.96 on training and test set was promising.
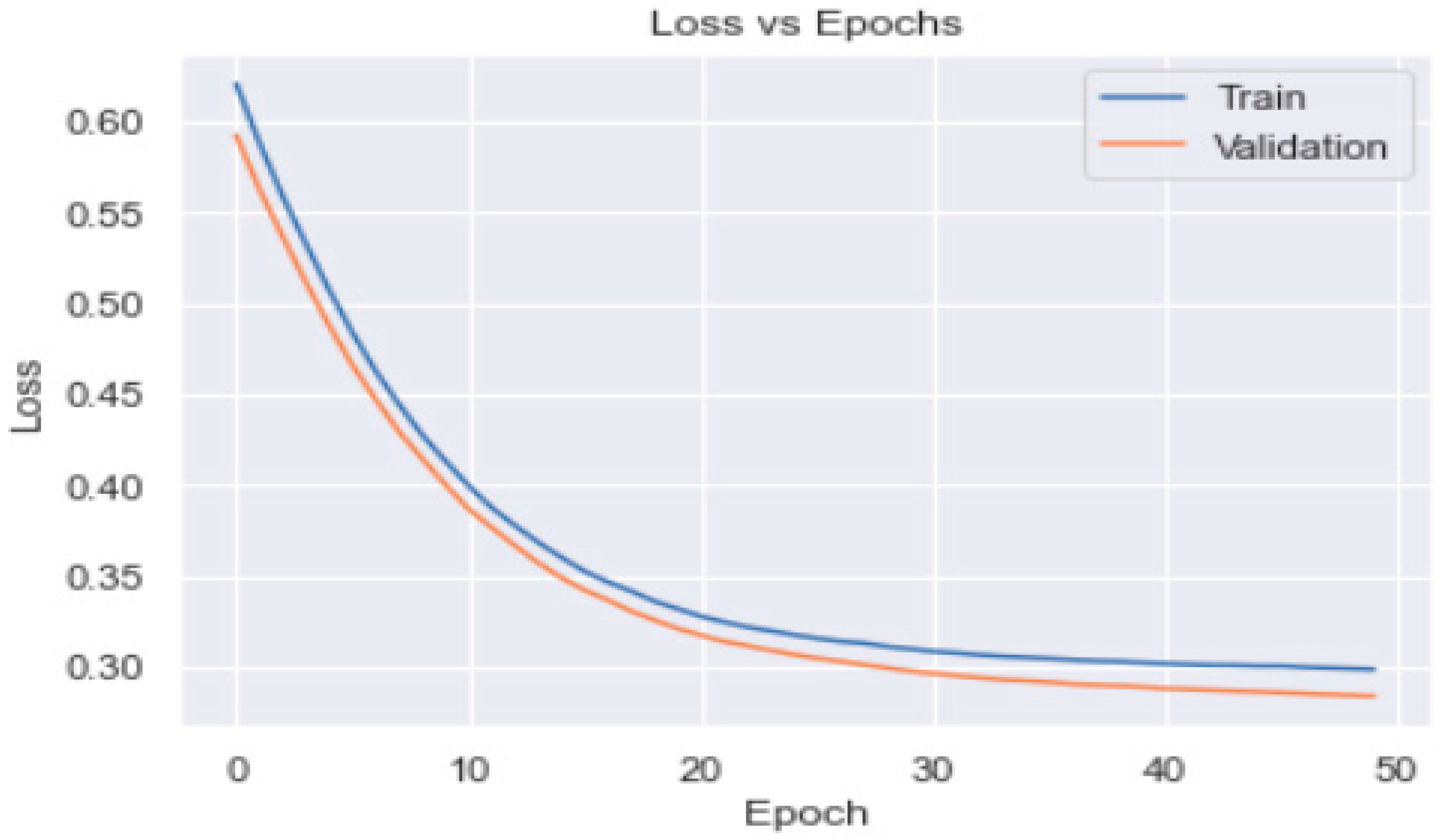
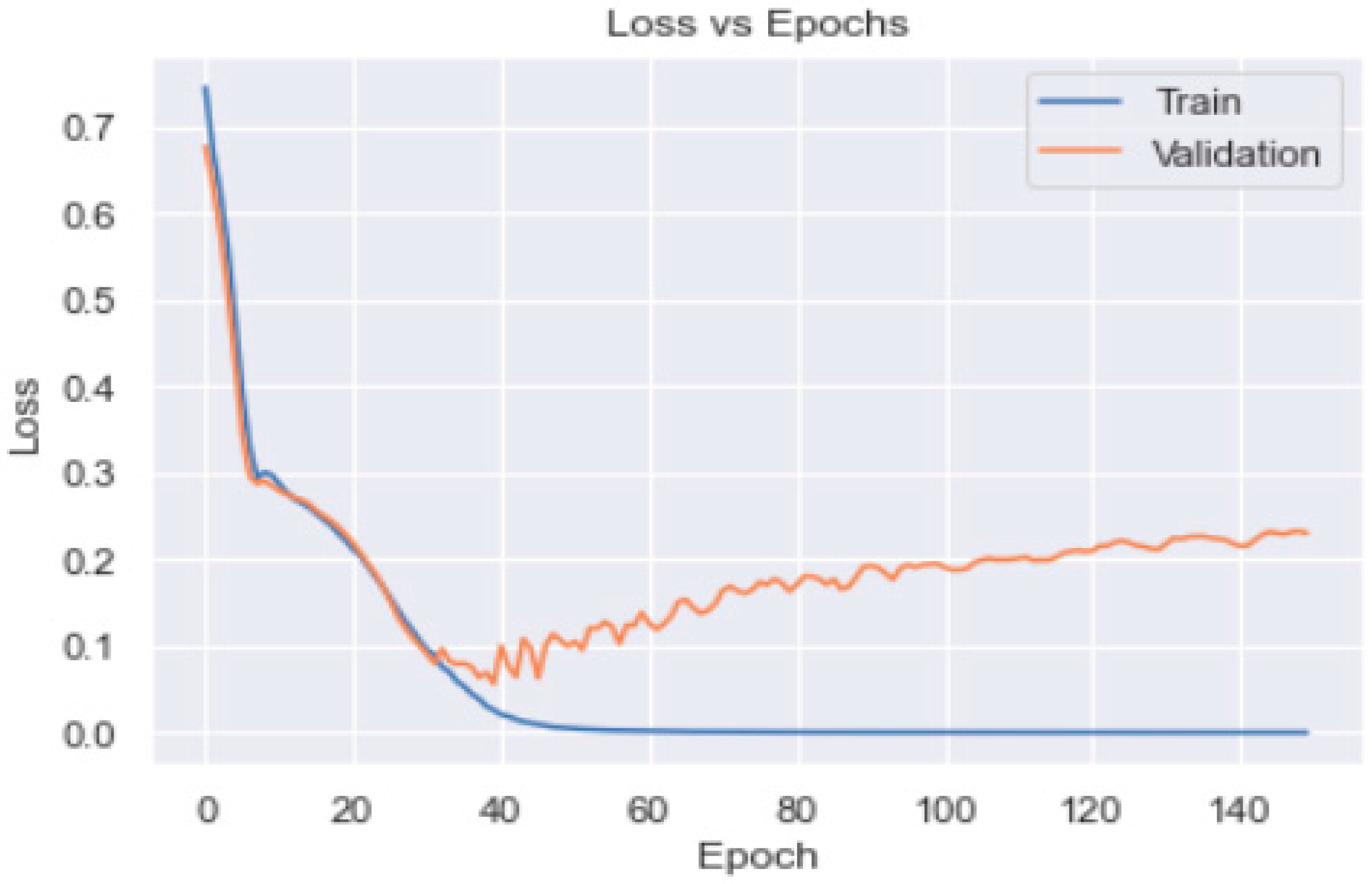
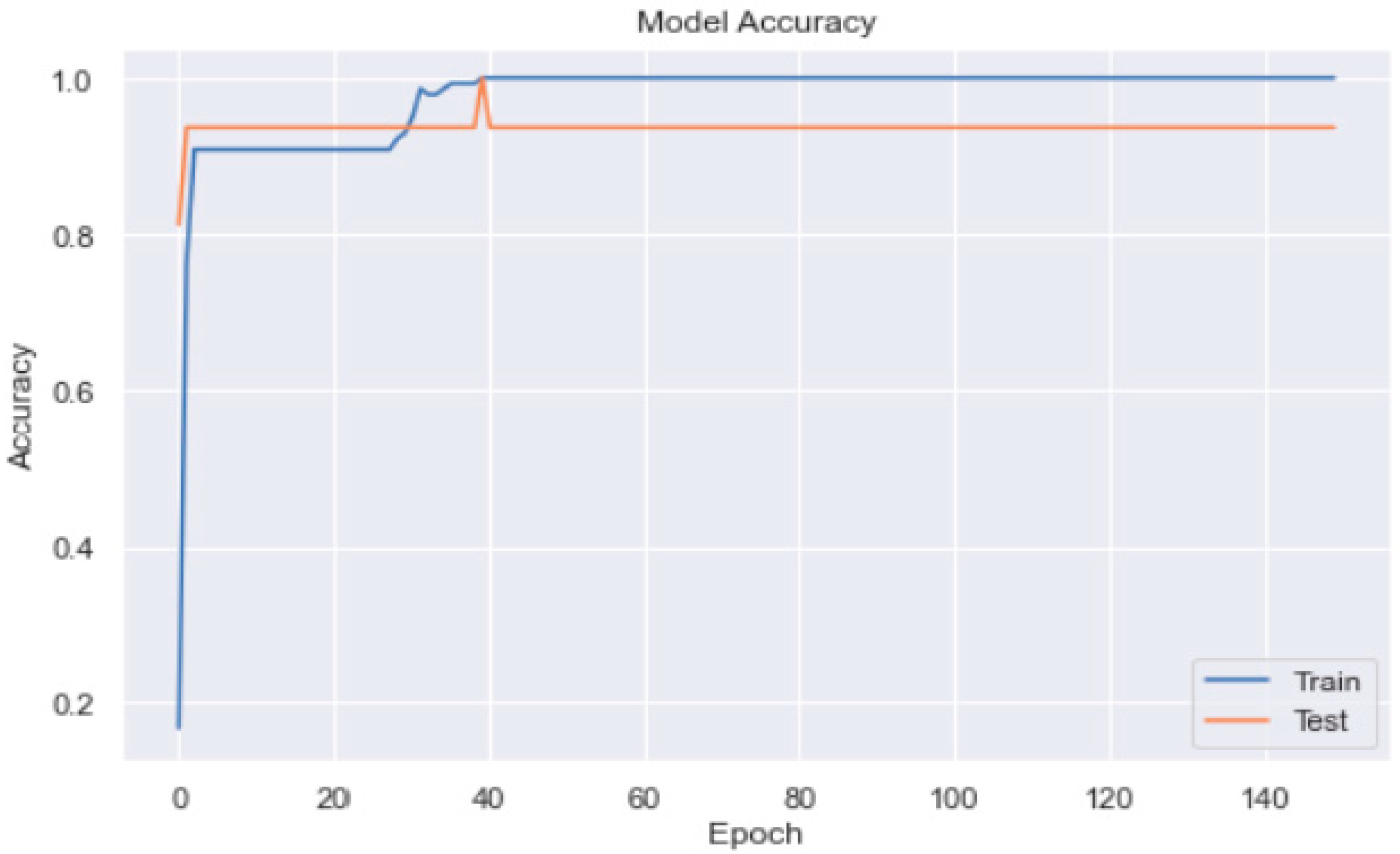
3.2. Artificial Neural Network
3.3. Artificial Neural Network Models
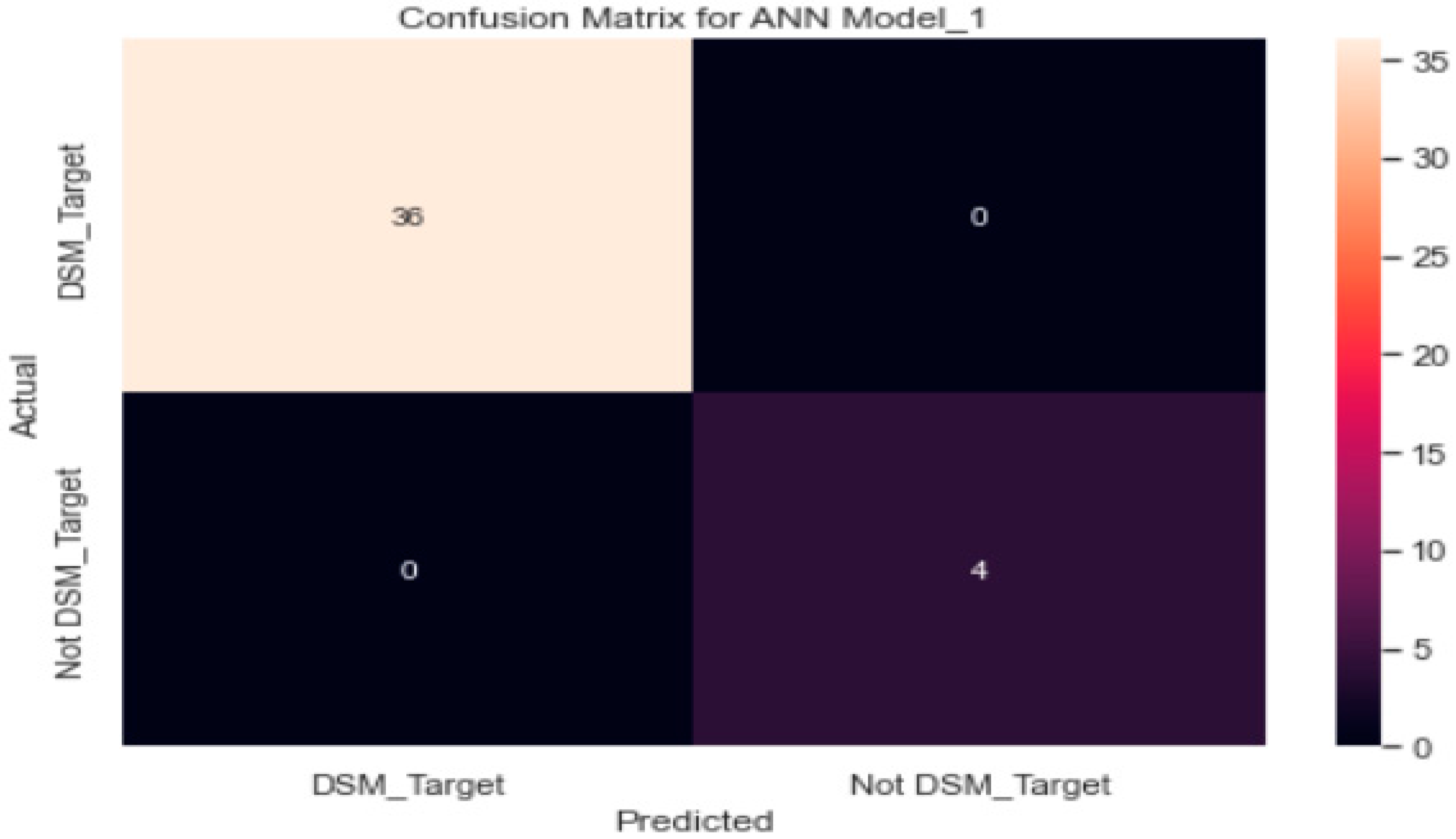
3.3.1. ANN Model_1 with SGD Optimiser
3.3.2. ANN Model_2 with Adam Optimiser
4. Discussions
4.1. Clinical Perspectives
- Data-driven precision care by AI will usher in a new era in the treatment of diabetes.
- The study has altered diabetes prognosis and self-management, which may contribute to reducing the worldwide prevalence of type 2 diabetes.
- Predictive risk stratification of populations, improved decision-making, and self-management are all made possible by artificial neural networks and machine learning.
- This research study will provide benefits to healthcare professionals in decision-making and remote monitoring of diabetes self-management activities. The precise AI application will contribute to reducing the prevalence of complications from type 2 diabetes. The AI applications will be deployed in other rural areas of Pakistan and may be extended to other countries in the sub-continent.
4.2. Strengths and Limitations
- The strength of this study is that by assessing diabetes self-management activities, AI will guide patients to improve their diabetes self-care and help health professionals in decision making and remotely monitoring patients’ activities.
- The limitation of this study is that AI poses a risk of de-skilling general practitioners due to their dependency on it. This may result in a vicious circle of inaccuracy because AI requires periodic refining by specialists [47].
- The ongoing cost, availability, and implementation of AI are obstacles to the use of AI in rural areas of Pakistan. Interoperability has been cited as a typical barrier to adopting devices and apps in diabetes self-management [47].
- The other limitation is the relatively small sample size of just 200 patients with type 2 diabetes. In diabetes self-management, a recurring difficulty is the dearth of data needed to develop rational and precise algorithms. The results of this study may not be generalised to other regions. The longer the length of future studies’ training sets, the more accurate and applicable to other regions their results will be. For digital applications to design meaningful solutions, datasets will need to be increasingly developed and organised. Concerns over security, data privacy, and regulatory issues hinder the implementation of technology in diabetes self-management.
- Using retrospective data, current AI models and applications in diabetes treatment have been validated. The prospective confirmation of these technological breakthroughs has the potential to automate diabetes care [48]. To include digital biomarkers and data from applications, monitors, and activity trackers, endpoints in clinical research will need to be reformulated.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayan, K. The Diabetes Pandemic: Looking for the silver lining. Clin. Diabetes 2005, 23, 51–52. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://www.diabetesatlas.org/ (accessed on 20 June 2021).
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. Available online: http://www.idf.org/home/index.cfm (accessed on 10 May 2020). [CrossRef]
- World Health Organization. STEPS Surveillance Manual: WHO STEP Wise Approach to Chronic Disease Risk Factor Surveillance; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Jafar, T.H.; Chaturvedi, N.; Pappas, G. Prevalence of overweight and obesity and their association with hypertension and diabetes mellitus in an Indo-Asian population. CMAJ 2006, 175, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, R.M. Effect of Physical Activity and Obesity on Type 2 Diabetes in a Middle-Aged Population. J. Environ. Public Health 2009, 2009, 195285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, M.H.; Auerbach, S.B.; Cook, S.; Harrison, J.F.; Koppert, J.; Jin, L.; Thiel, F.; Karrison, T.G.; Harrand, A.G.; Schaefer, C.T.; et al. Quality of diabetes care in community health centers. Am. J. Public Health 2000, 90, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Mensing, C.; Boucher, J.; Cypress, M.; Weinger, K.; Mulcahy, K.; Barta, P.; Hosey, G.; Kopher, W.; Lasichak, A.; Lamb, B.; et al. National standards for diabetes self-management education. Diabetes Care 2007, 2007 (Suppl. 1), S96–S103. [Google Scholar] [CrossRef] [Green Version]
- Norris, S.L.; Lau, J.; Smith, S.J.; Schmid, C.H.; Engelgau, M.M. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control. Diabetes Care 2002, 25, 1159–1171. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, R.E.; Strycker, L.A.; Toobert, D.J.; Eakin, E.G. The Chronic Illness Resources Surveys: A social-ecologic approach to assessing support for disease self-management. J. Behav. Med. 2000, 23, 559–583. [Google Scholar] [CrossRef]
- Panesar, A. Machine Learning and AI for Healthcare: Big Data for Improved Health Outcomes, 2nd ed.; Apress: Berkeley, CA, USA, 2021; Available online: https://savonia.finna.fi/Record/savonia.994859365406248 (accessed on 10 January 2023).
- Nomura, A.; Noguchi, M.; Kometani, M.; Furukawa, K.; Yoneda, T. Artificial Intelligence in Current Diabetes Management and Prediction. Curr. Diabetes Rep. 2021, 21, 61. [Google Scholar] [CrossRef]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef]
- Stojanov, D.; Lazarova, E.; Veljkova, E.; Rubartelli, P.; Giacomini, M. Predicting the outcome of heart failure against chronic-ischemic heart disease in elderly population—Machine learning approach based on logistic regression, case to Villa Scassi hospital Genoa, Italy. J. King Saud Univ. Sci. 2023, 35, 102573. [Google Scholar] [CrossRef]
- Edlitz, Y.; Segal, E. Prediction of type 2 diabetes mellitus onset using logistic regression-based scorecards. Elife 2022, 11, e71862. [Google Scholar] [CrossRef] [PubMed]
- Alzubi, A.; Najadat, H.; Doulat, W.; Al-Shari, O.; Zhou, L. Predicting the recurrence of breast cancer using machine learning algorithms. Multimed. Tools Appl. 2021, 80, 13787–13800. [Google Scholar] [CrossRef]
- Elsayad, A.M.; Nassef, A.M.; Al-Dhaifallah, M. Diagnosis of Hepatitis Disease with Logistic Regression and Artificial Neural Networks. J. Comput. Sci. 2020, 16, 364–377. [Google Scholar] [CrossRef]
- Meraihi, Y.; Gabis, A.B.; Mirjalili, S.; Ramdane-Cherif, A.; Alsaadi, F.E. Machine Learning-Based Research for COVID-19 Detection, Diagnosis, and Prediction: A Survey. SN Comput. Sci. 2022, 3, 286. [Google Scholar] [CrossRef] [PubMed]
- Rahimloo, P.; Jafarian, A. Prediction of Diabetes by Using Artificial Neural Network, Logistic Regression Statistical Model and Combination of Them. Bull. Soc. R. Sci. Liege 2016, 85, 1148–1164. [Google Scholar] [CrossRef]
- Karan, O.; Bayraktar, C.; Karlık, B. Diagnosing diabetes using neural networks devices. Expert Syst. Appl. 2012, 39, 54–60. [Google Scholar] [CrossRef]
- Choubey, D.K.; Paul, S.; Kumar, S. Classification of Pima Indian diabetes dataset using naive Bayes with the genetic algorithm as an attribute selection. In Communication and Computing Systems: Proceedings of the International Conference on Communication and Computing System (ICCCS 2016), Gurgaon, India, 9–11 September 2016; CRC Press: Boca Raton, FL, USA, 2016; pp. 451–455. [Google Scholar]
- Contreras, I.; Vehi, J. Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef]
- Nawi, N.M.; Atomi, W.H.; Rehman, M.Z. The effect of data pre-processing on optimized training of artificial neural networks. Procedia Technol. 2013, 11, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Boos, C.F.; Scolaro, G.R.; Pereira, M.C.V.; Azevedo, F.M. Analysis of Pre-Processing Methods for Artificial Neural Network Pattern Recognition of EEG Signals. IFMBE Proc. 2013, 39, 558–561. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Umair, M.; Khan, M.A.; Choudry, M.A. GA backing to STBC based MC-CDMA systems. In Proceedings of the Intelligent Systems Modelling & Simulation (ISMS), 2013 4th International Conference, Bangkok, Thailand, 29–31 January 2013; pp. 503–506. [Google Scholar]
- Atta, A.; Abbas, S.; Khan, M.A.; Ahmed, G.; Farooq, U. An adaptive approach: Smart traffic congestion control system. J. King Saud Univ. Comput. Inf. Sci. 2018, 32, 1012–1019. [Google Scholar] [CrossRef]
- Bukhari, M.M.; Alkhamees, B.F.; Hussain, S.; Gumaei, A.; Assiri, A.; Ullah, S.S. An improved Artificial Neural Network Model for Effective Diabetes Prediction. Complexity 2021, 10, 5525271. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Huang, J.T.; Yao, K.; Yu, D.; Seide, F.; Seltzer, M.; Zweig, G.; He, X.; Williams, J.; et al. Recent advances in deep learning for speech research at Microsoft. In Proceedings of the ICASSP 2013, 2013 IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Image net classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 1, 1097–1105. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for Stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Duchi, J.; Hazan, E.; Singer, Y. Adaptive subgradient methods for online learning and stochastic optimization. J. Mach. Learn. Res. 2011, 12, 2121–2159. [Google Scholar]
- Graves, A. Generating sequences with recurrent neural networks. arXiv 2013, arXiv:1308.0850. [Google Scholar]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the Dimensionality of Data with Neural Networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieleman, T.; Hinton, G. Lecture 6.5—RMSProp. COURSERA Neural Netw. Mach. Learn. 2012, 4, 26–31. [Google Scholar]
- Kamradt, M.; Bozorgmehr, K.; Krisam, J.; Freund, T.; Kiel, M.; Qreini, M.; Flum, E.; Berger, S.; Besier, W.; Szecsenyi, J.; et al. Assessing self-management in patients with diabetes mellitus type 2 in Germany: Validation of a German version of the Summary of Diabetes Self-Care Activities measure (SDSCA-G). Health Qual. Life Outcomes 2014, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.; Khader, Y.S.; Al-Khawaldeh, A.; Ajlouni, K. Factors associated with poor glycemic control among patients with Type 2 diabetes. J. Diabetes Complicat. 2010, 24, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Islahudin, F.; Paraidathathu, T. Factors associated with good glycemic control among patients with type 2 diabetes mellitus. J. Diabetes Investig. 2013, 5, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Savage, C.; Toobert, D.; Pan, W.; Whitmer, K. Adaptation and Testing of Instruments to Measure Diabetes Self-Management in People With Type 2 Diabetes in Mainland China. J. Transcult. Nurs. 2008, 19, 234–242. [Google Scholar]
- Zhong, X.; Tanasugarn, C.; Fisher, E.B.; Krudsood, S.; Nityasuddhi, D. Awareness and practices of self-management and influence factors among individuals with type 2 diabetes in urban community settings in Anhui Province, China. Southeast Asian J. Trop. Med. Public Health 2011, 42, 184–196. [Google Scholar] [PubMed]
- Ansari, R.M.; Hosseinzadeh, H.; Harris, M.; Zwar, N. Self-management experiences among middle-aged population of rural area of Pakistan with type 2 diabetes: A qualitative analysis. Clin. Epidemiol. Glob. Health 2019, 7, 177–183. [Google Scholar] [CrossRef] [Green Version]
- DeCoster, V.A. Challenges of Type 2 Diabetes and Role of Health Care Social Work: A Neglected Area of Practice. Health Soc. Work 2001, 26, 26–37. [Google Scholar] [CrossRef]
- Serour, M.; Alqhenaei, H.; Al-Saqabi, S.; Mustafa, A.-R.; Ben-Nakhi, A. Cultural factors and patients’ adherence to lifestyle measures. Br. J. Gen. Pract. 2007, 57, 291–295. [Google Scholar]
- Verma, M.; Paneri, S.; Badi, P.; Raman, P.G. Effect of increasing duration of diabetes mellitus Type 2 on glycated hemoglobin and insulin sensitivity. Indian J. Clin. Biochem. 2006, 21, 142–146. [Google Scholar] [CrossRef] [Green Version]
- EL-Kebbi, I.M.; Cook, C.B.; Ziemer, D.C.; Miller, C.D.; Gallina, D.L.; Phillips, L.S. Association of younger age with poor glycemic control and obesity in Urban African Americans with Type 2 diabetes. Arch. Intern. Med. 2003, 163, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Bukhsh, A.; Khan, T.M.; Nawaz, M.S.; Sajjad, H.; Chan, K.G.; Lee, L.-H.; Goh, B.-H. Association of diabetes related self-care activities with glycemic control of patients with type 2 diabetes in Pakistan. Patient Prefer. Adherence 2018, 12, 2377–2385. [Google Scholar] [CrossRef] [Green Version]
- Buch, V.; Varughese, G.; Maruthappu, M. Artificial intelligence in diabetes care. Diabet. Med. 2018, 35, 495–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagherazzi, G.; Ravaud, P. Digital diabetes: Perspectives for diabetes prevention, management and research. Diabetes Metab. 2018, 45, 322–329. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Male (n) | Female (n) | Mean ± SD | p-Value | Total | |
|---|---|---|---|---|---|---|
| Age (in years) | 51 | 53 | 51.40 ± 6.42 | 0.25 | 52 | |
| <60 years | 85 | 87 | 172 | |||
| ≥60 years | 15 | 13 | 28 | |||
| Diabetes Patients | 100 | 100 | 0.20 | 200 | ||
| Marital Status | ||||||
| Single | 15 | 5 | 20 | |||
| Married | 75 | 85 | 160 | |||
| Divorced | 10 | 2 | 12 | |||
| Widowed | 0 | 8 | 8 | |||
| Education | ||||||
| <grade 9 | 16 | 50 | 66 | |||
| High School | 65 | 40 | 105 | |||
| College degree | 10 | 7 | 17 | |||
| Professional | 9 | 3 | 0.70 | 12 | ||
| Employment | ||||||
| Full/part time | 75 | 65 | 0.05 | 140 | ||
| Unemployed | 10 | 35 | 45 | |||
| Retired | 15 | 0 | 15 | |||
| Diabetes Duration | ||||||
| <8 years | 36 | 42 | 7.72 ± 2.38 | 78 | ||
| ≥8 years | 64 | 58 | 8.1 ± 2.30 | 0.048 | 122 | |
| HbA1c (%) | ||||||
| Uncontrolled (>7%) | 91 | 91 | 9.03 ± 1.52 | 0.050 | 182 | |
| Controlled (≤7%) | 9 | 9 | 18 | |||
| Classification | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|
| Logistic regression | 98% | 97.3% | 79% | 88% |
| Artificial neural network | ||||
| SGD optimizer | 90% | 74% | 74% | 74% |
| Adam optimiser | 100% | 100% | 100% | 100% |
| RMSprop | 84% | 82% | 81% | 82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, R.M.; Harris, M.F.; Hosseinzadeh, H.; Zwar, N. Application of Artificial Intelligence in Assessing the Self-Management Practices of Patients with Type 2 Diabetes. Healthcare 2023, 11, 903. https://doi.org/10.3390/healthcare11060903
Ansari RM, Harris MF, Hosseinzadeh H, Zwar N. Application of Artificial Intelligence in Assessing the Self-Management Practices of Patients with Type 2 Diabetes. Healthcare. 2023; 11(6):903. https://doi.org/10.3390/healthcare11060903
Chicago/Turabian StyleAnsari, Rashid M., Mark F. Harris, Hassan Hosseinzadeh, and Nicholas Zwar. 2023. "Application of Artificial Intelligence in Assessing the Self-Management Practices of Patients with Type 2 Diabetes" Healthcare 11, no. 6: 903. https://doi.org/10.3390/healthcare11060903
APA StyleAnsari, R. M., Harris, M. F., Hosseinzadeh, H., & Zwar, N. (2023). Application of Artificial Intelligence in Assessing the Self-Management Practices of Patients with Type 2 Diabetes. Healthcare, 11(6), 903. https://doi.org/10.3390/healthcare11060903







