Cretan Aging Cohort-Phase III: Methodology and Descriptive Characteristics of a Long-Term Longitudinal Study on Predictors of Cognitive Decline in Non-Demented Elderly from Crete, Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
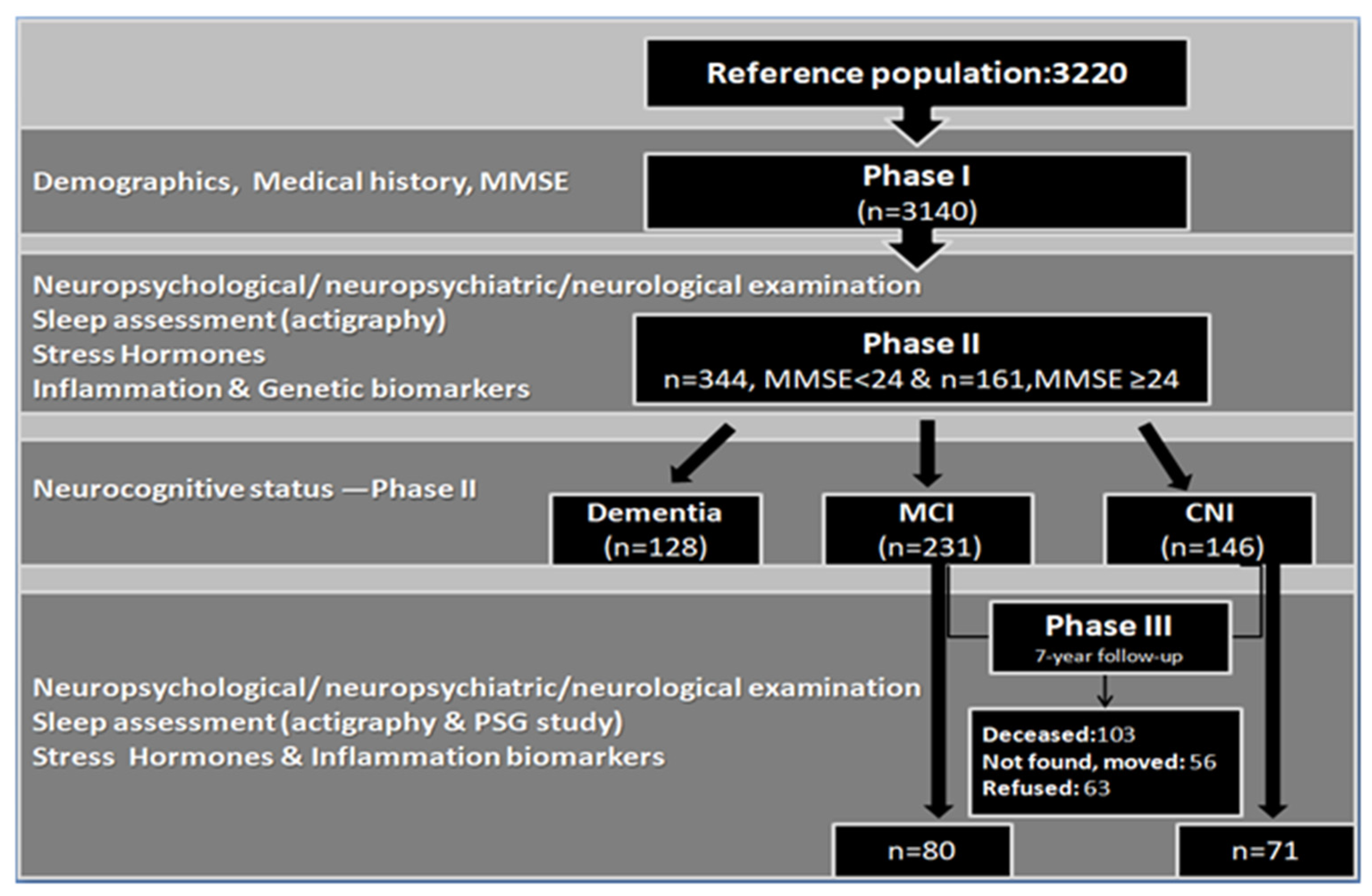
2.1.1. Phase I–Phase II
2.1.2. Phase III
2.2. Measurements
2.2.1. Sleep Measurements
- (i)
- Polysomnography (PSG)
- (ii)
- Actigraphy
2.2.2. Inflammatory Biomarkers
2.2.3. Diagnosis of Neurocognitive Impairment
- (i)
- Neuropsychological assessment
- (ii)
- Informant scales
2.2.4. Semi-Structured Interview
- -
- Current and past medical conditions, with emphasis on illnesses and operations occurring during the follow-up period, including Traumatic Brain Injury (TBI), stroke and pharmacotherapy (any type of treatment with a special focus on psychotropic substances). We then calculated total number of major medical morbidities (hypertension, diabetes, heart/pulmonary/hematological/liver diseases, gastrointestinal conditions, hyper/hypothyroidism, cancer, arthritis).
- -
- Mental morbidities (i.e., depression and anxiety diagnosis) were assessed according to the DSM-5 criteria, based on a clinical interview, neuropsychological evaluation, and existing diagnosis following the same procedures described previously [28].
- -
- Anthropometric measurements: weight, height, and Body Mass Index were assessed as previously described [8].
- -
- A frailty composite index was calculated based on level of physical activity, self-reported symptoms of exhaustion and decreased appetite, and objectively assessed upper limb weakness (using a dynamometer measurement). Frailty level was then recorded into 3 classes (absence of frailty, pre-frailty, frailty).
- -
- Overall subjective memory difficulties were assessed via a single question (“Do you have any memory problems?”), requiring a yes/no response, whereas domain-specific memory complaints (difficulty recalling recent information, words and names) were assessed using single questions requiring a binary response (WHICAP medical package: Medical Conditions and WHICAP survey).
- -
- Sleep problems: we used a shortened version of the Penn State Sleep Questionnaire comprising 12 items (answered on a 4-point Likert scale ranging from 0 = absence of symptoms to 3 = serious symptomatology) in order to assess presence and severity of self-reported sleep complaints, sleep duration and napping throughout the day (apnea, snoring, excessive movements during sleep, difficulty falling/staying asleep, early awakening, overall quality of sleep and, lastly, average night sleep duration and time required for falling asleep, as well as napping frequency and duration, if applicable) [41].
- -
- Lifestyle habits: we recorded current smoking and drinking habits (number of cigarettes if a current smoker, smoking cessation and year of quitting, as well as frequency of alcohol consumption on a daily basis). We also estimated level of physical activity during the previous week (including frequency of participation in particular activities such as gardening, housework, handiwork, shopping), as well as based on participants’ responses to the question “How many days did you walk for more than 10 min in a row in a brisk manner during the last week?”, as previously described in detail [41].
- -
- Social support and frequency of social contacts: we calculated the total number of social contacts (close relatives and friends) reported by participants during the last month, the availability of emotional and practical support, using two questions adapted from the Social Support Questionnaire–Short Form [42]: “Is there anyone you can really count on when you need help? Is there anyone you can really count on to help you feel more relaxed when you are under pressure/stress?” and the quality of perceived support (“How satisfied are you with the level of support you receive?”), answered on a 5-point Likert scale ranging from 0 (not at all) to 4 (completely satisfied).
2.2.5. Neuropsychiatric Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report. Available online: https://www.alzint.org/resource/world-alzheimer-report-2022/ (accessed on 21 September 2022).
- Daffner, K.R. Promoting Successful Cognitive Aging: A Comprehensive Review. Alzheimer’s Dis. 2010, 19, 1101–1122. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, C.; Yu, D.; Fawkes, S.; Ma, J.; Zhang, M.; Li, C. Prevalence of Mild Cognitive Impairment in Community-Dwelling Chinese Populations Aged Over 55 Years: A Meta-analysis and Systematic Review. BMC Geriatr. 2021, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.J.; Visser, P.J. Prevalence of Cerebral Amyloid Pathology in Persons without Dementia: A Meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- McGrattan, A.M.; Pakpahan, E.; Siervo, M.; Mohan, D.; Reidpath, D.D.; Prina, M.; Allotey, P.; Zhu, Y.; Shullin, C.; Yates, J.; et al. Risk of Conversion from Mild Cognitive Impairment to Dementia in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Alzheimer’s Dement. 2022, 8, e12267. [Google Scholar] [CrossRef]
- Gillis, G.; Mirzaei, F.; Potashman, M.; ArfanIkram, M.; Maserejian, N. The Incidence of Mild Cognitive Impairment. Alzheimers Dement. 2019, 11, 248–256. [Google Scholar] [CrossRef]
- Vlachos, G.S.; Kosmidis, M.H.; Yannakoulia, M.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Ntanasi, E.; Stefanis, L.; Scarmeas, N. Prevalence of Mild Cognitive Impairment in the Elderly Population in Greece: Results from the HELIAD Study. Alzheimer Dis. Assoc. Disord. 2020, 34, 156–162. [Google Scholar] [CrossRef]
- Zaganas, I.V.; Simos, P.; Basta, M.; Kapetanaki, S.; Panagiotakis, S.; Koutentaki, I.; Fountoulakis, N.; Bertsias, A.; Duijker, G.; Tziraki, C.; et al. The Cretan Aging Cohort: Cohort Description and Burden of Dementia and Mild Cognitive Impairment. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 23–33. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Li, C.; Tan, Y.; Li, G.; Xu, D.; Chen, L. Sleep Disturbance in Mild Cognitive Impairment: A Systematic Review of Objective Measures. Neurol. Sci. 2017, 38, 1363–1371. [Google Scholar] [CrossRef]
- Basta, M.; Simos, P.; Vgontzas, A.; Koutentaki, E.; Tziraki, S.; Zaganas, I.; Panagiotakis, S.; Kapetanaki, S.; Fountoulakis, N.; Lionis, C. Associations between Sleep Duration and Cognitive Impairment in Mild Cognitive Impairment. J. Sleep Res. 2019, 28, e12864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Espeland, M.A.; Brunner, R.L.; Lovato, L.C.; Wallace, R.B.; Leng, X.; Phillips, L.S.; Robinson, J.G.; Kotchen, J.M.; Johnson, K.C.; et al. Sleep Duration, Cognitive Decline and Dementia Risk in Older Women. Alzheimer’s Dement. 2016, 12, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D.; Tan, Y. A Systematic Review and Dose-Response Meta-analysis of Sleep Duration and the Occurrence of Cognitive Disorders. Sleep Breath 2018, 22, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.; Zaganas, I.; Simos, P.; Koutentaki, E.; Dimovasili, C.; Mathioudakis, L.; Bourbouli, M.; Panagiotakis, S.; Kapetanaki, S.; Vgontzas, A. Apolipoprotein E ε4 (APOE ε4) Allele is Associated with Long Sleep Duration among Elderly with Cognitive Impairment. J. Alzheimer’s Dis. 2021, 79, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Kelly, J.F.; Bennett, D.A. The APOE ε4 Allele is Associated with Incident Mild Cognitive Impairment among Community-Dwelling Older Persons. Neuroepidemiology 2010, 34, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.I.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Yee, K.L.; Sumbria, R.K. Tumor Necrosis Factor α Inhibition for Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517709278. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Brunner, E.; Kumari, M.; Shipley, M.; Elbaz, A.; Kivimaki, M. Interleukin-6 and C-Reactive Protein as Predictors of Cognitive Decline in Late Midlife. Neurology 2014, 83, 486–493. [Google Scholar] [CrossRef]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follet, H.; Kales, A.; Rousos, G.P. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance and Inflammatory Cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Stahl, S.T.; Smagula, S.F.; Rodakowski, J.; Dew, M.A.; Karp, J.F.; Albert, S.M.; Butters, M.; Gildengers, A.; Reynolds, C.F. Subjective Sleep Quality and Trajectories of Interleukin-6 in Older Adults. Am. J. Geriatr. Psychiatry 2021, 29, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.Z.; Louis, E.K.S.; Przybelski, S.A.; Morgenthaler, T.I.; Machulda, M.M.; Boeve, B.F.; Petersen, R.C.; Jack, C.R.; Graff-Radford, J.; Vemuri, P.; et al. Sleepiness in Cognitively Unimpaired Older Adults is Associated with CSF Biomarkers of Inflammation and Axonal Integrity. Front. Aging Neurosci. 2022, 14, 930315. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; Pruessner, J.C.; Hellhammer, D.H.; Pike, G.B.; Lupien, S.J. The Associations among Hippocampal Volume, Cortisol Reactivity, and Memory Performance in Healthy Young Men. Psychiatry Res. Neuroimaging 2007, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Antypa, D.; Basta, M.; Vgontzas, A.; Zaganas, I.; Panagiotakis, S.; Vogiatzi, E.; Kokosali, E.; Simos, P. The association of Basal Cortisol Levels with Episodic Memory in Older Adults is Mediated by Executive Function. Neurobiol. Learn. Mem. 2022, 190, 107600. [Google Scholar] [CrossRef]
- Hidaka, S.; Ikejima, C.; Kodama, C.; Nose, M.; Yamashita, F.; Sasaki, M.; Kinoshita, T.; Tanimukai, S.; Mizukami, K.; Takahashi, H.; et al. Prevalence of Depression and Depressive Symptoms among Older Japanese People: Comorbidity of Mild Cognitive Impairment and Depression. Int. J. Geriatr. Psychiatry 2012, 27, 271–279. [Google Scholar] [CrossRef]
- Basta, M.; Micheli, M.; Simos, P.; Zaganas, I.; Panagiotakis, S.; Koutra, K.; Krasanaki, C.; Lionis, C.; Vgontzas, A. Frequency and Risk Factors Associated with Depression in Elderly Visiting Primary Health Care (PHC) Settings: Findings from the Cretan Aging Cohort. J. Affect. Disord. Rep. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Ismail, Z.; Elbayoumi, H.; Fischer, C.E.; Hogan, D.B.; Millikin, C.P.; Schweizer, T.; Mortby, M.E.; Smith, E.E.; Patten, S.B.; Fiest, K.M. Prevalence of Depression in Patients with Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 58–67. [Google Scholar] [CrossRef]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro Costa, E.; Diniz, B.S. Depressive Symptoms Increase the Risk of Progression to Dementia in Subjects with Mild Cognitive Impairment: Systematic Review and Meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 905–911. [Google Scholar] [CrossRef]
- Geda, Y.E.; Knopman, D.S.; Mrazek, D.A.; Jicha, G.A.; Smith, G.E.; Negash, S.; Boeve, B.F.; Ivnik, R.J.; Petersen, R.C.; Pankratz, V.S.; et al. Depression, Apolipoprotein E Genotype, and the Incidence of Mild Cognitive Impairment: A Prospective Cohort Study. Arch. Neurol. 2006, 63, 435–440. [Google Scholar] [CrossRef]
- Rapp, M.A.; Schnaider-Beeri, M.; Wysocki, M.; Guerrero-Berroa, E.; Grossman, H.T.; Heinz, A.; Haroutunian, V. Cognitive Decline in Patients with Dementia as Function of Depression. Am. J. Geriatr. Psychiatry 2011, 19, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Donovan, N.J.; Locascio, J.J.; Marshall, G.A.; Gatchel, J.; Hanseeuw, B.J.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A. Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am. J. Psychiatry 2018, 175, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, Z.; Jiang, Z.; Zhou, F. Prevalence of Anxiety in Patients with Mild Cognitive Impairment: A Systematic Review and Meta-analysis. J. Affect. Disord. 2018, 236, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ma, L. Depression, Anxiety and Apathy in Mild Cognitive Impairment: Current Perspectives. Front. Aging Neurosci. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Gulpers, B.J.A. Anxiety in Older Adults: Correlates, Comorbidities and Prognosis with Lifespan Perspectives. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- McKinnon, A.C.; Beath, A.P.; Naismith, S.L. Relationships between Sleep Quality, Depressive Symptoms and MCI Diagnosis: A Path Analysis. J. Affect. Disord. 2019, 256, 26–32. [Google Scholar] [CrossRef]
- Tinios, P.; Valvis, Z. Defining Long-Term Care Need Levels for Older Adults: Towards a Standardized European Classification. J. Aging Soc. Policy 2022, 1–20. [Google Scholar] [CrossRef]
- Castro-Costa, E.; Dewey, M.; Stewart, R.; Banerjee, S.; Huppert, F.; Mendonca-Lima, C.; Bula, M.; Reisches, F.; Wancata, J.; Ritchie, K. Prevalence of Depressive Symptoms and Syndromes in Later Life in Ten European Countries: The SHARE Study. Br. J. Psychiatry 2007, 191, 393–401. [Google Scholar] [CrossRef]
- Dardiotis, E.; Kosmidis, M.H.; Yannakoulia, M.; Hadjigeorgiou, G.M.; Scarmeas, N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): Rationale, Study Design, and Cohort Description. Neuroepidemiology 2014, 43, 9–14. [Google Scholar] [CrossRef]
- Basta, M.; Belogianni, C.; Yannakoulia, M.; Zaganas, I.; Panagiotakis, S.; Simos, P.; Vgontzas, A.N. Poor Diet, Long Sleep, and Lack of Physical Activity are Associated with Inflammation among Non-Demented Community-Dwelling Elderly. Healthcare 2022, 10, 143. [Google Scholar] [CrossRef]
- Sarason, I.G.; Sarason, B.R.; Shearin, E.N.; Pierce, G.R. A Brief Measure of Social Support: Practical and Theoretical Implications. J. Soc. Pers. Relatsh. 1987, 4, 497–510. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Backman, L.; Albert, M.; Almkvist, O.; et al. Mild Cognitive Impairment- beyond Controversies, towards a Consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Int. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular Dementia: Diagnostic Criteria for Research Studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and Management of Dementia with Lewy bodies: Third Report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Fontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal Lobar Degeneration: A Consensus on Clinical Diagnostic Criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef]
- Roman, G.C. Defining dementia: Clinical Criteria for the Diagnosis of Vascular Dementia. Acta Neurol. Scand. 2002, 106, 6–9. [Google Scholar] [CrossRef]
- Peppou, E.L.; Economou, M.; Skali, T.; Papageorgiou, C. From economic crisis to the COVID-19 pandemic crisis: Evidence from a Mental Health Helpine in Greece. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 407–409. [Google Scholar] [CrossRef]
- Arnault, L.; Jusot, F.; Renaud, T. Economic Vulnerability and Unmet Healthcare Needs among the Population Aged 50+ years during the COVID-19 Pandemic in Europe. Eur. J. Ageing 2021, 19, 811–825. [Google Scholar] [CrossRef]
- Cheng, Y.; Thorpe, L.; Kabir, R.; Lim, H.J. Latent Class Growth Modeling of Depression and Anxiety in Older Adults: An 8-year Follow-up of a Population-Based Study. BMC Geriatr. 2021, 21, 550. [Google Scholar] [CrossRef]
- De Beurs, E.; Beekman, A.; Geerlings, S.; Deeg, D.; Van Dyck, R.; Van Tilburgh, W. On Becoming Depressed or Anxious in Late Life: Similar Vulnerability Factors but Different Effects of Stressful Life Events. Br. J. Psychiatry 2001, 179, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Saz, P.; Marcos, G.; Dias, J.L.; De-la-Camara, C.; Ventura, T.; Montanes, J.A.; Lobo-Escolar, A.; Aznar, S.; the ZARADEMP workgroup. The ZARADEMP Project on the Incidence, Prevalence, and Risk Factors of Dementia (and Depression) in the Elderly Community: Methods and First Results. Eur. J. Psychiatry 2005, 19, 40–54. [Google Scholar] [CrossRef]
| KERRYPNX | CNI (n = 71) | MCI (n = 80) | MCI vs. CNI | MCI vs. CNI | ||
|---|---|---|---|---|---|---|
| Phase II | Phase III | Phase II | Phase III | (Phase II) | (Phase III) | |
| Age (years) | 70.48 (6.31) | 78.32 (6.16) * | 75.03 (6.34) | 83.30 (6.27) † | <0.001 | <0.001 1 |
| Gender (Female, (%)) | 55 (77.5) | 62 (77.5) | 0.9 2 | |||
| RuralResidence (%) | 59 (83.1) | 68 (85.0) | 0.7 | |||
| Body Mass Index | 31.22 (4.22) | 31.10 (5.89) | 30.12 (4.55) | 30.05 (5.95) | 0.07 | 0.3 |
| Living alone (%) | 17 (23.2) | 23 (32.4) * | 16 (20.0) | 18 (22.5) | 0.6 | 0.2 |
| No of Illnesses | 2.55 (1.62) | 3.28 (1.62) * | 2.49 (1.37) | 3.18 (1.50) † | 0.8 | 0.5 |
| Education (years) | 5.49 (3.23) | 4.70 (2.55) | 0.06 | |||
| Previous occupation (%) | 0.6 | |||||
| Housekeeping | 13 (18.3) | 22 (27.5) | ||||
| Farmer | 28 (39.4) | 33 (41.2) | ||||
| Worker | 7 (9.9) | 9 (11.2) | ||||
| Technician | 1 (1.4) | 1 (1.3) | ||||
| Employee | 11 (15.5) | 6 (7.5) | ||||
| Self-employed | 9 (12.7) | 8 (10.0) | ||||
| Teacher | 2 (2.8) | 1 (1.3) | ||||
| Dementia Family history (%) | 20 (28.2) | 24 (30.0) | 0.8 | |||
| APOE ε4 allele (%) | 6 (8.5) | 19 (24.4) | 0.04 | |||
| Smoking (%) | 7 (9.9) | 6 (8.6) * | 3 (3.8) | 2 (2.5) † | 0.1 | 0.1 |
| Alcohol use (%) | 21 (29.6) | 13 (18.8) * | 35 (43.6) | 16 (20.0) † | 0.08 | 0.8 |
| CNI (n = 71) | MCI (n = 80) | MCI vs. CNI | MCI vs. CNI | |||
|---|---|---|---|---|---|---|
| PhaseII | Phase III | PhaseII | Phase III | (Phase II) | (Phase III) | |
| HADS-A subscale score | 3.57 (3.71) | 5.83 (4.28) * | 2.81 (3.09) | 4.79 (3.63) † | 0.5 | 0.2 1 |
| GDS score | 3.84 (3.62) | 3.59 (2.88) | 3.93 (3.07) | 3.58 (3.05) | 0.8 | 0.9 |
| Depression Diagnosis (%) | 20 (28.2) | 26 (36.6) * | 27 (33.8) | 30 (37.5) | 0.5 | 0.9 2 |
| Anxiety Diagnosis (%) | 19 (26.8) | 26 (36.6) | 26 (32.5) | 20 (25.0) | 0.4 | 0.1 |
| Psychotropic medication use (%) 3 | 26 (36.6) | 32 (45.1) * | 21 (26.3) | 36 (45.6) † | 0.2 | 0.9 |
| Persistent Depression (%) 4 | 15 (21.1) | 14 (17.5) | 0.6 | |||
| Major stressful events (%) 4 | 21 (29.6) | 30 (37.5) | 0.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basta, M.; Skourti, E.; Alexopoulou, C.; Zampetakis, A.; Ganiaris, A.; Aligizaki, M.; Simos, P.; Vgontzas, A.N. Cretan Aging Cohort-Phase III: Methodology and Descriptive Characteristics of a Long-Term Longitudinal Study on Predictors of Cognitive Decline in Non-Demented Elderly from Crete, Greece. Healthcare 2023, 11, 703. https://doi.org/10.3390/healthcare11050703
Basta M, Skourti E, Alexopoulou C, Zampetakis A, Ganiaris A, Aligizaki M, Simos P, Vgontzas AN. Cretan Aging Cohort-Phase III: Methodology and Descriptive Characteristics of a Long-Term Longitudinal Study on Predictors of Cognitive Decline in Non-Demented Elderly from Crete, Greece. Healthcare. 2023; 11(5):703. https://doi.org/10.3390/healthcare11050703
Chicago/Turabian StyleBasta, Maria, Eleni Skourti, Christina Alexopoulou, Alexandros Zampetakis, Andronikos Ganiaris, Marina Aligizaki, Panagiotis Simos, and Alexandros N. Vgontzas. 2023. "Cretan Aging Cohort-Phase III: Methodology and Descriptive Characteristics of a Long-Term Longitudinal Study on Predictors of Cognitive Decline in Non-Demented Elderly from Crete, Greece" Healthcare 11, no. 5: 703. https://doi.org/10.3390/healthcare11050703
APA StyleBasta, M., Skourti, E., Alexopoulou, C., Zampetakis, A., Ganiaris, A., Aligizaki, M., Simos, P., & Vgontzas, A. N. (2023). Cretan Aging Cohort-Phase III: Methodology and Descriptive Characteristics of a Long-Term Longitudinal Study on Predictors of Cognitive Decline in Non-Demented Elderly from Crete, Greece. Healthcare, 11(5), 703. https://doi.org/10.3390/healthcare11050703








