Treatment of Far-Advanced Otosclerosis: Stapedotomy Plus Hearing Aids to Maximize the Recovery of Auditory Function—A Retrospective Case Series
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
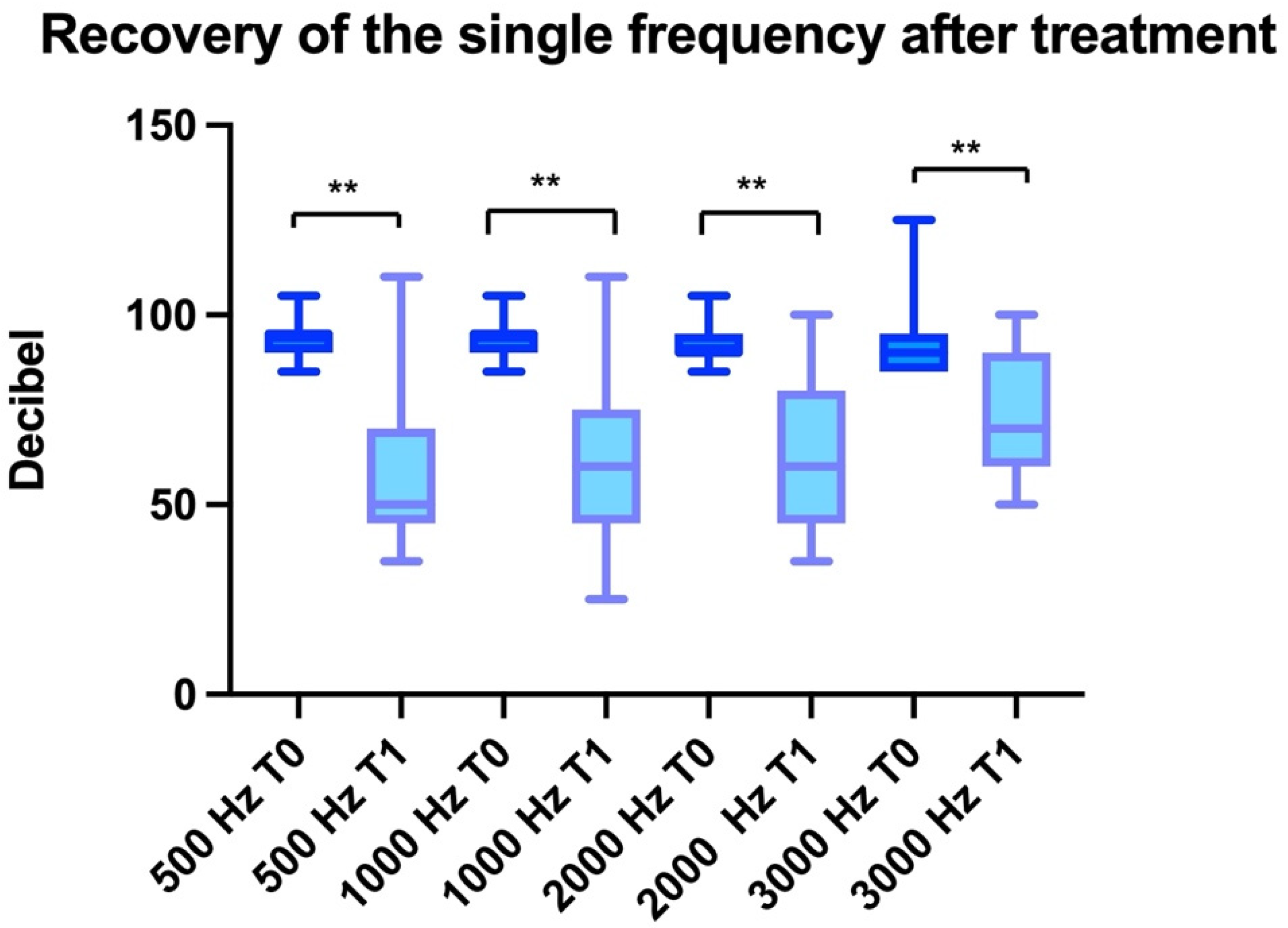
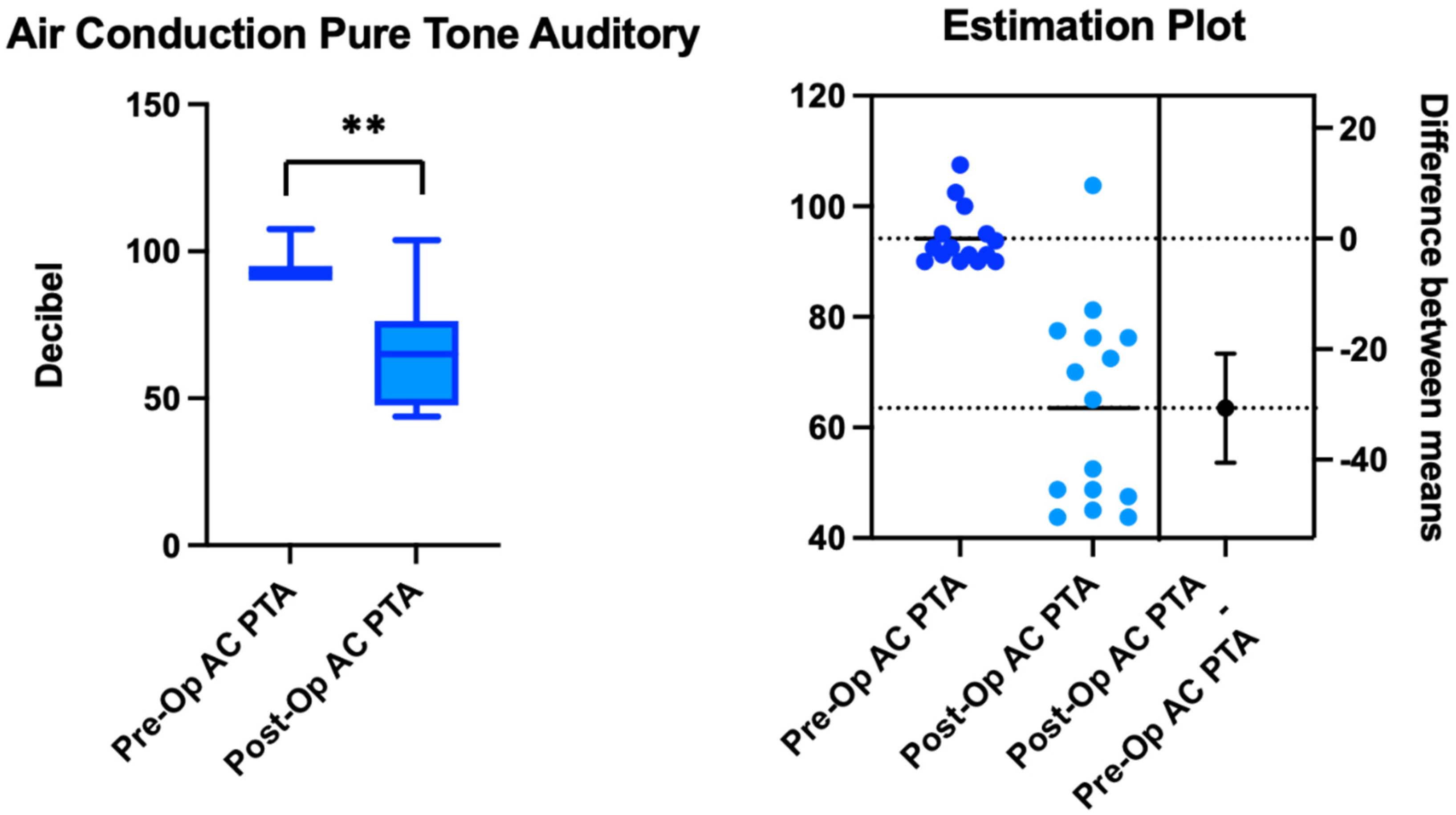
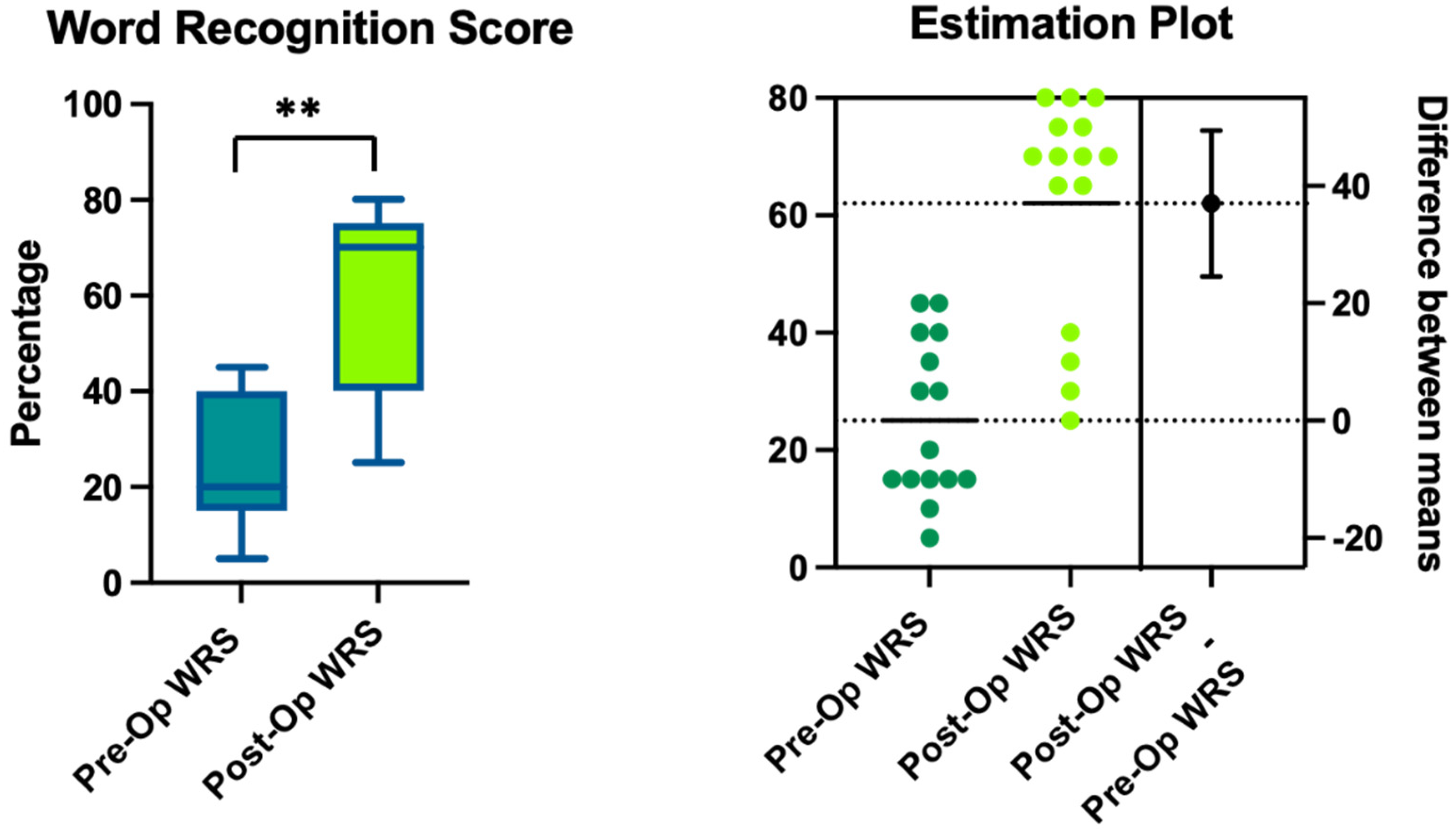
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eshragi, A.A.; Kadri, I.; Ocak, E.; Telischi, F.F. Advanced otosclerosis. Stapes surgery or cochlear implantation? Otolaryngol. Clin. N. Am. 2018, 51, 429–440. [Google Scholar]
- Liselotte, J.C.; David, W.P.; Richard, T.R. Cochlear implantation in 53 patients with otosclerosis: Demographics, computed tomographic scanning, surgery and complications. Otol. Neurotol. 2004, 25, 943–952. [Google Scholar]
- House, H.P.; Sheehy, J.L. Stapes surgery: Selection of the patient. Ann. Otol. Rhinol. Laryngol. 1961, 70, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Gillard, D.M.; Harris, J.P. Cost-effectiveness of Stapedectomy vs Hearing Aids in the Treatment of Otosclerosis. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 42–48. [Google Scholar] [CrossRef]
- Merkus, P.; Val Loon, M.V.; Smit, C.F.; De Cock, A.F.C.; Hensen, E.F. Decision making in advanced otosclerosis: An evidence-based strategy. Laringoscope 2011, 121, 1935–1941. [Google Scholar] [CrossRef]
- Kabbara, B.; Gauche, C.; Valmels, N.M.; Lepage, B.; Escude, B.; Deguine, O.; Fraysse, B.; Marx, M. Decisive criteria between stapedotomy and cochlear implantation in patients with far advanced otosclerosis. Otol. Neurotol. 2015, 36, 73–78. [Google Scholar] [CrossRef]
- Bajin, M.D.; Ergun, O.; Cinar, B.; Sennaroglu, L. Management of far-advanced otosclerosis: Stapes surgery or cochlear implant. Turk Arch. Otorhinolaryngol. 2020, 58, 35–40. [Google Scholar] [CrossRef]
- Moscillo, L.; Massimilla, E.A.; Mastella, A.; Nunziata, M.; Donadio, A.; Motta, G. Stapes Surgery in Far-Advanced Otosclerosis. Ear Nose Throat 2021, 1455613211013093. [Google Scholar]
- Verhaert, N.; Borgers, C.; De Voecht, K.; Boon, E.; Desloovere, C. A role for acoustic stimulation in advanced otosclerosis: Direct acoustic cochlear implant versus cochlear implant. Audio Neuro 2018, 23, 89–97. [Google Scholar] [CrossRef]
- Calmels, M.N.; Viana, C.; Wanna, G.; Marx, M.; James, C.; Deguine, O.; Fraysse, B. Very far-advanced otosclerosis: Stapedotomy or cochlear implantation. Acta Otolaryngol. 2007, 127, 574–578. [Google Scholar] [CrossRef]
- Messineo, D.; Ralli, M.; Greco, A.; Di Stadio, A. Double Ring in Cochlear Otosclerosis: A Limit to Cochlear Implantation? The Solution Is the Surgical Approach. Ear Nose Throat J. 2021, 100 (Suppl. S3), 235S–237S. [Google Scholar] [CrossRef]
- Lachance, S.; Bussieres, R.; Coté, M. Stapes surgery in profound hearing loss due to otosclerosis. Otol. Neurotol. 2012, 33, 721–723. [Google Scholar] [CrossRef]
- Ruckenstein, M.J.; Rafter, K.O.; Montes, M.; Bigelow, D.C. Management of far-advanced otosclerosis in the era of cochlear implantation. Otol. Neuro 2001, 22, 471–474. [Google Scholar] [CrossRef]
- Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol. Head Neck Surg. 1995, 113, 186–187. [CrossRef]
- Di Stadio, A.; Volpe, A.D.; Ralli, M.; Korsch, F.; Greco, A.; Ricci, G. Spiral Ganglions and Speech Perception in the Elderly. Which Turn of the Cochlea is the More Relevant? A Preliminary Study on Human Temporal Bones. J. Int. Adv. Otol. 2020, 16, 318–322. [Google Scholar] [CrossRef]
- Ricci, G.; Lapenna, R.; Gambacorta, V.; Faralli, M.; Di Stadio, A. OTOPLAN, Cochlear Implant, and Far-Advanced Otosclerosis: Could the Use of Software Improve the Surgical Final Indication? J. Int. Adv. Otol. 2022, 18, 74–78. [Google Scholar] [CrossRef]
- Van Loon, M.C.; Merkus, P.; Smit, C.F.; Smits, C.; Witte, B.I.; Hensen, E.F. Stapedotomy in cochlear candidates with far advanced otosclerosis: A systematic review of the literature and meta-analysis. Otol. Neurotol. 2014, 35, 1707–1714. [Google Scholar] [CrossRef]
- Teaima, A.A.; Elnashar, A.A.; Hakim, E.K.; Hadaey, H.S. Comparison of the efficacy of cochlear implantation and stapes surgery in far advanced otosclerosis: A meta-analysis study. Eur. Arch. OtoRhinoLaryngol. 2022, 280, 77–88. [Google Scholar] [CrossRef]
- Ricci, G.; Gambacorta, V.; Lapenna, R.; Della Volpe, A.; La Mantia, I.; Ralli, M.; Di Stadio, A. The effect of female hormone in otosclerosis. A comparative study and speculation about their effect on the ossicular chain based on the clinical results. Eur. Arch. Otorhinolaryngol. 2022, 279, 4831–4838. [Google Scholar] [CrossRef]
- Shea, P.F.; Ge, X.; Shea, J.J., Jr. Stapedectomy for far-advanced otosclerosis. Am. J. Otol. 1999, 20, 425–429. [Google Scholar]
- Redfors, Y.D.; Moller, C. Otosclerosis: Thirty-year follow-up after surgery. Ann. Otol. Rhinol. Laryngol. 2011, 120, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Abdurehim, Y.; Lehmann, A.; Zeitouni, A.G. Stapedotomy vs cochlear implantation for advanced otosclerosis: Systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2016, 155, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Rotteveel, L.J.; Snik, A.F.; Cooper, H.; Mawman, D.J.; Van Olphen, A.F.; Mylanus, E.A. Speech perception after cochlear implantation in 53 patients with otosclerosis: Multicenter results. Audiol. Neurotol. 2010, 15, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.; Garcia-Valdecasas, J.; Garofano, M.; Ballesteros, J.M. Otosclerosis: Mid-term results of cochlear implantation. Audiol. Neurootol. 2007, 12, 401–406. [Google Scholar] [CrossRef]
- Vashishth, A.; Fulcheri, A.; Rossi, G.; Prasad, S.C.; Caruso, A.; Sanna, M. Cochlear implantation in otosclerosis: Surgical and auditory outcomes with a brief on facial nerve stimulation. Otol. Neurotol. 2017, 38, 345353. [Google Scholar] [CrossRef]
- Nadol, J.B.; Young, Y.-S.; Glynn, R.J. Survival of Spiral Ganglion Cells in Profound Sensorineural Hearing Loss: Implications for Cochlear Implantation. Ann. Otol. Rhinol. Laryngol. 1989, 98, 411–416. [Google Scholar] [CrossRef]
- Free, R.H.; Falcioni, M.; Di Trapani, G.; Giannuzzi, A.L.; Russo, A.; Sanna, M. The Role of Subtotal Petrosectomy in Cochlear Implant Surgery–A Report of 32 Cases and Review on Indications. Otol. Neurotol. 2013, 34, 1033–1040. [Google Scholar] [CrossRef]
- Polo, R.; Del Mar Medina, M.; Arístegui, M.; Lassaletta, L.; Gutierrez, A.; Aránguez, G.; Prasad, S.C.; Alonso, A.; Gavilán, J.; Sanna, M. Subtotal Petrosectomy for Cochlear Implantation: Lessons Learned After 110 Cases. Ann. Otol. Rhinol. Laryngol. 2016, 125, 485–494. [Google Scholar] [CrossRef]
- Seeman, M.T.; Gehani, N.C.; Tummala, N.; Coughlan, C.; Fares, S.A.; Hsu, D.P.; Murray, G.S.; Lippy, W.H.; Megerian, C.A. Cochlear implantation outcomes in patients with far advanced otosclerosis. Am. J. Otolaryngol. 2012, 33, 608–614. [Google Scholar] [CrossRef]
- Castillo, F.; Polo, R.; Gutierrez, A.; Reyes, P.; Royuela, A.; Alonso, A. Cochlear implantation outcomes in advanced otosclerosis. Am. J. Otol. 2014, 35, 558–564. [Google Scholar] [CrossRef]
- Calvino, M.; Sanchez-Cuadrado, I.; Gavilan, J.; Lassalletta, L. Cochlear implant users with otosclerosis: Are hearing and quality of life outcomes worse than in cochlear implant users without otosclerosis? Audiol. Neurotol. 2019, 23, 345–355. [Google Scholar] [CrossRef]
- Munoz-Fernandez, N.; Morant-Ventura, A.; Achiques, M.T.; Dualde-Beltrán, D.; Garcia-Callejo, F.J.; Monrroy-Parada, M.V.; Pitarch, I.; Latorre, E.; Marco-Algarra, J. Evolution of otosclerosis to cochlear implantation. Acta Otorrinolaringol. Esp. 2012, 63, 265–271. [Google Scholar] [CrossRef]
- Assiri, M.; Khurayzi, T.; Alshalan, A.; Alsanosi, A. Cochlear implantation among patients with otosclerosis: A Systematic review of clinical characteristics and outcomes. Eur. Arch. OtoRhinoLryngol. 2022, 279, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Ulubil, S.A.; Hodges, A.V.; Balkany, T.J. Revision cochlear implantation for facial nerve stimulation in otosclerosis. Arch. Otolaryngol. 2006, 132, 398–404. [Google Scholar] [CrossRef]
- Matterson, A.G.; O’Leary, S.; Pinder, D.; Freidman, L.; Dowell, R.; Briggs, R. Otosclerosis: Selection of ear for cochlear implantation. Otol. Neurotol. 2007, 28, 438–446. [Google Scholar] [CrossRef]
- Berrettini, S.; Burdo, S.; Forli, F.; Ravecca, F.; Marcaccini, M.; Casani, A.P.; Franceschini, S.S. Far-advanced otosclerosis: Stapes surgery or cochlear implantation? J. Otolaryngol. 2004, 33, 165–171. [Google Scholar] [CrossRef]
- Heining, C.; Banga, R.; Irving, R.; Coulson, C.; Monksfield, P. Audiological outcome of stapes surgery for far advanced cochlear otosclerosis. J. Laryngol. Otol. 2017, 131, 961–964. [Google Scholar] [CrossRef]
- Andersen, S.A.; Öhman, M.C.; Sørensen, M.S. The stability of short-term hearing outcome after stapedotomy: A prospective database study. Acta Otolaryngol. 2015, 135, 871–879. [Google Scholar] [CrossRef]
- Di Stadio, A.; Messineo, D.; Ralli, M.; Roccamatisi, D.; Musacchio, A.; Ricci, G.; Greco, A. The impact of white matter hyperintensities on speech perception. Neurol. Sci. 2020, 41, 1891–1898. [Google Scholar] [CrossRef]
- Di Stadio, A.; Ralli, M.; Roccamatisi, D.; Scarpa, A.; Della Volpe, A.; Cassandro, C.; Ricci, G.; Greco, A.; Bernitsas, E. Hearing loss and dementia: Radiologic and biomolecular basis of their shared characteristics. A systematic review. Neurol. Sci. 2021, 42, 579–588. [Google Scholar] [CrossRef]
| Patient | Gender | Age | Side | AC-PTA (dB HL) T0 | AC-PTA (dB HL) T1 | WRS T0 | WRS T1 | WRS Gain |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | Right | 91.25 | 43.75 | 40 | 80 | 40 |
| 2 | M | 42 | Left | 100 | 72.5 | 30 | 70 | 40 |
| 3 | F | 64 | Left | 92.5 | 65 | 15 | 70 | 55 |
| 4 | F | 61 | Left | 90 | 43.75 | 40 | 80 | 40 |
| 5 | M | 51 | Left | 91.25 | 48.75 | 35 | 75 | 40 |
| 6 | M | 65 | Left | 107.5 | 76.25 | 15 | 65 | 50 |
| 7 | F | 52 | Left | 90 | 76.25 | 15 | 40 | 25 |
| 8 | F | 58 | Right | 92.5 | 52.5 | 45 | 75 | 30 |
| 9 | M | 57 | Right | 95 | 47.5 | 15 | 70 | 55 |
| 10 | F | 64 | Right | 90 | 70 | 20 | 65 | 45 |
| 11 | M | 48 | Left | 90 | 81.25 | 10 | 25 | 15 |
| 12 | F | 64 | Right | 91.25 | 77.5 | 15 | 35 | 20 |
| 13 | F | 70 | Right | 95 | 45 | 45 | 80 | 35 |
| 14 | M | 68 | Right | 102.5 | 103.75 | 5 | 30 | 25 |
| 15 | F | 72 | Right | 93.75 | 48.75 | 30 | 70 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, G.; Ferlito, S.; Gambacorta, V.; Faralli, M.; De Luca, P.; Di Giovanni, A.; Di Stadio, A. Treatment of Far-Advanced Otosclerosis: Stapedotomy Plus Hearing Aids to Maximize the Recovery of Auditory Function—A Retrospective Case Series. Healthcare 2023, 11, 676. https://doi.org/10.3390/healthcare11050676
Ricci G, Ferlito S, Gambacorta V, Faralli M, De Luca P, Di Giovanni A, Di Stadio A. Treatment of Far-Advanced Otosclerosis: Stapedotomy Plus Hearing Aids to Maximize the Recovery of Auditory Function—A Retrospective Case Series. Healthcare. 2023; 11(5):676. https://doi.org/10.3390/healthcare11050676
Chicago/Turabian StyleRicci, Giampietro, Salvatore Ferlito, Valeria Gambacorta, Mario Faralli, Pietro De Luca, Alfredo Di Giovanni, and Arianna Di Stadio. 2023. "Treatment of Far-Advanced Otosclerosis: Stapedotomy Plus Hearing Aids to Maximize the Recovery of Auditory Function—A Retrospective Case Series" Healthcare 11, no. 5: 676. https://doi.org/10.3390/healthcare11050676
APA StyleRicci, G., Ferlito, S., Gambacorta, V., Faralli, M., De Luca, P., Di Giovanni, A., & Di Stadio, A. (2023). Treatment of Far-Advanced Otosclerosis: Stapedotomy Plus Hearing Aids to Maximize the Recovery of Auditory Function—A Retrospective Case Series. Healthcare, 11(5), 676. https://doi.org/10.3390/healthcare11050676








