Detection of Adverse Drug Reactions in COVID-19 Hospitalized Patients in Saudi Arabia: A Retrospective Study by ADR Prompt Indicators
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Population
2.2. Selection of “ADR Prompt Indicators”
2.3. Active Data Collection and Surveillance
2.4. Causality Assessment of ADRs
2.5. Pilot Study
2.6. Comprehensive Correlation between ADRs and Characteristics of the Patients
2.7. Determination of Preventable ADRs
2.8. Identification of the Severity of ADRs
2.9. Statistical Analysis
3. Results
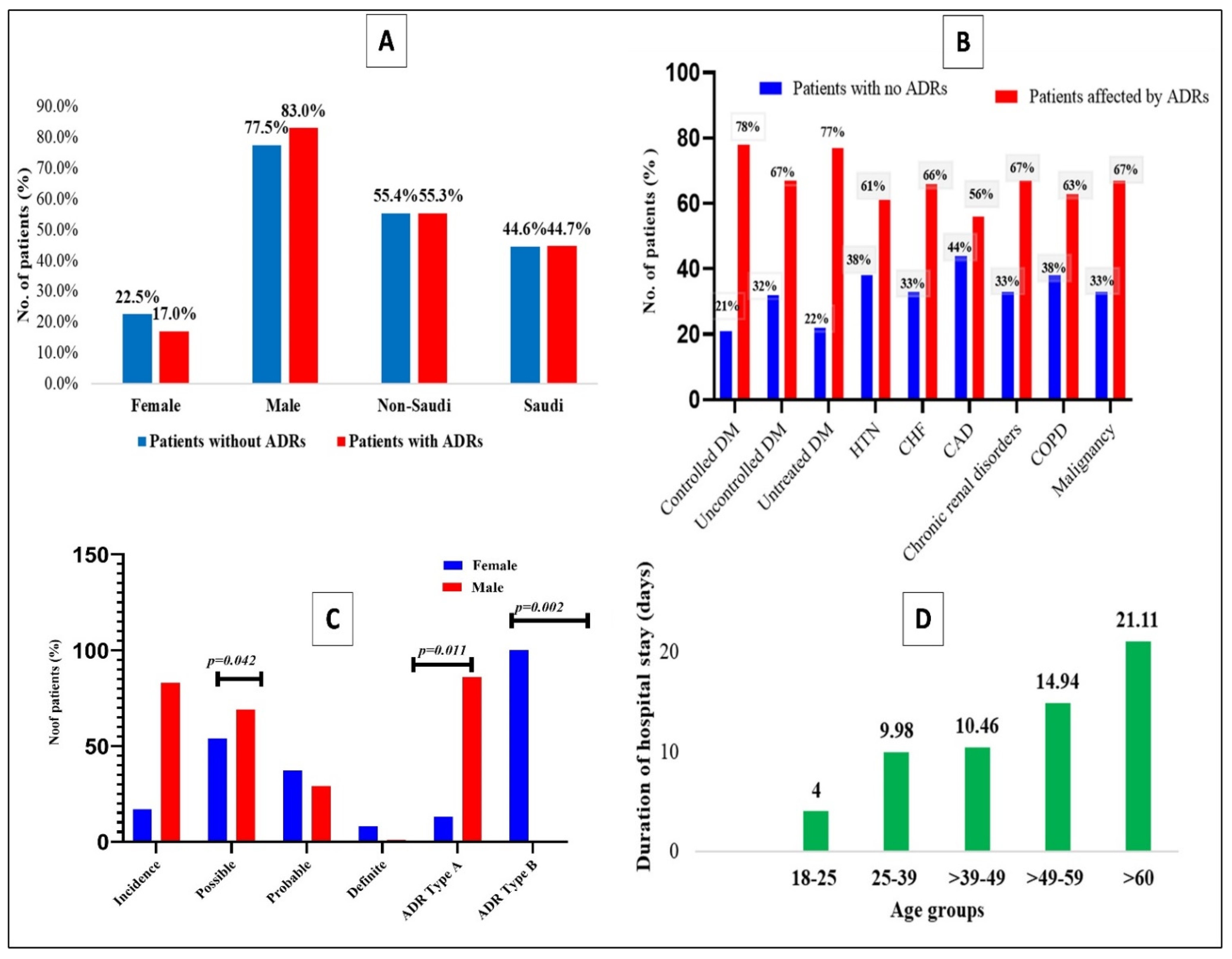
3.1. Demographic Characteristics of Patients
3.2. Clinical Characteristics of Patients of Those with and without ADRs
3.3. Influence of Comorbidities over the Development of ADRs among the COVID-19 Patients
3.4. Identification of ADRs Using Medications and Laboratory Prompt Indicators
3.5. Causality, Incidence, and Type of Adverse Drug Reactions
3.6. Adverse Drug Reaction (ADR) with Regards to Age in the Current Study
3.7. The Organs and Systems Involved in Patients with ADRs
3.8. Potentiality of the Most Common Drugs Involved in ADRs of COVID-19 Patients
3.9. Association of ADRs and Medications Used for COVID-19
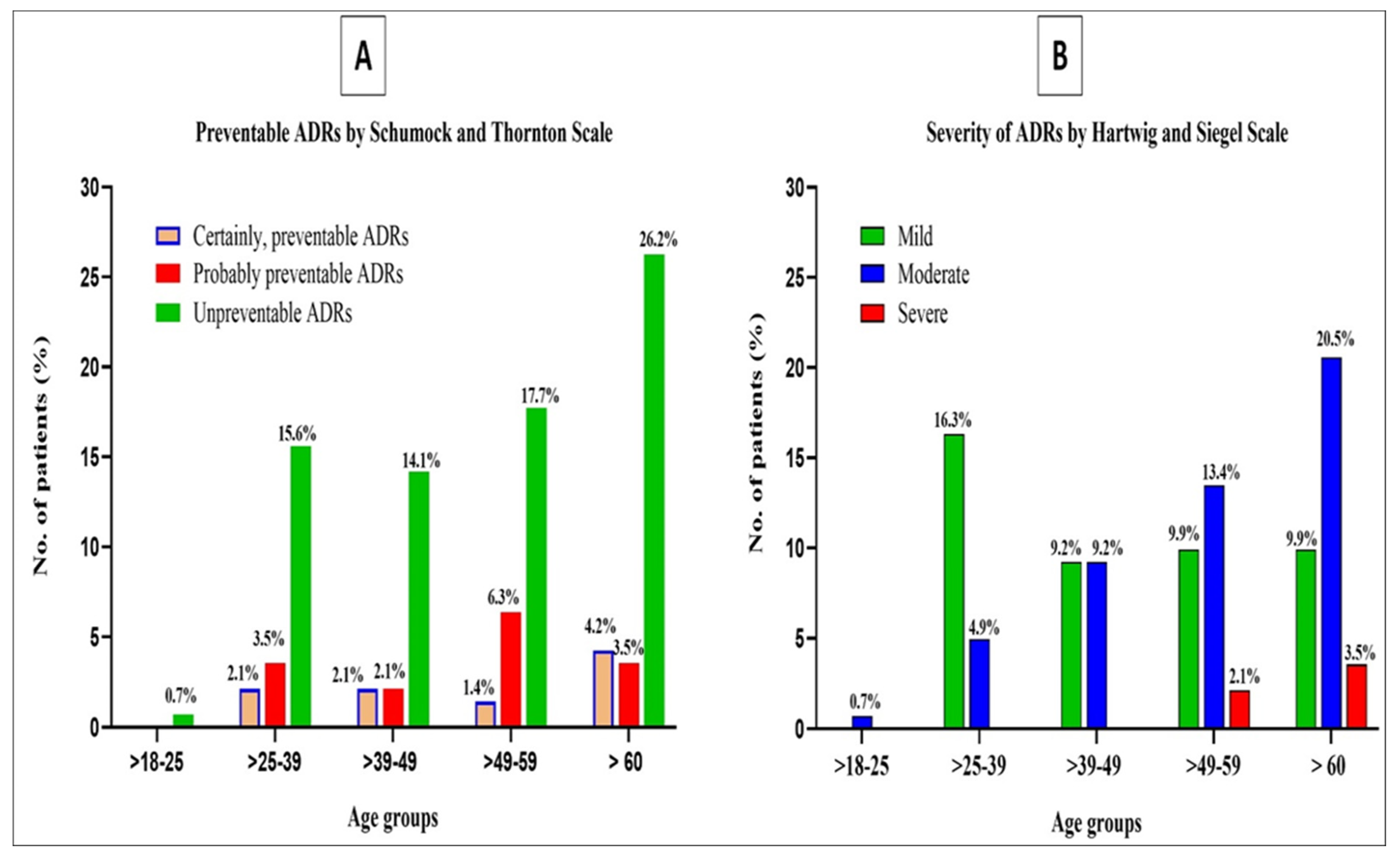
3.10. Preventability and Severity of ADRs
4. Discussion
4.1. Characteristics of the Patients
4.2. Relationship of Age to the Development of ADRs
4.3. Drug-Induced ADRs in Hospitalized Patients of COVID-19 Are Directly Proportional to the Total Amount of Drug Intake and Simultaneously Prolonged Hospitalization
4.4. The Impact of Comorbidities on the Development of ADRs in COVID-19 Patients
4.5. Relationship of Medication and Laboratory Prompt Indicators in Hospitalized ADRs Due to COVID-19
4.6. Causality Assessment of ADRs in the Current Study
4.7. Organs and Systems Involved in Hospitalized ADR in COVID-19 Patients
4.8. Potentiality of the Most Common Drugs Involved in ADRs of Patients Treated for COVID-19
4.9. Relation between ADRs and Medications Used for Treatment of COVID-19
4.10. Preventability and Severity of ADRs
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ramírez, E.; Urroz, M.; Rodríguez, A.; González-Muñoz, M.; Martín-Vega, A.; Villán, Y.; Seco, E.; Monserrat, J.; Frías, J.; Carcas, A.J.; et al. Incidence of suspected serious adverse drug reactions in coronavirus disease-19 patients detected by a pharmacovigilance program by laboratory signals in a tertiary hospital in Spain: Cautionary Data. Front. Pharmacol. 2020, 11, 602841. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.G.; Caxito, S.M.; Xavier, A.R.E.; Xavier, M.A.; Brandão, F. COVID-19: Therapeutic approaches description and discussion. An. Acad. Bras. Ciênc. 2020, 92, e20200466. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 16 May 2020).
- Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 November 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard in Saudi Arabia. Available online: https://covid19.who.int/region/emro/country/sa (accessed on 14 November 2022).
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Britannica, The Editors of Encyclopaedia. “COVID-19”. Encyclopedia Britannica. Available online: https://www.britannica.com/science/COVID-19 (accessed on 21 February 2023).
- Majmundar, N.; Ducruet, A.; Prakash, T.; Nanda, A.; Khandelwal, P. Incidence, Pathophysiology, and Impact of Coronavirus Disease 2019 (COVID-19) on Acute Ischemic Stroke. World Neurosurg. 2020, 142, 523–525. [Google Scholar] [CrossRef]
- Mongia, A.; Saha, S.K.; Chouzenoux, E.; Majumdar, A. A computational approach to aid clinicians in selecting anti-viral drugs for COVID-19 trials. Sci. Rep. 2021, 11, 9047. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Hashimoto, K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020, 87, 59–73. [Google Scholar] [CrossRef]
- Rodrigues, L.; Cunha, R.B.; Vassilevskaia, T.; Viveiros, M.; Cunha, C. Drug Repurposing for COVID-19: A Review and a Novel Strategy to Identify New Targets and Potential Drug Candidates. Molecules 2022, 27, 2723. [Google Scholar] [CrossRef]
- Serafin, M.B.; Bottega, A.; Foletto, V.S.; da Rosa, T.F.; Hörner, A.; Hörner, R. Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105969. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, W.; Simeonov, A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2018, 175, 181–191. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Q.; Li, L. Pharmacological care strategy for antivirals in patients with COVID-19 complicated by underlying disorders. Chin. J. Hosp. Pharm. 2020, 40, 612–616. [Google Scholar]
- Osorio, T.L.; Rivera, C.M.; Pino-Marín, D.; Giraldo, N.; Amariles, P. Relevancia clínica de las interacciones medicamentosas en pacientes infectados con el virus de la inmunodeficiencia humana: Actualización 2015–2017. (The clinical relevance of drug interactions in patients with human immunodeficiency virus infection: Update 2015–2017). Organo Of. Soc. Chil. Infectol. 2019, 36, 475–489. [Google Scholar] [CrossRef]
- Poutanen, S.M.; Low, D.E.; Henry, B.; Finkelstein, S.; Rose, D.; Green, K.; Tellier, R.; Draker, R.; Adachi, D.; Ayers, M.; et al. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003, 348, 1995–2005. [Google Scholar] [CrossRef]
- Khan, L.M. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay—A systematic review. Eur. J. Clin. Pharmacol. 2013, 69, 1985–1996. [Google Scholar] [CrossRef]
- Alj, L.; Touzani, M.; Benkirane, R.; Soulaymani, R. Detecting Medication Errors in Pharmacovigilance Database: Capacities and Limits. Int. J. Risk Saf. Med. 2007, 30, 919–990. [Google Scholar] [CrossRef]
- Khan, L.M.; Al-Harthi, S.; Alkreathy, H.; Osman, A.-M.M.; Ali, A.S. Detection of adverse drug reactions by medication antidote signals and comparison of their sensitivity with common methods of ADR detection. Saudi Pharm. J. 2014, 23, 515–522. [Google Scholar] [CrossRef]
- Khan, L.M.; Al-Harthi, S.; Saadah, O.; Al-Amoudi, A.; Sulaiman, M.; Ibrahim, I. Impact of pharmacovigilance on adverse drug reactions reporting in hospitalized internal medicine patients at Saudi Arabian teaching hospital. Saudi Med. J. 2012, 33, 863–868. [Google Scholar]
- Khan, L.M.; Kamel, F.O.; Alkreathy, H.; Al-Harthi, S.; Saadah, O.I.; Osman, A.-M.M.; Allibaih, M. Benefits of Medication Antidote Signals for the Detection of Potential Adverse Drug Reactions over Contemporary Methods of Pharmacovigilance in Hospitalized Children. Int. J. Pharmacol. 2016, 13, 64–73. [Google Scholar] [CrossRef]
- Rozich, J.D.; Haraden, C.R.; Resar, R.K. Adverse drug event trigger tool: A practical methodology for measuring medication related harm. BMJ Qual. Saf. 2003, 12, 194–200. [Google Scholar] [CrossRef]
- Morimoto, T.; Gandhi, T.K.; Seger, A.C.; Hsieh, T.C.; Bates, D.W. Adverse drug events and medication errors: Detection and classification methods. BMJ Qual. Saf. 2004, 13, 306–314. [Google Scholar] [CrossRef]
- Griffin, F.A.; Resar, R.K. IHI Global Trigger Tool for Measuring Adverse Events, 2nd ed.; IHI Innovation Series white paper; Institute for Healthcare Improvement: Cambridge, MA, USA, 2009. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.C.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Health Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef]
- Schumock, G.T.; Thornton, J.P. Focusing on the preventability of adverse drug reactions. Hosp. Pharm. 1992, 27, 538. [Google Scholar] [PubMed]
- Li, X.; Li, H.; Deng, J.; Zhu, F.; Liu, Y.; Chen, W.; Yue, Z.; Ren, X.; Xia, J. Active pharmacovigilance in China: Recent development and future perspectives. Eur. J. Clin. Pharmacol. 2018, 74, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Allen-Duck, A.; Robinson, J.C.; Stewart, M.W. Healthcare Quality: A Concept Analysis. Nurs. Forum. 2017, 52, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, V.A.; Kirchhoff, J.W.; Andersen, K.L.; Sørby, L.A.; Andreassen, H.M.; Skaug, E.-A.; Roos, A.K.; Tvete, L.S.; Helgesen, A.K. Health care quality from the patients’ perspective: A comparative study between an old and a new, high-tech hospital. J. Multidiscip. Healthc. 2018, 11, 591–600. [Google Scholar] [CrossRef]
- Alyami, M.H.; Naser, A.Y.; Orabi, M.A.A.; Alwafi, H.; Alyami, H.S. Epidemiology of COVID-19 in the Kingdom of Saudi Arabia: An Ecological Study. Front. Public Health 2020, 8, 506. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Alqassim, A.Y.; Muaddi, M.A.; Alghamdi, S.S. Regional Differences in COVID-19 Mortality Rates in the Kingdom of Saudi Arabia: A Simulation of the New Model of Care. Cureus 2021, 13, e20797. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ang, A.S.Y.; Ali, N.M.; Ang, L.M.; Omar, A. Incidence of adverse reaction of drugs used in COVID-19 management: A retrospective, observational study. J. Pharm. Policy Pract. 2021, 14, 84. [Google Scholar] [CrossRef]
- Sun, J.; Deng, X.; Chen, X.; Huang, J.; Huang, S.; Li, Y.; Feng, J.; Liu, J.; He, G. Incidence of Adverse Drug Reactions in COVID-19 Patients in China: An Active Monitoring Study by Hospital Pharmacovigilance System. Clin. Pharmacol. Ther. 2020, 108, 791–797. [Google Scholar] [CrossRef]
- Al-Shehail, B.; Al Jamea, Z.; Chacko, R.; Alotaibi, F.; Ismail, N.; Alshayban, D. Incidence and risk factors of adverse drug reactions in patients with coronavirus disease 2019: A pharmacovigilance experience utilizing an ADR trigger tool. Saudi Pharm. J. 2022, 30, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.M.; Musa, I.; Salah, S.; Elnur, M.; Al-Wutayd, O.; Adam, I. High Mortality Rate in Adult COVID-19 Inpatients in Eastern Sudan: A Retrospective Study. J. Multidiscip. Healthc. 2020, 13, 1887–1893. [Google Scholar] [CrossRef]
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Lorenzin, M.; Felicetti, M.; Doria, A.; Ramonda, R. Does gender influence clinical expression and disease outcomes in COVID-19? A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020, 34, 339–343. [Google Scholar] [CrossRef]
- Bwire, G.M. Coronavirus: Why Men are More Vulnerable to COVID-19 Than Women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef]
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef]
- Xia, W.; Shao, J.; Guo, Y.; Peng, X.; Li, Z.; Hu, D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr. Pulmonol. 2020, 55, 1169–1174. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Barreno, L.G.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; He, Y.; Liu, X.; Liu, M.; Tang, Y.; Li, X.; Yang, G.; Liang, G.; Xu, S.; et al. Age-Related Risk Factors and Complications of Patients With COVID-19: A Population-Based Retrospective Study. Front. Med. 2022, 8, 757459. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J.; Knox, K.S.; Rios, C.T.; Natt, B.; Bhattacharya, D.; Fain, M.J. SARS-CoV-2 and COVID-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience 2020, 42, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Amelung, S.; Meid, A.D.; Nafe, M.; Thalheimer, M.; Hoppe-Tichy, T.; Haefeli, W.E.; Seidling, H.M. Association of preventable adverse drug events with inpatients’ length of stay-A propensity-matched cohort study. Int. J. Clin. Pract. 2017, 71, e12990. [Google Scholar] [CrossRef] [PubMed]
- Sahilu, T.; Getachew, M.; Melaku, T.; Sheleme, T. Adverse Drug Events and Contributing Factors Among Hospitalized Adult Patients at Jimma Medical Center, Southwest Ethiopia: A Prospective Observational Study. Curr. Ther. Res. 2020, 93, 100611. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Williams, J.L.; Burnham, T.G.; Prevost, T.; Schiff, R.; Erskine, S.D.; Davies, J.G. Predicting adverse drug reactions in older adults; a systematic review of the risk prediction models. Clin. Interv. Aging 2014, 9, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, M.; Majeed, A. Current and future perspectives on the management of polypharmacy. BMC Fam. Pract. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Suggett, E.; Marriott, J. Risk Factors Associated with the Requirement for Pharmaceutical Intervention in the Hospital Setting: A Systematic Review of the Literature. Drugs Real World Outcomes 2016, 3, 241–263. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 12 November 2022).
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020, 92, 1915–1921. [Google Scholar] [CrossRef]
- Melo, J.R.R.; Duarte, E.C.; de Moraes, M.V.; Fleck, K.; e Silva, A.S.D.N.; Arrais, P.S.D. Adverse drug reactions in patients with COVID-19 in Brazil: Analysis of spontaneous notifications of the Brazilian pharmacovigilance system. Cad. Saúde Pública 2021, 37, e00245820. [Google Scholar] [CrossRef]
- Clark, R.; Waters, B.; Stanfill, A.G. Elevated liver function tests in COVID-19: Causes, clinical evidence, and potential treatments. Nurse Pract. 2021, 46, 21–26. [Google Scholar] [CrossRef]
- Su, Y.-J.; Chang, C.-W.; Chen, M.-J.; Lai, Y.-C. Impact of COVID-19 on liver. World J. Clin. Cases 2021, 9, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Balzano, T.; El Hiba, O.; del Rey, N.L.-G.; El Amine, S.; Smimih, K. Liver Injury in COVID-19 Patients: An Overview of the Current Evidence. In Handbook of Research on Pathophysiology and Strategies for the Management of COVID-19; El Hiba, O., Ed.; IGI Globa: Hershey, PA, USA, 2022; pp. 141–158. [Google Scholar] [CrossRef]
- Alqahtani, S.; Schattenberg, J.M. Liver injury in COVID-19: The current evidence. United Eur. Gastroenterol. J. 2020, 8, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.M.; Al-Harthi, S.E.; Osman, A.-M.M.; Sattar, M.A.A.A.; Ali, A.S. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm. J. 2015, 24, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Varallo, F.R.; Planeta, C.D.S.; Herdeiro, M.T.; Mastroianni, P.D.C. Imputation of adverse drug reactions: Causality assessment in hospitals. PLoS ONE 2017, 12, e0171470. [Google Scholar] [CrossRef]
- Nazir, N.; Chopra, D.; Sidhu, J.; Bhandari, B. Adverse Drug Reactions in COVID-19 Patients Admitted to Intensive care Unit: Analysis of Individual Case Study Reports. 09 June 2021, preprint (version 1) available at Research Square. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Alshammari, T.M.; Al-Kathiri, W.H.; Le Louet, H.; Aljadhey, H.S. Completeness of adverse drug reactions reports of the Saudi adverse event reporting system. Saudi Med. J. 2015, 36, 821–828. [Google Scholar] [CrossRef]
- Crescioli, G.; Brilli, V.; Lanzi, C.; Burgalassi, A.; Ieri, A.; Bonaiuti, R.; Romano, E.; Innocenti, R.; Mannaioni, G.; Vannacci, A.; et al. Adverse drug reactions in SARS-CoV-2 hospitalised patients: A case-series with a focus on drug–drug interactions. Intern. Emerg. Med. 2020, 16, 697–710. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Dairem, A.; Moore, W.J.; Shah, A.; Postelnick, M.J.; Badowski, M.E.; Michienzi, S.M.; Borkowski, J.L.; Polisetty, R.S.; Fong, K.; et al. Multicenter point prevalence evaluation of the utilization and safety of drug therapies for COVID-19 at the onset of the pandemic timeline in the United States. Am. J. Health Pharm. 2021, 78, 568–577. [Google Scholar] [CrossRef]
- Almazrou, D.; Egunsola, O.; Ali, S.; Bagalb, A. Medication Misadventures Among COVID-19 Patients in Saudi Arabia. Cureus 2021, 13, e15513. [Google Scholar] [CrossRef] [PubMed]
- Olry, A.; Meunier, L.; Délire, B.; Larrey, D.; Horsmans, Y.; Le Louët, H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020, 43, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Cabral, F.F.; Pereira, M.; Borges, K.; de Brito Passos, A.; Francelino, E.; Monteiro, M.; Arrais, P. Eventos Adversos A Medicamentos No Tratamento Da COVID-19 No Ceará: Adverse Events to Medicines in the Treatment of COVID-19 in Ceará. Cad. ESP-Rev. Científica Esc. Saúde Pública Ceará 2020, 14, 30–37. [Google Scholar]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Yang, S.; Liang, J.; Liu, T. Analysis of diarrhea associated with lopinavir-ritonavir for COVID-19 and its prevention. Med. J. West China 2020, 32, 485–488. [Google Scholar] [CrossRef]
- Chouchana, L.; Boujaafar, S.; Gana, I.; Preta, L.-H.; Regard, L.; Legendre, P.; Azoulay, C.; Canouï, E.; Zerbit, J.; Carlier, N.; et al. Plasma Concentrations and Safety of Lopinavir/Ritonavir in COVID-19 Patients. Ther. Drug Monit. 2021, 43, 131–135. [Google Scholar] [CrossRef]
- Iftikhar, S.; Sarwar, M.R.; Saqib, A.; Sarfraz, M. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: A multicenter, cross-sectional study in Lahore, Pakistan. PLoS ONE 2018, 13, e0199456. [Google Scholar] [CrossRef]
- Alenzi, K.A.; Alanazi, N.S.; Almalki, M.; Alomrani, H.; Alatawi, F.O. The evaluation of adverse drug reactions in Saudi Arabia: A retrospective observational study. Saudi Pharm. J. 2022, 30, 735–741. [Google Scholar] [CrossRef] [PubMed]
- AlKhamees, O.A.; AlNemer, K.A.; Maneea, M.W.B.; AlSugair, F.A.; AlEnizi, B.H.; Alharf, A.A. Top 10 most used drugs in the Kingdom of Saudi Arabia 2010–2015. Saudi Pharm. J. 2018, 26, 211–216. [Google Scholar] [CrossRef] [PubMed]
| ADR Prompt Indicators | |
|---|---|
| Laboratory indicators | |
| Platelet count < 3.5 × 100/L | Drug-influenced platelet diminution |
| Serum ALT, AST, T Bilirubin or ALP > 2ULN | Drug-influenced liver injury |
| Serum cholesterol > 6 mmol/L | Drug-influenced hypercholesterolemia |
| Serum Triglyceride > 1.7 mmol/L | Drug-influenced hyperlipidemia |
| Medication indicators | |
| Dextrose 50% | Hypoglycemia |
| Metoclopramide and Ondansetron | Drug-influenced nausea and vomiting |
| Promethazine and chlorpheniramine | Skin allergy |
| Phytonadione | Drug-influenced coagulation disorder |
| Sodium polystyrene and Calcium polystyrene | Drug-influenced Hyperkalemia |
| Loperamide | Drug-influenced diarrhea |
| Variables | All Patients (n = 381) | Patients without ADRs (n = 240) | Patients with ADRs (n = 141) | p-Value | Univariate Analysis | |
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | p-Value | |||||
| Age | 48.93 ± 14.63 | 48.46 ± 14.74 | 49.74 ± 14.45 | 0.409 | 1.006 (0.992–1.020) | p = 0.408 |
| Gender | 0.125 | |||||
| Female | 78 (20.5%) | 54 (22.5%) | 24 (17.0%) | * A | ||
| Male | 303 (79.5%) | 186 (77.5%) | 117 (83.0%) | 1.415 (0.830–2.413) | p = 0.202 | |
| Nationality | 0.024 | |||||
| Non-Saudi | 211 (55.4%) | 133 (55.4%) | 78 (55.3%) | * B | ||
| Saudi | 170 (44.5%) | 107 (44.6%) | 63 (44.7%) | 1.004 (0.661–1.525) | p = 0.985 | |
| Variables | Total Patients (n = 381) | Patients with No ADRs (n = 240) | Patients Affected by ADRs (n = 141) | p-Value | Univariate Analysis | |
|---|---|---|---|---|---|---|
| Relative Risk (95% CI) | p-Value | |||||
| Duration of hospital stay | 11.25 ± 12.7 | 9.55 ± 7.90 | 14.13 ± 7.87 | 0.001 | 1.038 (1.014–1.063) | p = 0.002 |
| Quantity of drug intake | 8.00 ± 4.99 | 6.98 ± 4.36 | 9.74 ± 5.51 | 0.0001 | 1.127 (1.075–1.182) | p < 0.0001 |
| Use of antiviral agent | 0.0001 | |||||
| Combined use of antiviral | 251 (65.9%) | 176 (73.3%) | 75 (53.2%) | * C | ||
| Single-use of antiviral | 130 (34.1%) | 64 (26.7%) | 66 (46.8%) | 2.420 (1.563–3.748) | p < 0.0001 | |
| History of past drug allergies | 0.507 | |||||
| No | 274 (71.9%) | 173 (72.1%) | 101 (71.6%) | * D | ||
| Yes | 107 (28.1%) | 67 (27.9%) | 40 (28.4%) | 1.023 (0.644–1.623) | p = 0.924 | |
| History of chronic diseases | 0.117 | |||||
| No | 219 (57.5%) | 184 (76.7%) | 35 (24.8%) | * E | ||
| Yes | 162 (42.5%) | 56 (23.3%) | 106 (75.2%) | 1.320 (0.868–2.008) | p = 0.195 | |
| COVID-19 Patients with Comorbidities | Total Patients (n = 381) | Patients with No ADRs (n = 240) | Patients Affected by ADRs (n = 141) | Univariate Analysis | |
|---|---|---|---|---|---|
| Relative Risk (95% CI) | p-Value | ||||
| Without comorbidity | 219 (57.5%) | 184 (76.7%) | 35 (24.8%) | * E | |
| With comorbidity | 162 (42.5%) | 56 (23.3%) | 106 (75.2%) | 1.320 (0.868–2.008) | 0.195 |
| Controlled DM | 14 | 3 (21.4%) | 11(78.6%) | 6.685 (1.832–24.391) | 0.001 |
| Uncontrolled DM § | 37 | 12 (32.4%) | 25 (67.7%) | 4.095 (1.986–8.444) | 0.001 |
| Untreated DM § | 9 | 2 (22.2%) | 7 (77.8%) | 3.511 (0.864–14.266) | 0.062 |
| HTN | 54 | 21 (38.9%) | 33 (61.1%) | 0.295 (0.140–0.625) | 0.001 |
| CHF § | 12 | 4 (33.3%) | 8 (66.7%) | 3.549 (1.049–12.007) | 0.031 |
| CAD § | 16 | 7 (43.7%) | 9 (56.3%) | 2.269 (0.826–6.234) | 0.103 |
| Chronic renal disorder | 09 | 3 (33.3%) | 6 (66.7%) | 3.511 (0.864–14.266) | 0.062 |
| COPD§ | 8 | 3 (37.5%) | 5 (62.5%) | 2.904 (0.683–12.342) | 0.131 |
| Malignancy | 03 | 1 (33.3%) | 2 (66.7%) | 3.439 (0.309–38.271) | 0.285 |
| Medication Prompt Indicators (MPI) | MPI Confirmed as ADRs (n = 62; 43.9%) | p-Value | Lab Prompt Indicators (LPI) | LPI Confirmed as ADRs (n = 79; 56.1%) | p-Value |
|---|---|---|---|---|---|
| Dextrose 50% | - | - | Platelet count < 120 PLT/μL | 5 (3.5%) | p < 0.0001 |
| Promethazine | - | - | Serum ALT > 55 U/L | 21 (14.89%) | p < 0.0001 |
| Chlorpheniramine | 4 (2.8%) | p < 0.0001 | Serum AST > 34 U/L | 28 (19.9%) | p < 0.0001 |
| Metoclopramide | 23 (16.3%) | p < 0.0001 | Serum T Bilirubin > 20.5 μmol/L | 5 (3.5%) | p < 0.0001 |
| Phytonadione | 2 (1.4%) | p < 0.0001 | Serum ALP > 150 U/L | 5 (3.5%) | p < 0.0001 |
| Sodium polystyrene | - | - | Serum Cholesterol > 6 mmol/L | 9 (6.38%) | p < 0.0001 |
| Calcium polystyrene | 5 (3.5%) | p < 0.0001 | Serum Triglyceride > 2.4 mmol/L | 6 (4.3%) | p < 0.0001 |
| Loperamide | 8 (5.7%) | p < 0.0001 | |||
| Ondansetron | 20 (14.2%) | p < 0.0001 |
| Character | Total (n = 381) | Female (n = 78) | Male (n = 303) | p-Value |
|---|---|---|---|---|
| Incidence | 141 (37.0%) | 24 (17%) | 117 (83%) | 0.188 |
| Definite | 04 (2.8%) | 02 (8.3%) | 02 (1.7%) | 0.187 |
| Probable | 43 (30.5%) | 9 (37.5%) | 34 (29%) | 0.536 |
| Possible | 94 (66.66%) | 13 (54%) | 81 (69.2%) | 0.042 |
| ADR Type A | 137 (97.1%) | 19 (13.8%) | 118 (86.1%) | 0.011 |
| ADR Type B | 04 (2.8%) | 04 (100%) | - | 0.002 |
| Age Groups | Total (n = 141) | Female (n = 24) 17.1% | Male (n = 117) 82.9% | p-Value | Duration of Hospital Stay (Days) (Mean ± SD) | p-Value |
|---|---|---|---|---|---|---|
| 18–25 (n = 1) | 1 | 1 (4.2%) | - | 0.0001 | 4.00 ± 0.00 | 0.001 |
| 25–39 (n = 30) | 30 | 3 (12.5%) | 27 (23.1%) | 9.98 ± 5.82 | ||
| 39–49 (n = 26) | 26 | 4 (16.7%) | 22 (18.8%) | 10.46 ± 4.98 | ||
| 49–59 (n = 36) | 36 | 7 (29.2%) | 29 (24.8%) | 14.94 ± 7.99 | ||
| >60 (n = 48) | 48 | 9 (37.5%) | 39 (33.3%) | 21.11 ± 8.50 |
| Organ and the Systems Involved | Medication Groups & Their Intake | No of the ADRs (n = 141) | Incidence of ADRs (%) | p-Value |
|---|---|---|---|---|
| Skin reactions (n = 4) | Antibiotics (n = 34) | 2.1% | 0.044 | |
| Antiviral (n = 58) | 1 | 0.7% | 0.454 | |
| GIT disorders (n = 51) | Antibiotics (n = 34) | 12 | 8.5% | 0.537 |
| Antiviral (n = 58) | 21 | 14.9% | 0.567 | |
| Antimalarials (n = 18) | 13 | 9.2% | 0.001 | |
| Monoclonal antibodies (n = 11) | 2 | 1.4% | 0.168 | |
| Other drugs (n = 10) | 3 | 2.1% | 0.48 | |
| Hepatobiliary disorders (n = 59) | Antibiotics (n = 34) | 15 | 10.6% | 0.454 |
| Antiviral (n = 11) | 33 | 23.4% | 0.002 | |
| Antimalarials (n = 10) | 4 | 2.84% | 0.058 | |
| Monoclonal antibodies (n = 11) | 5 | 3.6% | 0.52 | |
| Other drugs (n = 10) | 2 | 1.4% | 0.130 | |
| Hyperkalemia (n = 5) | Antiviral (n = 58) | 1 | 0.7% | 0.314 |
| Other drugs (n = 10) | 4 | 2.8% | 0.0001 | |
| Hypercholesterolemia (n = 9) | Corticosteroids (n = 10) | 9 | 6.4% | 0.0001 |
| Hypertriglyceridemia (n = 6) | Corticosteroids (n = 10) | 1 | 0.7% | 0.362 |
| Antiviral (n = 58) | 2 | 1.4% | 0.52 | |
| Monoclonal antibodies (n = 2) | 3 | 2.1% | 0.006 | |
| Thrombocytopenia (n = 5) | Antibiotics (n = 34) | 3 | 2.1% | 0.091 |
| Antimalarials (n = 18) | 1 | 0.7% | 0.5 | |
| Monoclonal antibodies (n = 11) | 1 | 0.71% | 0.338 | |
| Bleeding disorder (n = 2) | Other drugs (n = 10) | 2 | 1.4% | 0.005 |
| Potential Drugs to Produce ADRs | All ADRs (n = 141) 100% | Serious ADRs (n = 8) 5.7% | Definite (n = 4) 2.8% | Probable (n = 43) 30.4% | Possible (n = 94) 66.6% | Outcome | |
|---|---|---|---|---|---|---|---|
| Cure (n = 99) 70.2% | Recovery (n = 42) 29.8% | ||||||
| Lopinavir/ritonavir | 23 (16.3%) | 4 (2.8%) | 1 (0.7%) | 10 (7.1%) | 12(8.5%) | 16 (11.4%) | 7 (4.9%) |
| Favipiravir | 14 (9.9%) | 2 (1.4%) | 1 (0.7%) | 5 (3.5%) | 8 (5.67%) | 9 (6.3%) | 5 (3.5%) |
| Oseltamivir | 21 (14.9%) | 1(0.7%) | - | 3 (2.1%) | 18 (12.77%) | 13 (9.2%) | 8 (5.6%) |
| Hydroxychloroquine | 18 (12.8%) | - | 6 (4.2%) | 12 (8.51%) | 11(7.8%) | 7 (5.0%) | |
| Tocilizumab | 11 (7.8%) | 1 (0.7%) | 2 (1.4%) | 4 (2.8%) | 5 (3.55%) | 9 (6.4%) | 2 (1.4%) |
| Corticosteroids | 10 (7.1%) | - | 7 (4.9%) | 3 (2.13%) | 6 (4.3%) | 4 (2.84%) | |
| Antibiotics | 34 (24.1%) | - | 7 (4.9%) | 27 (19.2%) | 27 (19.2%) | 7 (5.0%) | |
| Medications used for chronic disorders | 10 (7.1%) | - | - | 1 (0.7%) | 9 (6.4%) | 8 (5.7%) | 2 (1.4%) |
| Total | 141 (100%) | 8 (5.7%) | 4 (2.8%) | 43 (30.4%) | 94 (66.6%) | 99 (70.2%) | 42 (29.8%) |
| Class of Drug in ADR n (%) | Skin ADR n (%) 4 (2.8) | GIT ADR n (%) 51 (36.2) | Hepatobiliary Disorder n (%) 59 (41.8) | Hyperkalemia n (%) 5 (3.5) | Hypertriglyceridemia n (%) 6 (4.2) | Hypercholesterolemia n (%) 9 (6.4) | Thrombocytopenia n (%) 5 (3.5) | Bleeding Disorder n (%) 2 (1.4) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics n = 34 (24.1%) | 3 (2.1%) | 12 (8.5%) | 15 (10.6%) | - | - | - | 3 (2.1%) | - | p < 0.0001 |
| Corticosteroids n = 10 (7.1%) | - | - | - | - | 1 (0.7%) | 9 (6.4%) | - | - | p < 0.0001 |
| Antiviral n = 58 (41.1%) | 1 (0.7%) | 21 (14.9%) | 33 (23.4%) | 1 (0.7%) | 2 (1.4%) | - | - | - | p < 0.0001 |
| Antimalarials n = 18 (12.8%) | - | 13 (9.2%) | 4 (2.8%) | - | - | - | 1 (0.7%) | p < 0.0001 | |
| Monoclonal antibodies n = 11 (7.8%) | - | 2 (1.4%) | 5 (3.6%) | - | 3 (2.13%) | - | 1 (0.7%) | - | p > 0.050 |
| Chronic disorder medications n = 10 (7.1%) | 3 (2.1%) | 2 (1.4%) | 4 (2.8%) | - | - | - | 2 (1.4%) | p > 0.050 |
| The System Involved | Schumock and Thornton Scale of Preventable ADRs | Hartwig and SIEGEL Scale of Severity of ADRs | ||||
|---|---|---|---|---|---|---|
| ADRs n = 141 (%) | Certainly, Preventable ADRs 14 (9.9%) | Probably Preventable ADRs 22 (15.6%) | Unpreventable ADRs 105 (74.5%) | Mild 64 (45.4%) | Moderate 69 (48.9%) | Severe 8 (5.7%) |
| Skin reactions 4 (2.8%) | - | - | 4 (2.8%) | - | 4 (2.8%) | - |
| Gastrointestinal disorders 51 (36.2%) | - | - | 51 (48.6%) | 51 (36.2%) | - | |
| Hepatobiliary disorders 59 (41.8%) | 12 (8.51%) | 6 (4.3%) | 41 (29.1%) | 10 (7.1%) | 46 (32.6%) | 3 (2.1%) |
| Electrolyte disorders (Hyperkalemia) 5 (3.5%) | - | 3 (2.1%) | 2(1.4%) | 2 (1.4%) | 3 (2.1%) | - |
| Hyperlipidemic disorders (Hypercholesterolemia 9 (6.4%) & Hypertriglyceridemia 6 (4.2%)) | - | 13 (9.2%) | 2 (1.4%) | 1 (0.7%) | 14 (9.9%) | - |
| Hematological disorders (Thrombocytopenia 5 (4.3%) & Bleeding disorder 2 (1.4%)) | 2 (1.4%) | - | 5 (3.55%) | - | 2 (1.4%) | 5 (3.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shareef, E.; Khan, L.M.; Alsieni, M.; Karim, S.; Kamel, F.O.; Alkreathy, H.M.; Bafail, D.A.; Ibrahim, I.M.; Burzangi, A.S.; Bazuhair, M.A. Detection of Adverse Drug Reactions in COVID-19 Hospitalized Patients in Saudi Arabia: A Retrospective Study by ADR Prompt Indicators. Healthcare 2023, 11, 660. https://doi.org/10.3390/healthcare11050660
Al-Shareef E, Khan LM, Alsieni M, Karim S, Kamel FO, Alkreathy HM, Bafail DA, Ibrahim IM, Burzangi AS, Bazuhair MA. Detection of Adverse Drug Reactions in COVID-19 Hospitalized Patients in Saudi Arabia: A Retrospective Study by ADR Prompt Indicators. Healthcare. 2023; 11(5):660. https://doi.org/10.3390/healthcare11050660
Chicago/Turabian StyleAl-Shareef, Ebtihal, Lateef M. Khan, Mohammed Alsieni, Shahid Karim, Fatemah O. Kamel, Huda M. Alkreathy, Duaa A. Bafail, Ibrahim M. Ibrahim, Abdulhadi S. Burzangi, and Mohammed A. Bazuhair. 2023. "Detection of Adverse Drug Reactions in COVID-19 Hospitalized Patients in Saudi Arabia: A Retrospective Study by ADR Prompt Indicators" Healthcare 11, no. 5: 660. https://doi.org/10.3390/healthcare11050660
APA StyleAl-Shareef, E., Khan, L. M., Alsieni, M., Karim, S., Kamel, F. O., Alkreathy, H. M., Bafail, D. A., Ibrahim, I. M., Burzangi, A. S., & Bazuhair, M. A. (2023). Detection of Adverse Drug Reactions in COVID-19 Hospitalized Patients in Saudi Arabia: A Retrospective Study by ADR Prompt Indicators. Healthcare, 11(5), 660. https://doi.org/10.3390/healthcare11050660






