Metformin Therapy and Breast Cancer Incidence in the Ha’il Region
Abstract
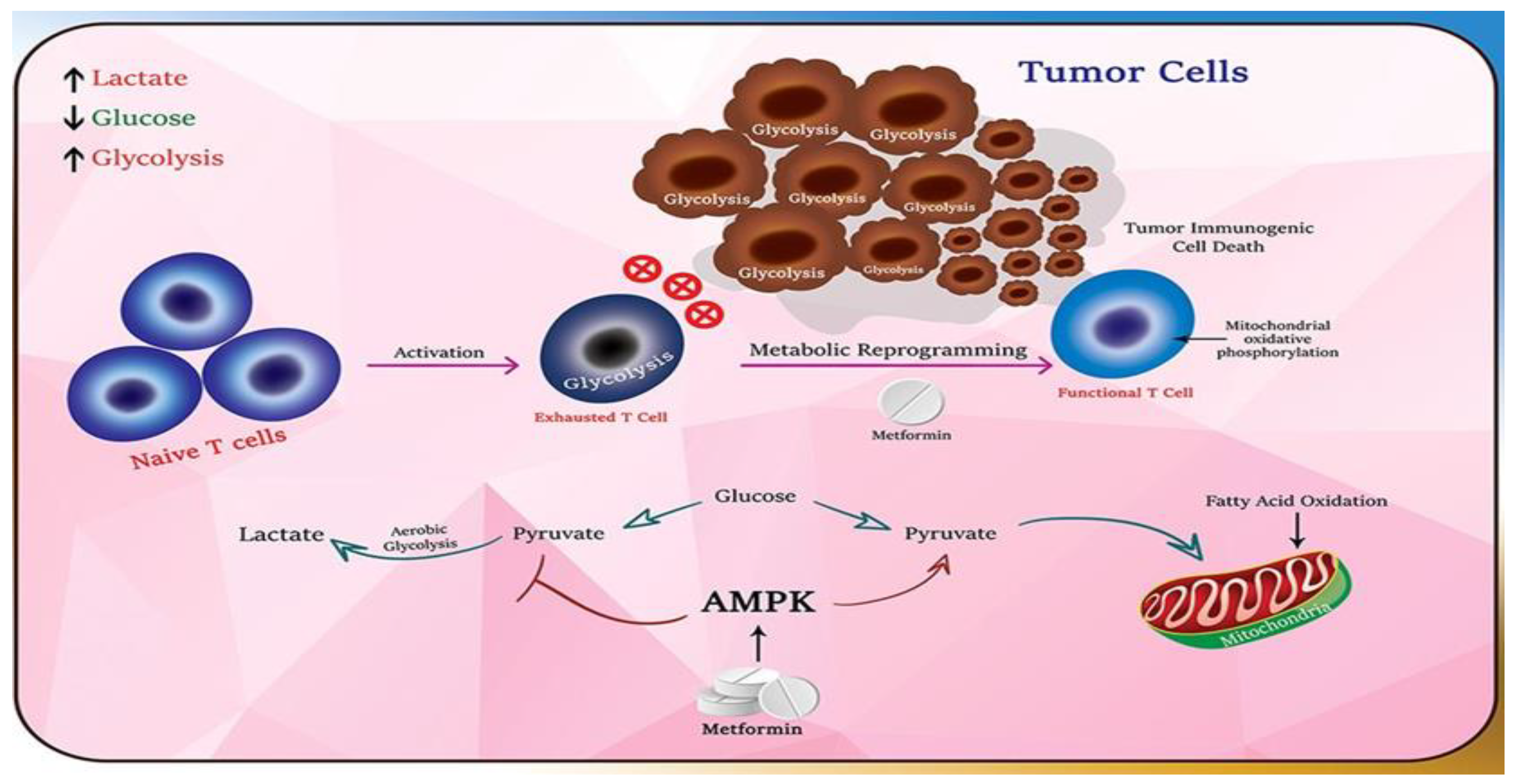
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients’ Demographic Data
3.2. Medication Information
3.3. Comorbidities
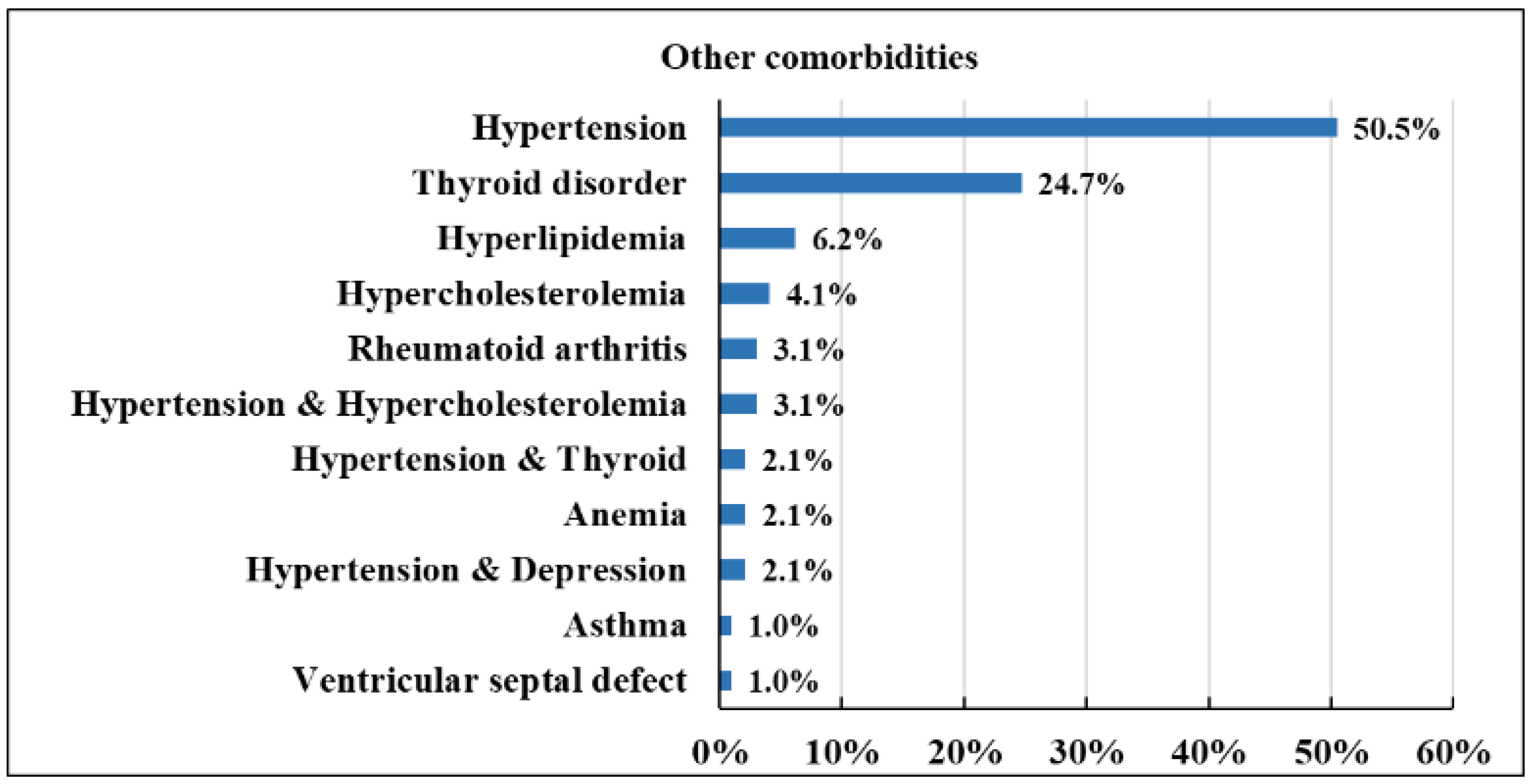
3.4. The Type of Other Comorbid Conditions
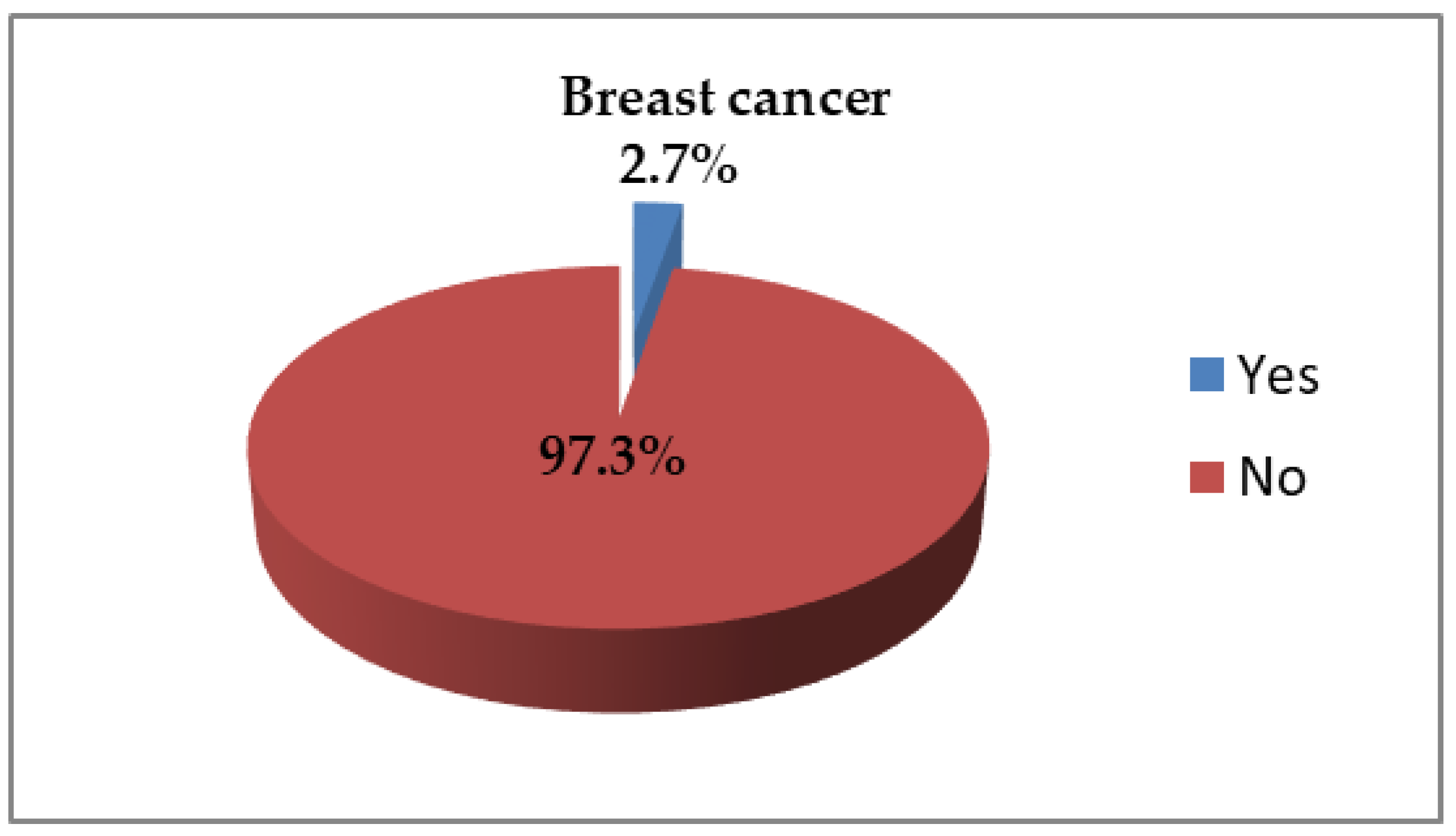
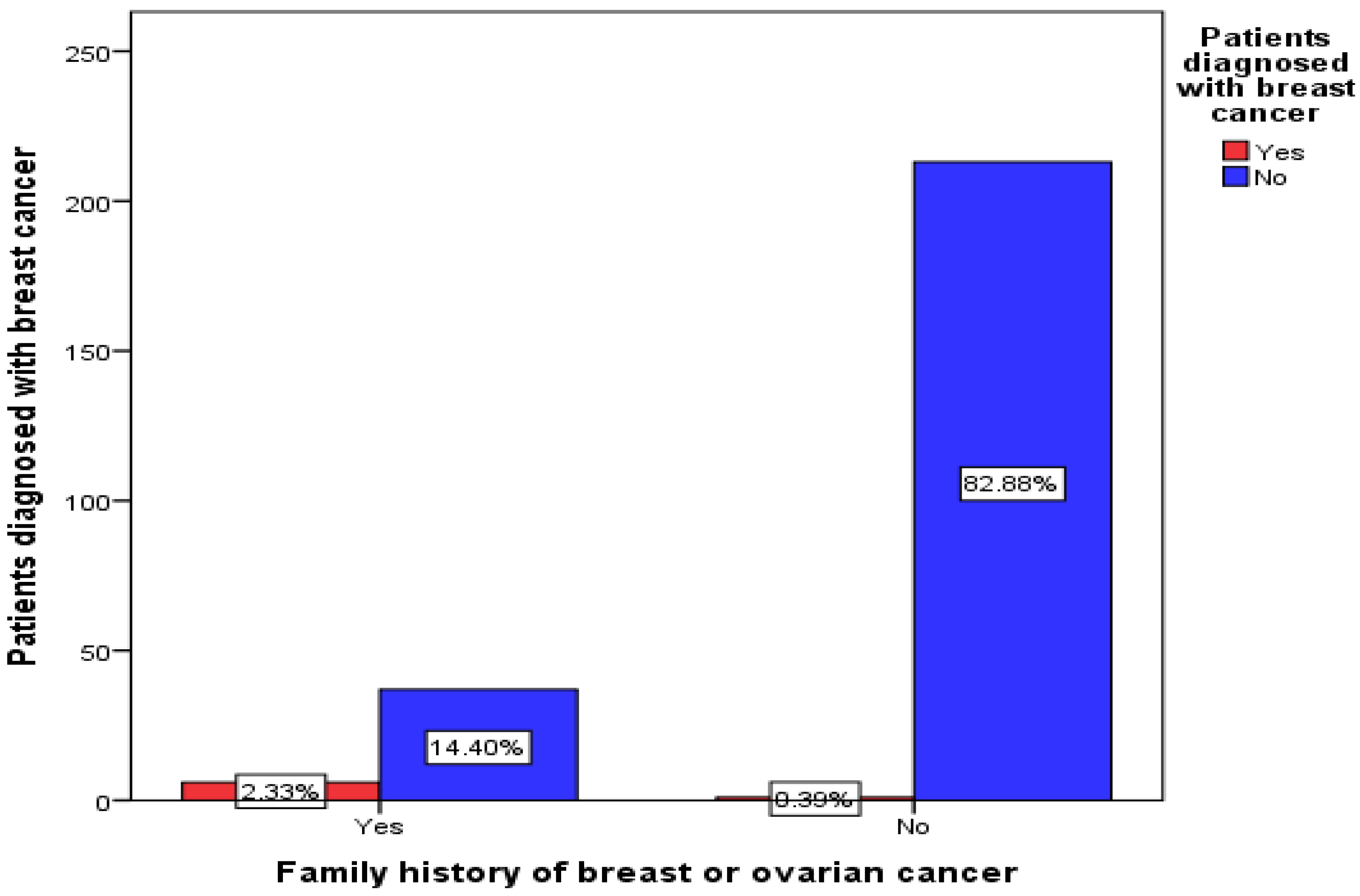
3.5. Breast Cancer Diagnosis
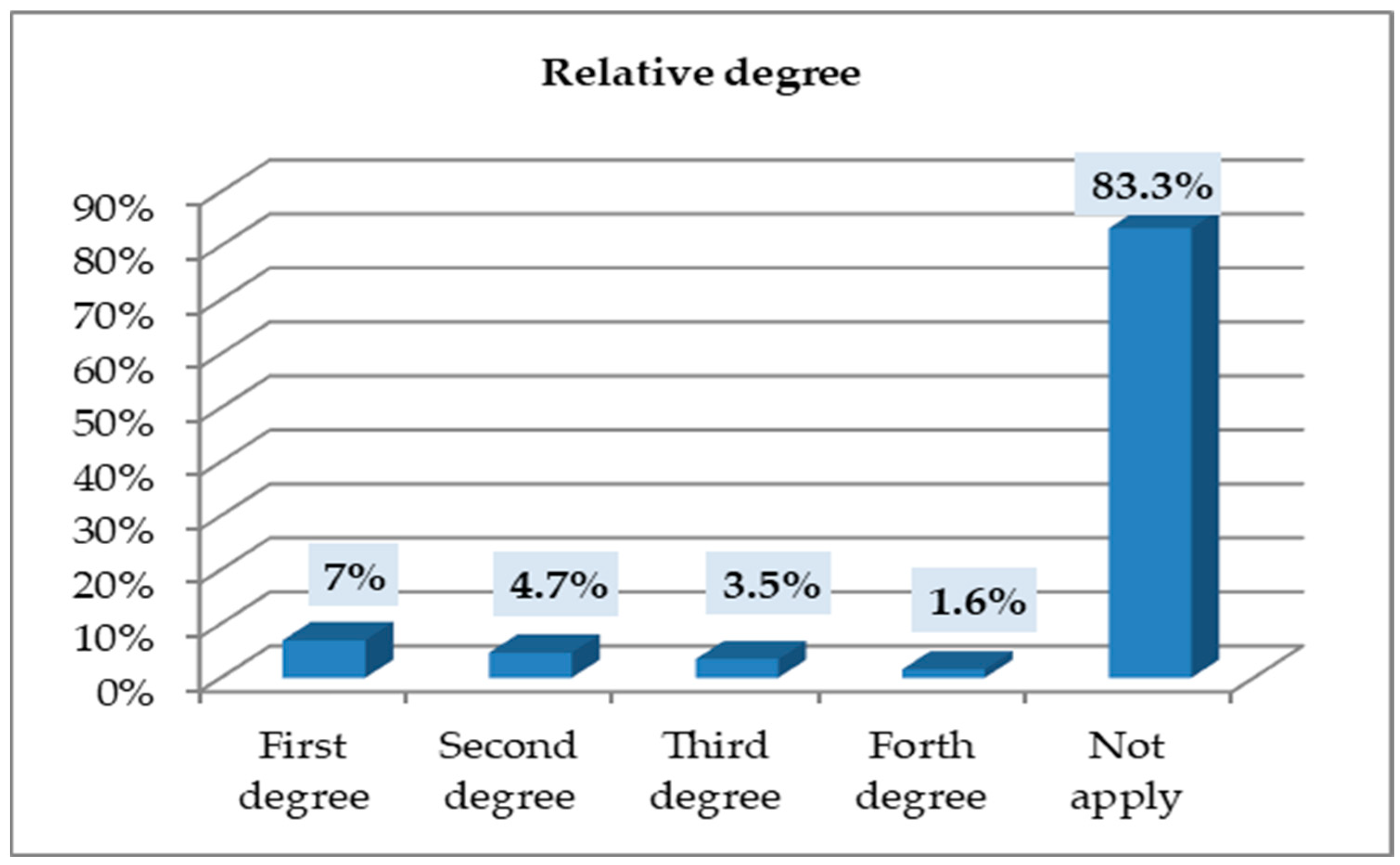
3.6. Relationship Degree (for Participants Who Had a Family History of Breast or Ovarian Cancer)
3.7. The Relationship between Medication Group and Patient Diagnosis of Breast Cancer
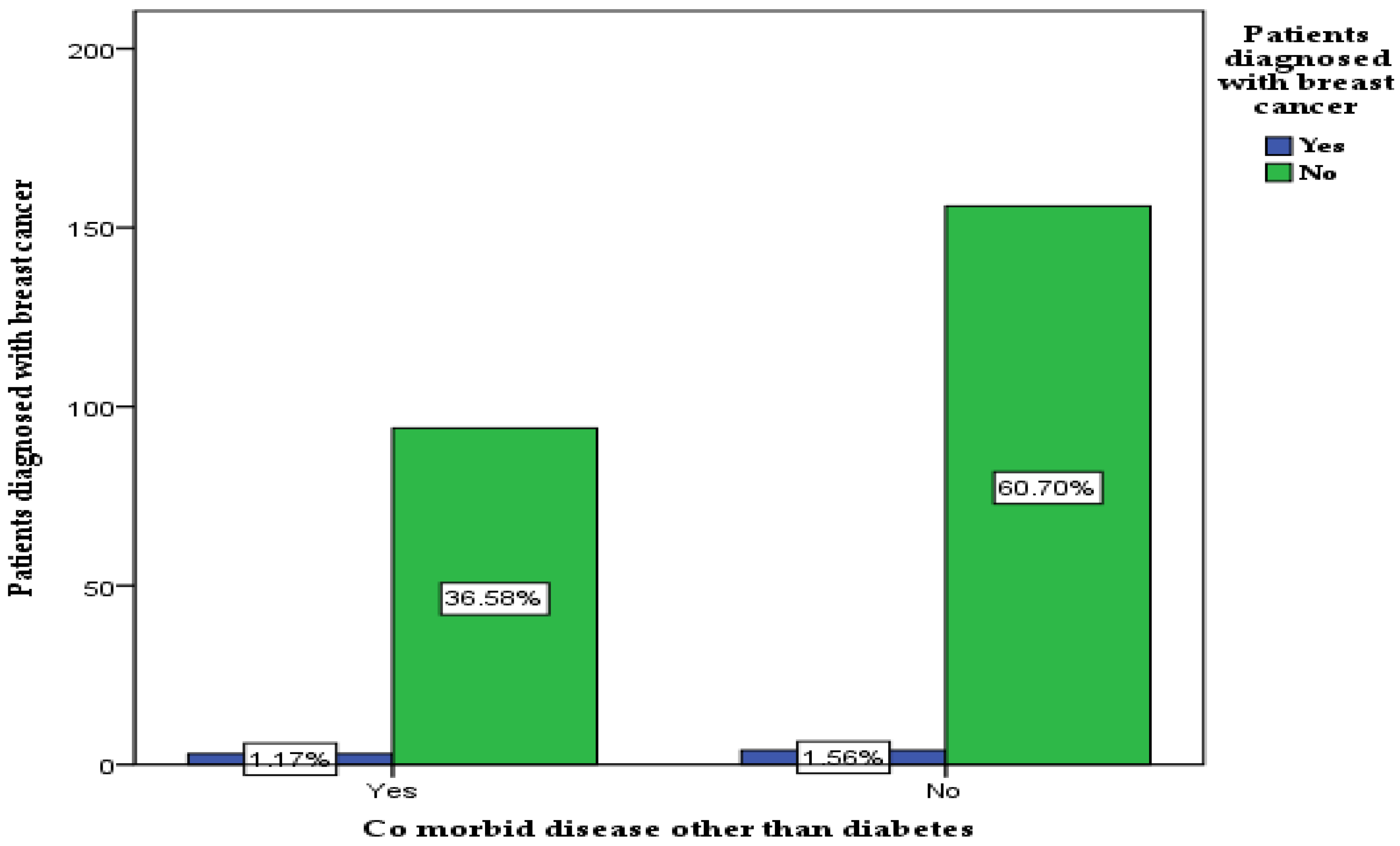
3.8. The Relationship between Comorbid Disease and Patient Diagnosis of Breast Cancer
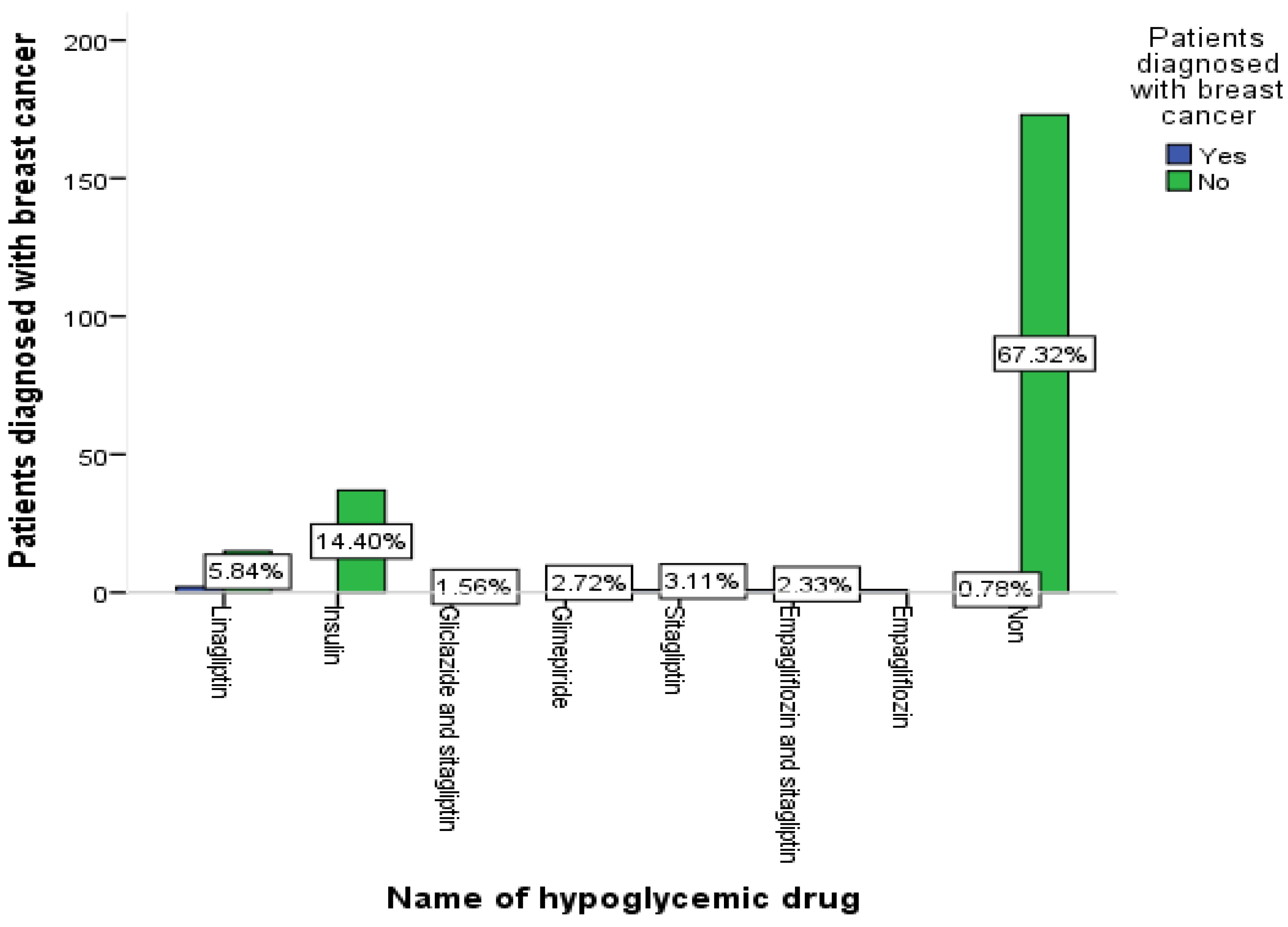
3.9. The Relationship between Medication Group and Patient Diagnosis of Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar]
- Lamanna, C.; Monami, M.; Marchionni, N.; Mannucci, E. Effect of metformin on cardiovascular events and mortality: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2011, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [PubMed]
- Brown, T.J.; Gupta, A. Management of cancer therapy–associated oral mucositis. JCO Oncol. Pract. 2020, 16, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Meier, C.; Krähenbühl, S.; Jick, S.S.; Meier, C.R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 2010, 33, 1304–1308. [Google Scholar] [CrossRef]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; McTiernan, A.; Wactawski-Wende, J.; Manson, J.E.; Aragaki, A.K.; Rohan, T.; Ipp, E.; Kaklamani, V.G.; Vitolins, M.; Wallace, R. Diabetes, metformin, and breast cancer in postmenopausal women. J. Clin. Oncol. 2012, 30, 2844. [Google Scholar] [CrossRef]
- Ugwueze, C.V.; Ogamba, O.J.; Young, E.E.; Onyenekwe, B.M.; Ezeokpo, B.C. Metformin: A possible option in cancer chemotherapy. Anal. Cell. Pathol. 2020, 2020, 7180923. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Abdel-Ghany, S.E.; Said, O.A.; Mostafa, M.A.; El-Zawahry, M. Metformin reshapes the methylation profile in breast and colorectal cancer cells. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2991. [Google Scholar]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.-C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009, 27, 3297. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Bahrambeigi, S.; Shafiei-Irannejad, V. Immune-mediated anti-tumor effects of metformin; targeting metabolic reprogramming of T cells as a new possible mechanism for anti-cancer effects of metformin. Biochem. Pharmacol. 2020, 174, 113787. [Google Scholar] [PubMed]
- Alimova, I.N.; Liu, B.; Fan, Z.; Edgerton, S.M.; Dillon, T.; Lind, S.E.; Thor, A.D. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009, 8, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Arif, J.M.; Al-Saif, A.M.; Al-Karrawi, M.A.; Al-Sagair, O.A. Causative relationship between diabetes mellitus and breast cancer in various regions of Saudi Arabia: An overview. Asian Pac J. Cancer Prev. 2011, 12, 589–592. [Google Scholar]
- Ahmed, A.A.; Elfeil, M.E.; Ahmed, S.K.; Elsammani, T.O. The in vitro Pharmacological Effects of Fagonia cretica Linn Ethanolic Extract on Isolated Rabbit Intestine. Technol. Innov. Pharm. Res. 2021, 9, 23–36. [Google Scholar]
- Roshan, M.H.; Shing, Y.K.; Pace, N.P. Metformin as an adjuvant in breast cancer treatment. SAGE Open Med. 2019, 7, 2050312119865114. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.S.; Thor, A.D. Metformin Activity against Breast Cancer: Mechanistic Differences by Molecular Subtype and Metabolic Conditions. In Metformin; IntechOpen: London, UK, 2020. [Google Scholar]
- Becker, J.; Nora, D.B.; Gomes, I.; Stringari, F.F.; Seitensus, R.; Panosso, J.S.; Ehlers, J.A.C. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin. Neurophysiol. 2002, 113, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.S.; Inzucchi, S.E. Metformin. Drugs 2003, 63, 1879–1894. [Google Scholar] [PubMed]
- Colmers, I.; Bowker, S.; Johnson, J. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012, 38, 475–484. [Google Scholar]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar]
- Andújar-Plata, P.; Pi-Sunyer, X.; Laferrere, B. Metformin effects revisited. Diabetes Res. Clin. Pract. 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Gong, J.; Robbins, L.A.; Lugea, A.; Waldron, R.T.; Jeon, C.Y.; Pandol, S.J. Diabetes, pancreatic cancer, and metformin therapy. Front. Physiol. 2014, 5, 426. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Zhou, X.-C.; Du, P.; Yin, M.-Y.; Xu, L.; Chen, W.-J.; Xu, C.-F. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine 2020, 99, e21687. [Google Scholar] [CrossRef]
- Wu, A.H.; Kurian, A.W.; Kwan, M.L.; John, E.M.; Lu, Y.; Keegan, T.H.; Gomez, S.L.; Cheng, I.; Shariff-Marco, S.; Caan, B.J.; et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: The California Breast Cancer Survivorship Consortium (CBCSC). CancerEpidemiol. Biomark. Prev. 2015, 24, 361–368. [Google Scholar] [CrossRef]
- Salinas-Martínez, A.M.; Flores-Cortés, L.I.; Cardona-Chavarría, J.M.; Hernández-Gutiérrez, B.; Abundis, A.; Vázquez-Lara, J.; González-Guajardo, E.E. Prediabetes, diabetes, and risk of breast cancer: A case-control study. Arch. Med. Res. 2014, 45, 432–438. [Google Scholar] [CrossRef]

| Variable | Frequency (Percent) | p Value |
|---|---|---|
| Age (Years) | 0.180 | |
| 25–35 | 8 (3.1%) | |
| 35–45 | 70 (27.2%) | |
| 46–55 | 79 (30.7%) | |
| More than 55 | 100 (38.9%) | |
| Education level | 1.000 | |
| Primary | 70 (27.2%) | |
| Intermediate | 21 (8.2%) | |
| Secondary | 56 (21.8%) | |
| University | 110 (42.8%) |
| Variable | Frequency (Percent) | p Value |
|---|---|---|
| Taking metformin for diabetes | 1.000 | |
| Yes | 254 (98.8%) | |
| No | 3 (1.2%) | |
| Duration of taking the medication | 0.652 | |
| ≤5 years | 94 (37%) | |
| 5–10 years | 98 (38.6%) | |
| >10 years | 62 (24.4%) | |
| Taking other hypoglycemic medication | 0.036 * | |
| Yes | 82 (31.9%) | |
| No | 175 (68.1%) | |
| Name of hypoglycemic drug | 0.002 ** | |
| Linagliptin | 17 (20.7%) | |
| Insulin | 37 (45.1%) | |
| Gliclazide and sitagliptin | 4 (4.9%) | |
| Glimepiride | 7 (8.5%) | |
| Sitagliptin | 9 (11%) | |
| Empagliflozin and sitagliptin | 7 (8.5%) | |
| Empagliflozin | 1 (1.2%) | |
| Take medication regularly | 0.736 | |
| Yes | 173 (67.3%) | |
| Forgetting sometimes | 84 (32.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.; Muqbel, T.; Abduallh, A.; Alanazi, S.; Khalifa, N.E.; Khojali, W.M.A.; Elagib, H.M.; Hussein, W.; Abdallah, M.H. Metformin Therapy and Breast Cancer Incidence in the Ha’il Region. Healthcare 2023, 11, 321. https://doi.org/10.3390/healthcare11030321
Osman M, Muqbel T, Abduallh A, Alanazi S, Khalifa NE, Khojali WMA, Elagib HM, Hussein W, Abdallah MH. Metformin Therapy and Breast Cancer Incidence in the Ha’il Region. Healthcare. 2023; 11(3):321. https://doi.org/10.3390/healthcare11030321
Chicago/Turabian StyleOsman, Mhdia, Taif Muqbel, Ahad Abduallh, Shuruq Alanazi, Nasrin E. Khalifa, Weam M. A. Khojali, Halima Mustafa Elagib, Weiam Hussein, and Marwa H. Abdallah. 2023. "Metformin Therapy and Breast Cancer Incidence in the Ha’il Region" Healthcare 11, no. 3: 321. https://doi.org/10.3390/healthcare11030321
APA StyleOsman, M., Muqbel, T., Abduallh, A., Alanazi, S., Khalifa, N. E., Khojali, W. M. A., Elagib, H. M., Hussein, W., & Abdallah, M. H. (2023). Metformin Therapy and Breast Cancer Incidence in the Ha’il Region. Healthcare, 11(3), 321. https://doi.org/10.3390/healthcare11030321






