What If the Clinical and Older Adults’ Perspectives about Frailty Converge? A Call for a Mixed Conceptual Model of Frailty: A Traditional Literature Review
Abstract
1. Introduction
2. Methods
2.1. Information Sources
2.2. Literature Search
2.3. Data Extraction
2.4. Data Synthesis
3. Results
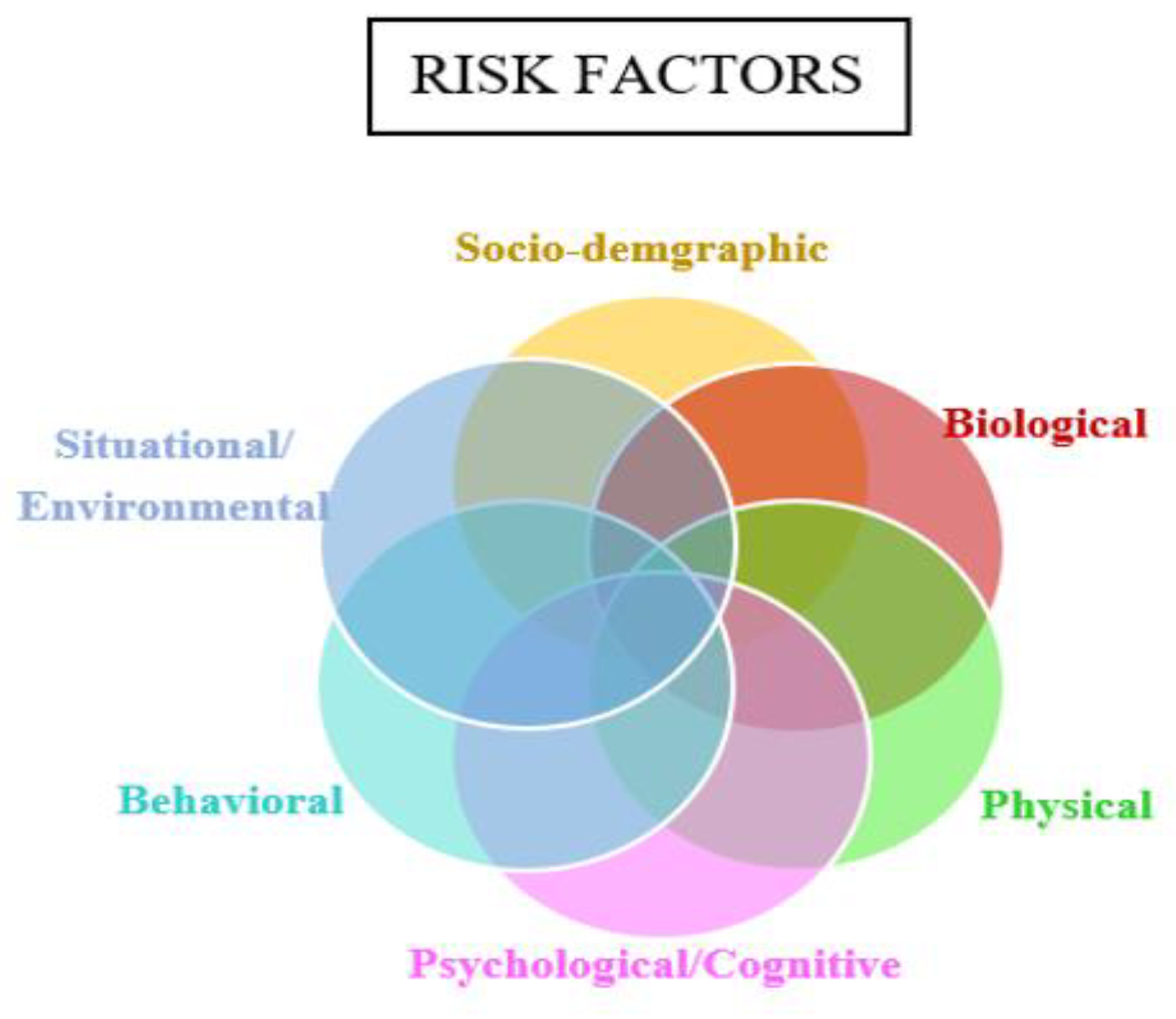
3.1. Defining Attributes of Frailty
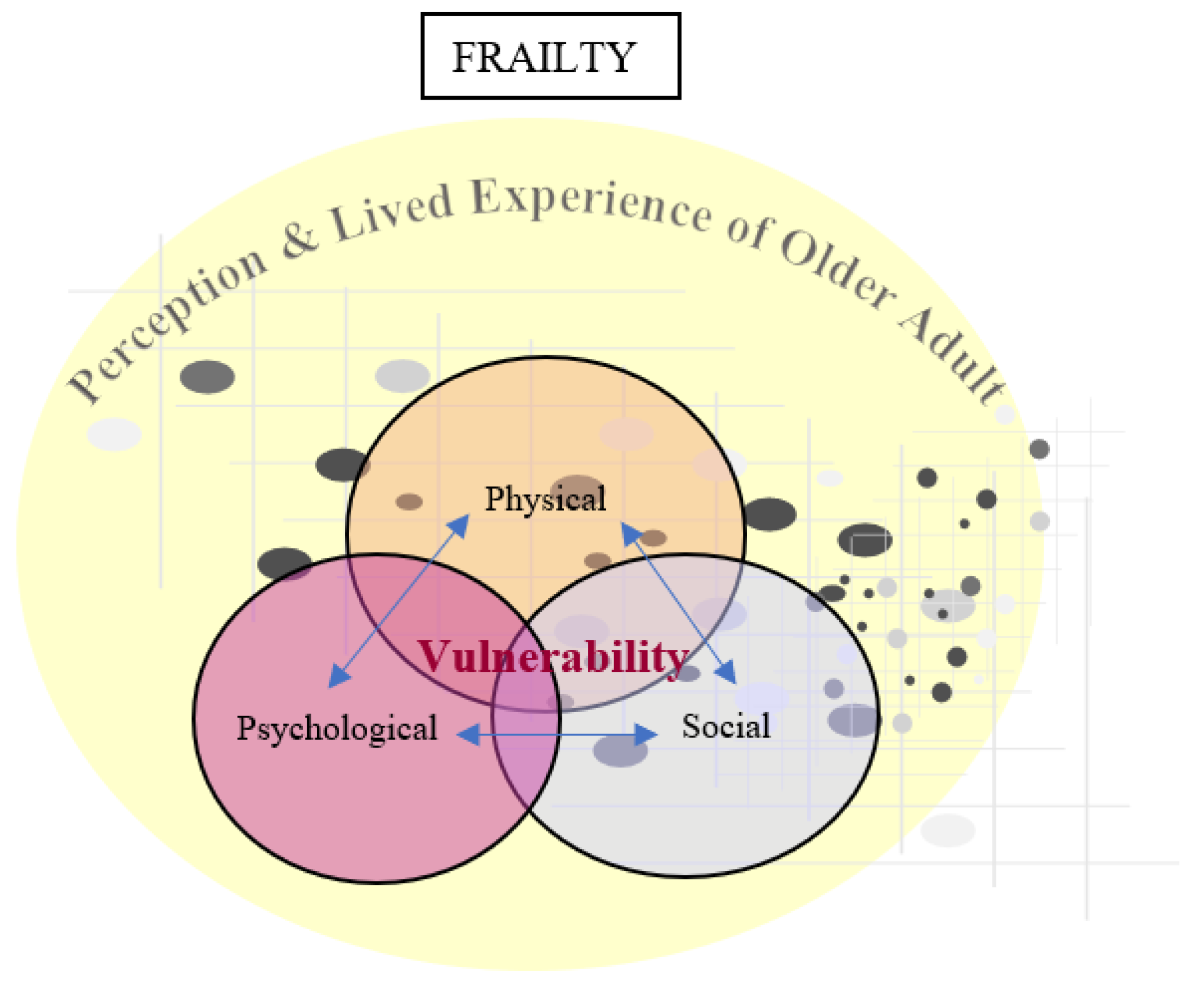
3.2. Perceptions and Lived Experiences of Frailty in Older Adults
3.2.1. Refusing Frailty Labeling Explicitly [8,9,10,11,12] and Additional Labeling Subthemes
3.2.2. Negative Impacts and Experiences of Frailty
- ▪
- ▪
- ▪
- Synergy between low mood and incapable body: When older adults with incapable bodies experienced low mood, their physical limitations intensified [31].
- ▪
- ▪
- Dependency in daily activities: Some older adults living with frailty were dependent on their family members or requested the continuous help of professionals [32].
- ▪
- Shrinkage in social network: Older adults living with frailty experienced a gradual decrease in their in-person contact with others, especially on days when their bodies had functional limitations [32].
3.2.3. Positive Impacts and Experiences of Frailty
- ▪
- ▪
- Rebuilding, fighting, and keeping going: Older adults with frailty and multimorbidity maximized their quality of life through rebuilding and fighting to maintain social relationships and engaging in self-care [33]. Rebuilding refers to how older adults rearrange their lifestyles to reach stability and how they use gratitude and positive life attitudes to enhance their psychological prosperity [31,33].
- ▪
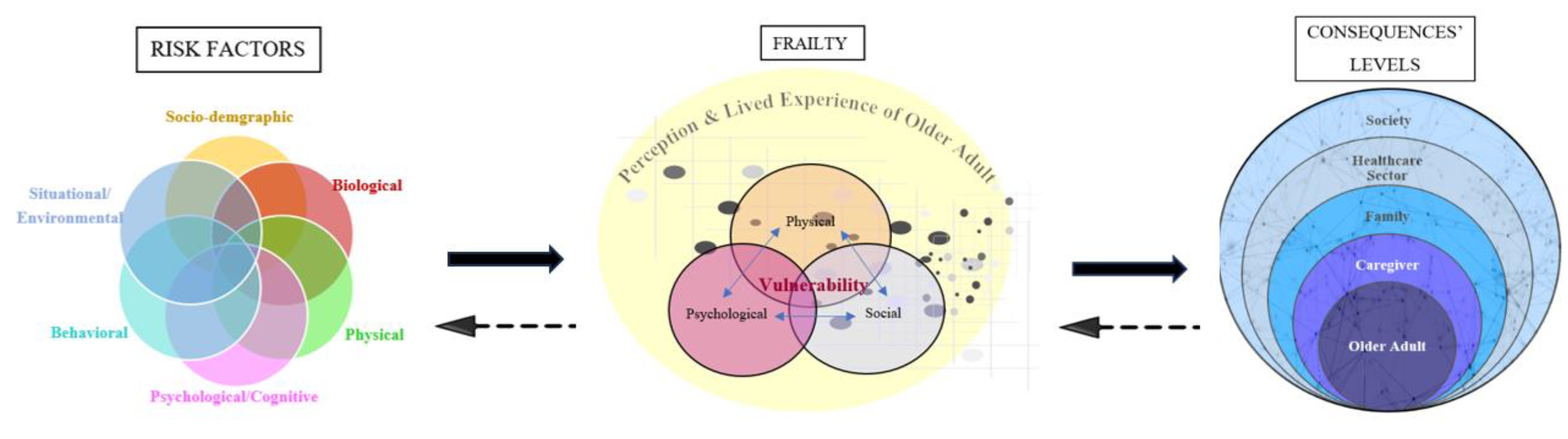
3.3. Frailty Risk Factors
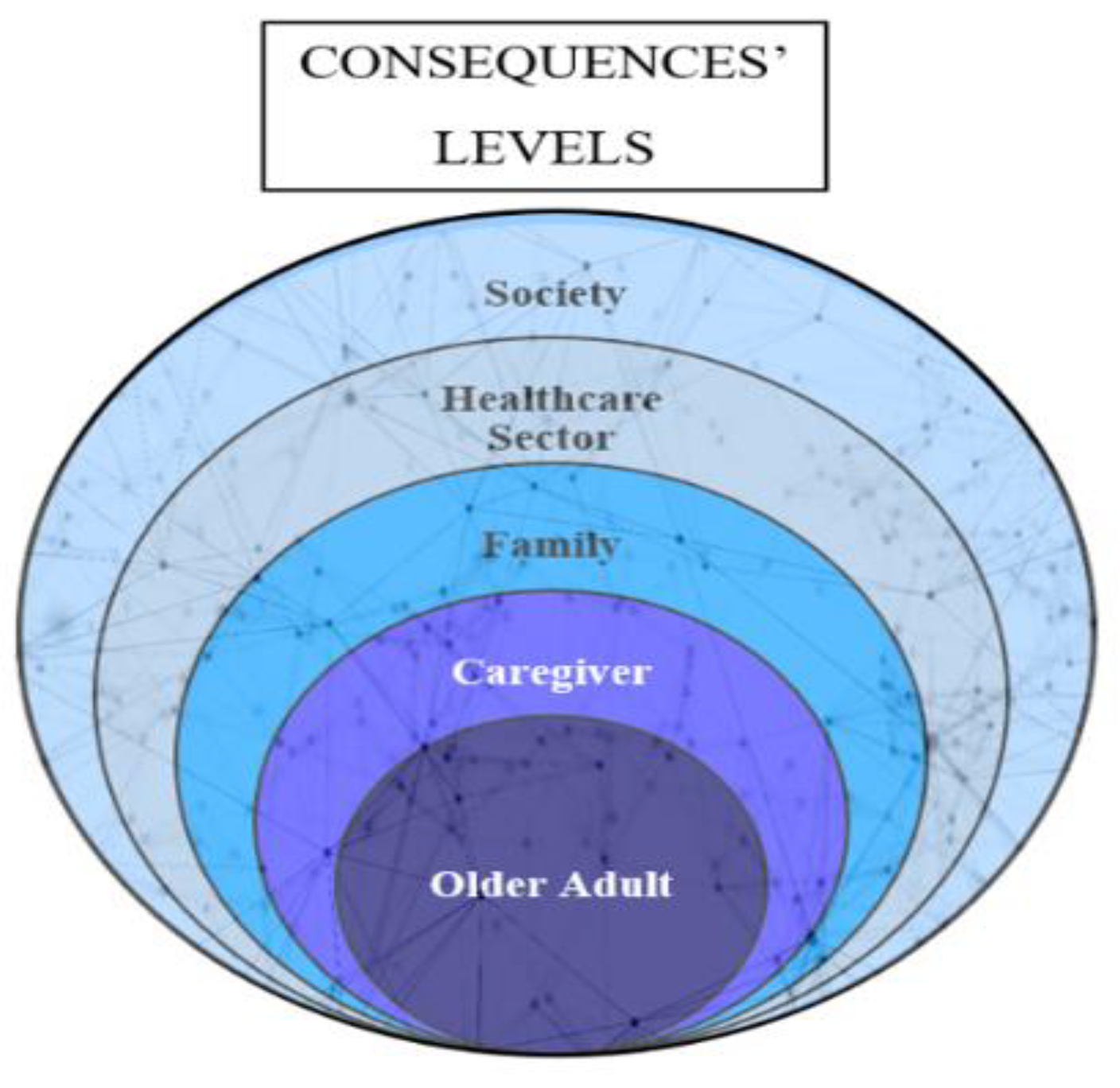
3.4. Frailty Consequences
4. Discussion
The Mixed Conceptual Model of Frailty
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, Q.D.; Moodie, E.M.; Desmarais, P.; Forget, M.-F.; Wang, H.T.; Keezer, M.R.; Wolfson, C. The state of frailty in research: A mapping review of its clinical applicability to practice. Ageing Res. Rev. 2021, 72, 101493. [Google Scholar] [CrossRef]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef]
- Campbell, A.J.; Buchner, D.M. Unstable disability and the fluctuations of frailty. Age Ageing 1997, 26, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Towards an integral conceptual model of frailty. J. Nutr. Health Aging 2010, 14, 175–181. [Google Scholar] [CrossRef]
- Durepos, P.; Sakamoto, M.; Alsbury, K.; Hewston, P.; Borges, J.; Takaoka, A. Older Adults’ Perceptions of Frailty Language: A Scoping Review. Can. J. Aging 2022, 41, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Bloomfield, K.; Boyd, M. Resilience, not frailty: A qualitative study of the perceptions of older adults towards “frailty”. Int. J. Older People Nurs. 2019, 14, e12261. [Google Scholar] [CrossRef]
- Schoenborn, N.L.; Van Pilsum Rasmussen, S.E.; Xue, Q.-L.; Walston, J.D.; McAdams-Demarco, M.A.; Segev, D.L.; Boyd, C.M. Older adults’ perceptions and informational needs regarding frailty. BMC Geriatr. 2018, 18, 46. [Google Scholar] [CrossRef]
- Archibald, M.; Lawless, M.; Ambagtsheer, R.C.; Kitson, A. Older adults’ understandings and perspectives on frailty in community and residential aged care: An interpretive description. BMJ Open 2020, 10, e035339. [Google Scholar] [CrossRef]
- Cluley, V.; Martin, G.; Radnor, Z.; Banerjee, J. Talking about frailty: The role of stigma and precarity in older peoples’ constructions of frailty. J. Aging Stud. 2021, 58, 100951. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Junius-Walker, U.; Onder, G.; Soleymani, D.; Wiese, B.; Albaina, O.; Bernabei, R.; Marzetti, E. The essence of frailty: A systematic review and qualitative synthesis on frailty concepts and definitions. Eur. J. Intern. Med. 2018, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tocchi, C. Frailty in older adults: An evolutionary concept analysis. Res. Theory Nurs. Pract. 2015, 29, 66–84. [Google Scholar] [CrossRef]
- Waldon, M. Frailty in older people: A principle-based concept analysis. Br. J. Community Nurs. 2018, 23, 482–494. [Google Scholar] [CrossRef]
- Wallington, S.L. Frailty: A term with many meanings and a growing priority for community nurses. Br. J. Community Nurs. 2016, 21, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Oliveira, F.; Leal, N.P.d.R.; Medeiros, F.d.A.L.; Oliveira, J.d.S.; Nóbrega, M.M.L.d.; Leadebal, O.D.C.P.; Fernandes, M.d.G.M. Clinical validation of nursing diagnosis Fragile Elderly Syndrome. Rev. Bras. Enferm. 2021, 74. [Google Scholar] [CrossRef]
- Simon, H.A. The architecture of complexity. In Facets of Systems Science; Springer: New York, NY, USA, 1991; pp. 457–476. [Google Scholar]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Oliveira, F.; Barbosa, K.T.F.; Rodrigues, M.M.P.; Fernandes, M. Frailty syndrome in the elderly: Conceptual analysis according to Walker and Avant. Rev. Bras. Enferm. 2020, 73 (Suppl. S3), e20190601. [Google Scholar] [CrossRef]
- Fitten, L.J. Psychological frailty in the aging patient. Frailty Pathophysiol. Phenotype Patient Care 2015, 83, 45–54. [Google Scholar]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; Abellan Van Kan, G.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.; Provencher, V. Cognitive frailty: Rational and definition from an (IANA/IAGG) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Bunt, S.; Steverink, N.; Olthof, J.; van der Schans, C.P.; Hobbelen, J.S.M. Social frailty in older adults: A scoping review. Eur. J. Ageing 2017, 14, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Freer, K.; Wallington, S.L. Social frailty: The importance of social and environmental factors in predicting frailty in older adults. Br. J. Community Nurs. 2019, 24, 486–492. [Google Scholar] [CrossRef]
- Warmoth, K.; Lang, I.A.; Phoenix, C.; Abraham, C.; Andrew, M.K.; Hubbard, R.E.; Tarrant, M. ‘Thinking you’re old and frail’: A qualitative study of frailty in older adults. Ageing Soc. 2016, 36, 1483–1500. [Google Scholar] [CrossRef]
- Dury, S.; Dierckx, E.; van der Vorst, A.; Van der Elst, M.; Fret, B.; Duppen, D.; Hoeyberghs, L.; De Roeck, E.; Lambotte, D.; Smetcoren, A.S.; et al. Detecting frail, older adults and identifying their strengths: Results of a mixed-methods study. BMC Public Health 2018, 18, 191. [Google Scholar] [CrossRef]
- Archibald, M.M.; Lawless, M.T.; Ambagtsheer, R.C.; Kitson, A.L. Understanding consumer perceptions of frailty screening to inform knowledge translation and health service improvements. Age Ageing 2021, 50, 227–232. [Google Scholar] [CrossRef]
- Søvde, B.E.; Sandvoll, A.M.; Natvik, E.; Drageset, J. In the borderland of the body: How home-dwelling older people experience frailty. Scand. J. Caring Sci. 2022, 36, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Skilbeck, J.K.; Arthur, A.; Seymour, J. Making sense of frailty: An ethnographic study of the experience of older people living with complex health problems. Int. J. Older People Nurs. 2018, 13, e12172. [Google Scholar] [CrossRef]
- Johansson, M.M.; Nätt, M.; Peolsson, A.; Öhman, A. Frail community-dwelling older persons’ everyday lives and their experiences of rehabilitation—A qualitative study. Scand. J. Occup. Ther. 2022, 30, 65–75. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, E.Y.; Son, Y.J. Home-dwelling older adults’ experiences of living with both frailty and multimorbidity: A meta-ethnography. Geriatr. Nurs. 2022, 47, 191–200. [Google Scholar] [CrossRef]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef]
- Qin, Y.; Hao, X.; Lv, M.; Zhao, X.; Wu, S.; Li, K. A global perspective on risk factors for frailty in community-dwelling older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2022, 105, 104844. [Google Scholar] [CrossRef]
- Fugate Woods, N.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2005, 53, 1321–1330. [Google Scholar] [CrossRef]
- Usher, T.; Buta, B.; Thorpe Jr, R.J.; Huang, J.; Samuel, L.J.; Kasper, J.D.; Bandeen-Roche, K. Dissecting the racial/ethnic disparity in frailty in a nationally representative cohort study with respect to health, income, and measurement. J. Gerontol. Ser. A 2021, 76, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.H.; Upenieks, L.; MacNeil, A. Disorderly Households, Self-Presentation, and Mortality: Evidence From a National Study of Older Adults. Res. Aging 2018, 40, 762–790. [Google Scholar] [CrossRef] [PubMed]
- Mulasso, A.; Roppolo, M.; Giannotta, F.; Rabaglietti, E. Associations of frailty and psychosocial factors with autonomy in daily activities: A cross-sectional study in Italian community-dwelling older adults. Clin. Interv. Aging 2016, 11, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Chen, C.-Y.; Lue, B.-H.; Tseng, M.-Y.; Wu, S.-C. Prevalence and associated factors of frailty among elderly people in Taiwan. Int. J. Gerontol. 2014, 8, 114–119. [Google Scholar] [CrossRef]
- Hubbard, R.E.; O’Mahony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and frailty measures in older people. J. Cell Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef]
- Rodríguez Mañas, L. Determinants of Frailty and Longevity: Are They the Same Ones? Nestle Nutr. Inst. Workshop Ser. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Shamsi, K.S.; Pierce, A.; Ashton, A.S.; Halade, D.G.; Richardson, A.; Espinoza, S.E. Proteomic screening of glycoproteins in human plasma for frailty biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.H.; Semba, R.D.; Leng, S.X.; Woodman, R.C.; Ferrucci, L.; Guralnik, J.M.; Fried, L.P. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: The Women’s Health and Aging Studies I and II. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm-Leen, E.R.; Hall, Y.N.; Tamura, M.K.; Chertow, G.M. Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am. J. Med. 2009, 122, 664–671.e2. [Google Scholar] [CrossRef] [PubMed]
- Topinková, E. Aging, disability and frailty. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 6–11. [Google Scholar] [CrossRef]
- Velázquez-Olmedo, L.B.; Borges-Yáñez, S.A.; Palos, P.A.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Sánchez-García, S. Oral health condition and development of frailty over a 12-month period in community-dwelling older adults. BMC Oral Health 2021, 21, 355. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, F.; Li, H.; Lin, S.; Yuan, Y.; Zhu, P. Examining the independent and interactive association of physical activity and sedentary behaviour with frailty in Chinese community-dwelling older adults. BMC Public Health 2022, 22, 1414. [Google Scholar] [CrossRef]
- Jang, W.; Shin, Y.; Kim, Y. Dietary Pattern Accompanied with a High Food Variety Score Is Negatively Associated with Frailty in Older Adults. Nutrients 2021, 13, 3164. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Navas-Acien, A.; Pérez-Gómez, B.; Artalejo, F.R. Association of lead and cadmium exposure with frailty in US older adults. Environ. Res. 2015, 137, 424–431. [Google Scholar] [CrossRef]
- Stow, D.; Hanratty, B.; Matthews, F.E. The relationship between deprivation and frailty trajectories over 1 year and at the end of life: A case-control study. J. Public Health 2022, 44, 844–850. [Google Scholar] [CrossRef]
- Salem, B.E.; Nyamathi, A.; Phillips, L.R.; Mentes, J.; Sarkisian, C.; Brecht, L. Identifying frailty among vulnerable populations. ANS. Adv. Nurs. Sci. 2014, 37, 70. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, e1161–e1163. [Google Scholar] [CrossRef] [PubMed]
- Furtado, G.E.; Letieri, R.; Hogervorst, E.; Teixeira, A.B.; Ferreira, J.P. Physical Frailty and cognitive performance in older populations, part I: Systematic review with meta-analysis. Cienc. Saude Coletiva 2019, 24, 203–218. [Google Scholar] [CrossRef]
- Viljanen, A.; Salminen, M.; Irjala, K.; Korhonen, P.; Wuorela, M.; Isoaho, R.; Kivelä, S.L.; Vahlberg, T.; Viitanen, M.; Löppönen, M.; et al. Frailty, walking ability and self-rated health in predicting institutionalization: An 18-year follow-up study among Finnish community-dwelling older people. Aging Clin. Exp. Res. 2021, 33, 547–554. [Google Scholar] [CrossRef]
- Saragih, I.D.; Advani, S.; Saragih, I.S.; Suarilah, I.; Susanto, I.; Lin, C.J. Frailty as a mortality predictor in older adults with COVID-19: A systematic review and meta-analysis of cohort studies. Geriatr. Nurs. 2021, 42, 983–992. [Google Scholar] [CrossRef]
- Gok Metin, Z.; Karadas, C.; Balci, C.; Cankurtaran, M. The Perceived Caregiver Burden Among Turkish Family Caregivers Providing Care for Frail Older Adults. J. Transcult. Nurs. 2019, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef]
- Bock, J.-O.; König, H.-H.; Brenner, H.; Haefeli, W.E.; Quinzler, R.; Matschinger, H.; Saum, K.-U.; Schöttker, B.; Heider, D. Associations of frailty with health care costs–results of the ESTHER cohort study. BMC Health Serv. Res. 2016, 16, 128. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, K.; Jiang, Y.; Yu, Q.; Huang, X.; Wang, J.; Liu, N.; Huang, P. The Impact of Frailty on COVID-19 Outcomes: A Systematic Review and Meta-analysis of 16 Cohort Studies. J. Nutr. Health Aging 2021, 25, 702–709. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Zhao, S.; Liu, X. Factors associated with frailty transitions among the old-old in a community. Geriatr. Gerontol. Int. 2023, 23, 405–410. [Google Scholar] [CrossRef]
- Shinohara, T.; Saida, K.; Tanaka, S.; Murayama, A.; Higuchi, D. Factors for the change in frailty status during the COVID-19 pandemic: A prospective cohort study over six- and 12-month periods in Japan. Geriatr. Nurs. 2022, 48, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mielke, N.; Schneider, A.; Huscher, D.; Ebert, N.; Schaeffner, E. Gender differences in frailty transition and its prediction in community-dwelling old adults. Sci. Rep. 2022, 12, 7341. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.Y.W.; Cheung, D.S.K.; Kwan, R.Y.C.; Wong, A.S.W.; Lai, C.K.Y. Factors associated with frailty transition at different follow-up intervals: A scoping review. Geriatr. Nurs. 2021, 42, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.S.K.; Kwan, R.Y.C.; Wong, A.S.W.; Ho, L.Y.W.; Chin, K.C.; Liu, J.Y.W.; Tse, M.M.Y.; Lai, C.K.Y. Factors Associated With Improving or Worsening the State of Frailty: A Secondary Data Analysis of a 5-Year Longitudinal Study. J. Nurs. Scholarsh. 2020, 52, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Jivraj, S.; Walters, K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 50, 81–88. [Google Scholar] [CrossRef]
- Fan, S.; Ye, J.; Xu, Q.; Peng, R.; Hu, B.; Pei, Z.; Yang, Z.; Xu, F. Digital health technology combining wearable gait sensors and machine learning improve the accuracy in prediction of frailty. Front. Public Health 2023, 11, 1169083. [Google Scholar] [CrossRef]
- da Cunha Leme, D.E.; De Oliveira, C. Machine Learning Models to Predict Future Frailty in Community-dwelling Middle-aged and Older Adults: The ELSA cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2023. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, M.; Xiang, C.; Fang, Y. Latent trajectories of frailty and risk prediction models among geriatric community dwellers: An interpretable machine learning perspective. BMC Geriatr. 2022, 22, 900. [Google Scholar] [CrossRef]
- Aprahamian, I.; Xue, Q.-L. Shaping the next steps of research on frailty: Challenges and opportunities. BMC Geriatr. 2021, 21, 432. [Google Scholar] [CrossRef]
- Yin, L.; Sawaya, Y.; Sato, R.; Shiba, T.; Onoda, K. Characteristics of falls and their fear in older adults requiring long-term care. J. Phys. Ther. Sci. 2023, 35, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Auais, M.; French, S.; Alvarado, B.; Pirkle, C.; Belanger, E.; Guralnik, J. Fear of Falling Predicts Incidence of Functional Disability 2 Years Later: A Perspective from an International Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Huo, B.; McKechnie, T.; Agzarian, J.; Hong, D. Impact of frailty on hiatal hernia repair: A nationwide analysis of in-hospital clinical and healthcare utilization outcomes. Dis. Esophagus 2023. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hou, X.Y.; Liu, Y.; Chen, S.; Wang, Q.; Du, W. Catastrophic Health Expenditure Associated with Frailty in Community-Dwelling Chinese Older Adults: A Prospective Cohort Analysis. Front. Public Health 2021, 9, 718910. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Differences in frailty in older men and women. Med. J. Aust. 2020, 212, 183–188. [Google Scholar] [CrossRef]
- Pickard, S.; Cluley, V.; Danely, J.; Laceulle, H.; Leon-Salas, J.; Vanhoutte, B.; Romero-Ortuno, R. New horizons in frailty: The contingent, the existential and the clinical. Age Ageing 2019, 48, 466–471. [Google Scholar] [CrossRef]
| Frailty Risk Factors | ||
|---|---|---|
| Socio-demographic | Increased age [34,35,36] | |
| Female sex [34,35] | ||
| Non-Hispanic Black and Hispanic American [37] | ||
| African American [34] | ||
| Low income [34,35,36] | ||
| Low socioeconomic status [34] | ||
| Low educational level [34,35] | ||
| Living alone [35] | ||
| Being widowed or single [35] | ||
| Disorderly households [38] | ||
| Social isolation [39] | ||
| Poor social interaction [40] | ||
| Decreased social support [22] | ||
| Biological | Inflammation | Increase in Interleukin-6 [34,41,42] |
| Increase in TNF-α [34,41,42] | ||
| Increase in C-reactive protein [34,41,42] | ||
| Increase in fibrinogen [34,42] | ||
| Increase in D-dimer [34,42] | ||
| Increase in leukocytes [34,42] | ||
| Increase in monocytes and lymphocytes [34,42] | ||
| Hormonal Changes | Low levels of free testosterone [34,42] | |
| Low level of estradiol [34,42] | ||
| Lowering in dehydroepiandrosterone sulfate (DHEAS) [34,42] | ||
| Elevated cortisol–DHEAS ratio [34,42] | ||
| Decreased growth hormone [34,42] | ||
| Decreased insulin-like growth factor-1 [34,42] | ||
| Other Biological Factors | Abnormal albumin level [34] | |
| Increased uric acid [34] | ||
| Lower levels of 25-hydroxyvitamin D [34] | ||
| Multiple deficiencies in micronutrients [34] | ||
| Changes in glycoproteins, including HbA1c [43] | ||
| Variability in resting metabolic rate [22] | ||
| Physical | Chronic diseases and comorbidity mainly: | |
| Malnutrition [35] | ||
| Being underweight, overweight, or obese [34,36] | ||
| Being overweight or obese in adulthood [42] | ||
| Sarcopenic obesity in older adults [22,42] | ||
| Genetic factors [42] | ||
| Functional limitations as per ADL and IADL [35] | ||
| Limitation in extremities’ function [34] | ||
| High allostatic load [34] | ||
| Urinary incontinence [35] | ||
| Edentulism and poor oral health [47] | ||
| Psychological and Cognitive | Cognitive impairment [34,35] | |
| Dementia [42] | ||
| Depression [34,35,36] | ||
| Spouse’s depression [34] | ||
| Loneliness feeling [36,39] | ||
| Poor self-reported health [34,35,36] | ||
| Low positive affect [34] | ||
| Behavioral | Smoking [34,36] | |
| Alcohol consumption [34,36] | ||
| Physical inactivity and sedentary lifestyle [48] | ||
| Low mental activity [14] | ||
| Irregular eating habits and low variety in food [22,49] | ||
| Messy body—self-presentation in the form of a disheveled appearance or poor hygiene [26] | ||
| Situational and Environmental | Polypharmacy [35] | |
| Crowded neighborhood [34] | ||
| Increased number of falls in the previous year [34] | ||
| Use of hormones [34] | ||
| Cold home [26] | ||
| Increased blood lead level [50] | ||
| Living in deprived areas [51] | ||
| Neighborhood safety [15] | ||
| Proximity to shops and healthcare services [15] | ||
| Physical/sexual/verbal abuse [52] | ||
| Incarceration [52] | ||
| Homelessness [52] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.H.; Gobbens, R.J.J. What If the Clinical and Older Adults’ Perspectives about Frailty Converge? A Call for a Mixed Conceptual Model of Frailty: A Traditional Literature Review. Healthcare 2023, 11, 3174. https://doi.org/10.3390/healthcare11243174
Khalil AH, Gobbens RJJ. What If the Clinical and Older Adults’ Perspectives about Frailty Converge? A Call for a Mixed Conceptual Model of Frailty: A Traditional Literature Review. Healthcare. 2023; 11(24):3174. https://doi.org/10.3390/healthcare11243174
Chicago/Turabian StyleKhalil, Asya Hani, and Robbert J. J. Gobbens. 2023. "What If the Clinical and Older Adults’ Perspectives about Frailty Converge? A Call for a Mixed Conceptual Model of Frailty: A Traditional Literature Review" Healthcare 11, no. 24: 3174. https://doi.org/10.3390/healthcare11243174
APA StyleKhalil, A. H., & Gobbens, R. J. J. (2023). What If the Clinical and Older Adults’ Perspectives about Frailty Converge? A Call for a Mixed Conceptual Model of Frailty: A Traditional Literature Review. Healthcare, 11(24), 3174. https://doi.org/10.3390/healthcare11243174







