Effectiveness of Virtual Reality and Feedback to Improve Gait and Balance in Patients with Diabetic Peripheral Neuropathies: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
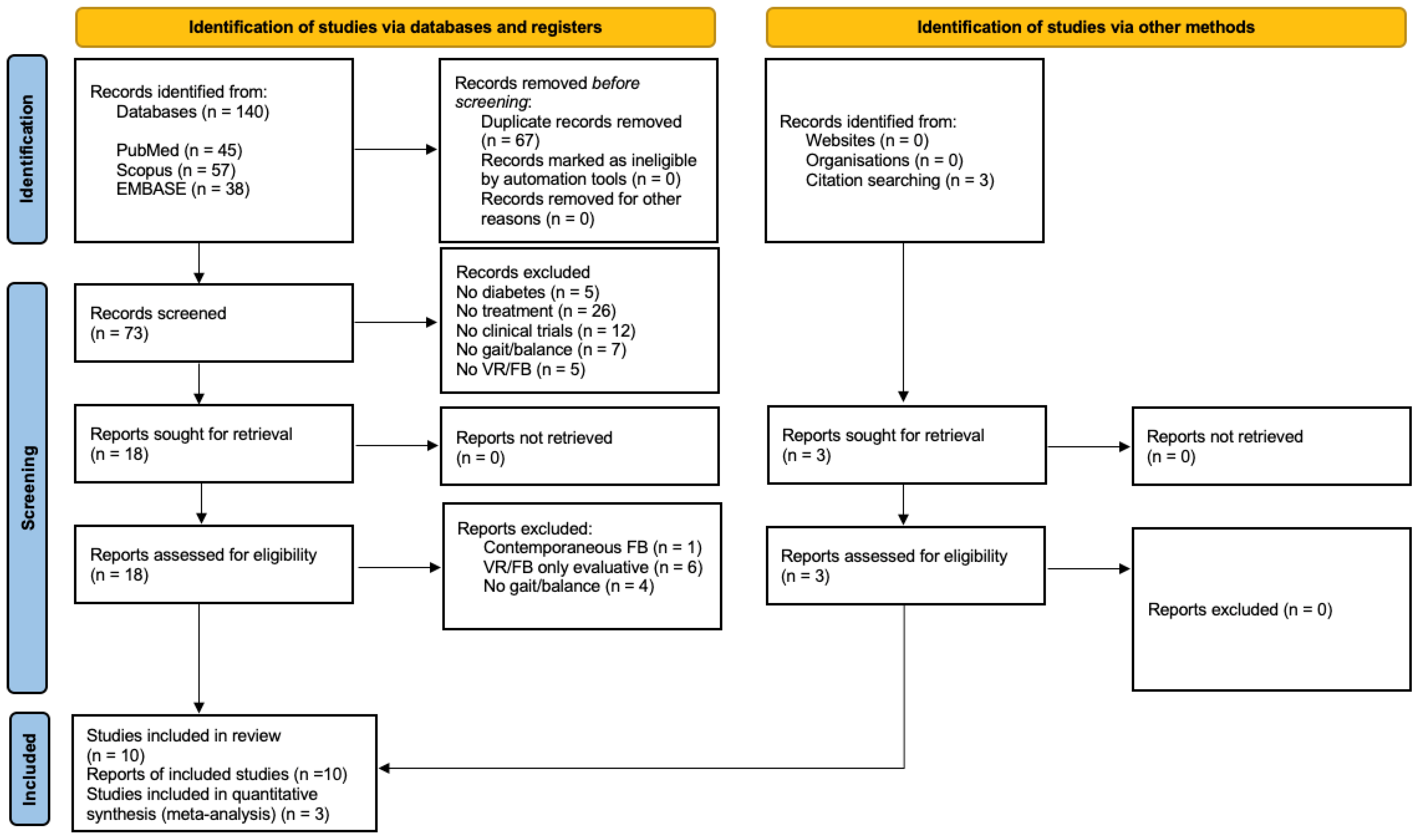
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Assessment of the Methodological Quality and Risk of Bias
2.4. Selection Process and Data Extraction
2.5. Data Synthesis and Statistical Analysis
3. Results
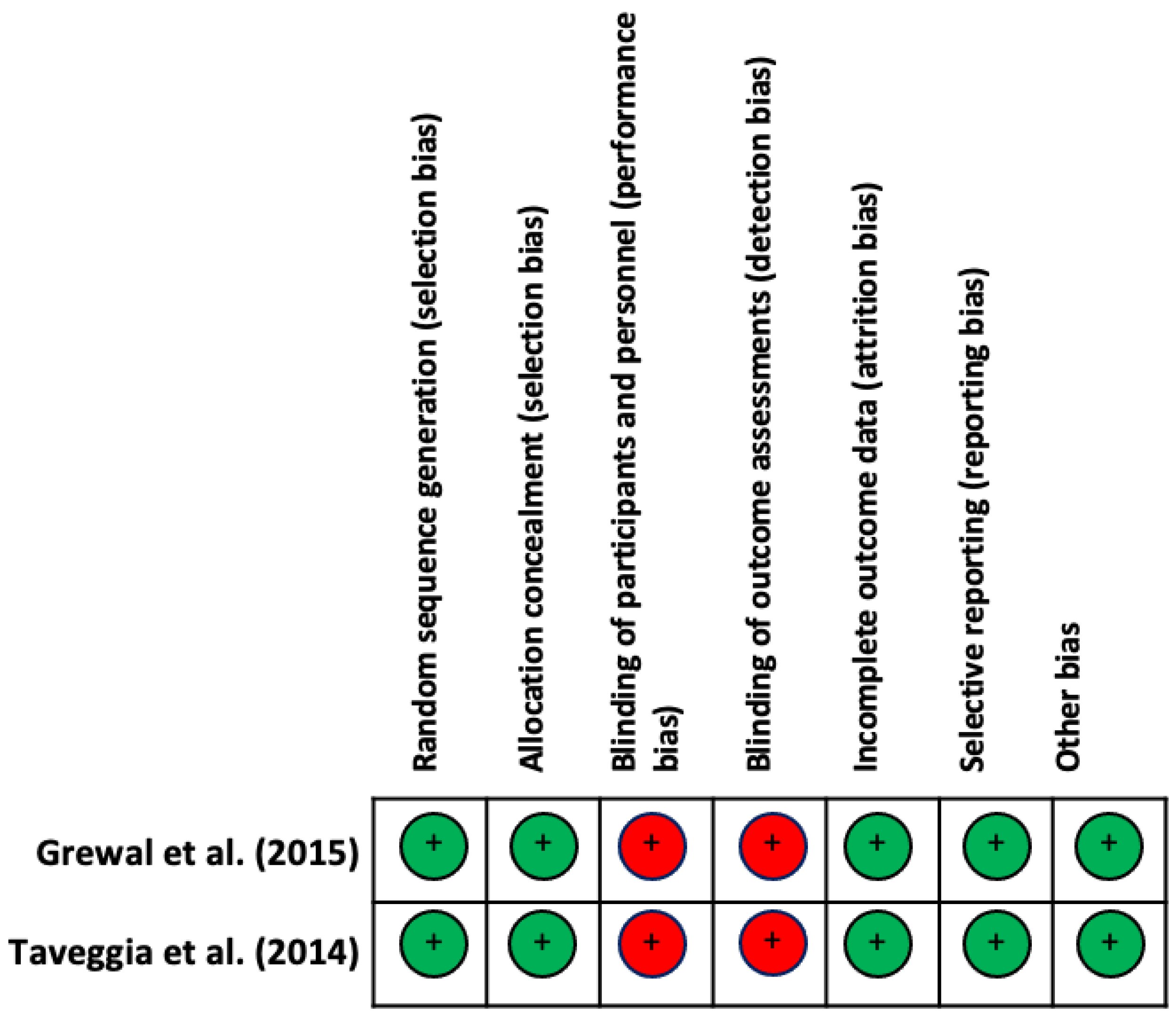
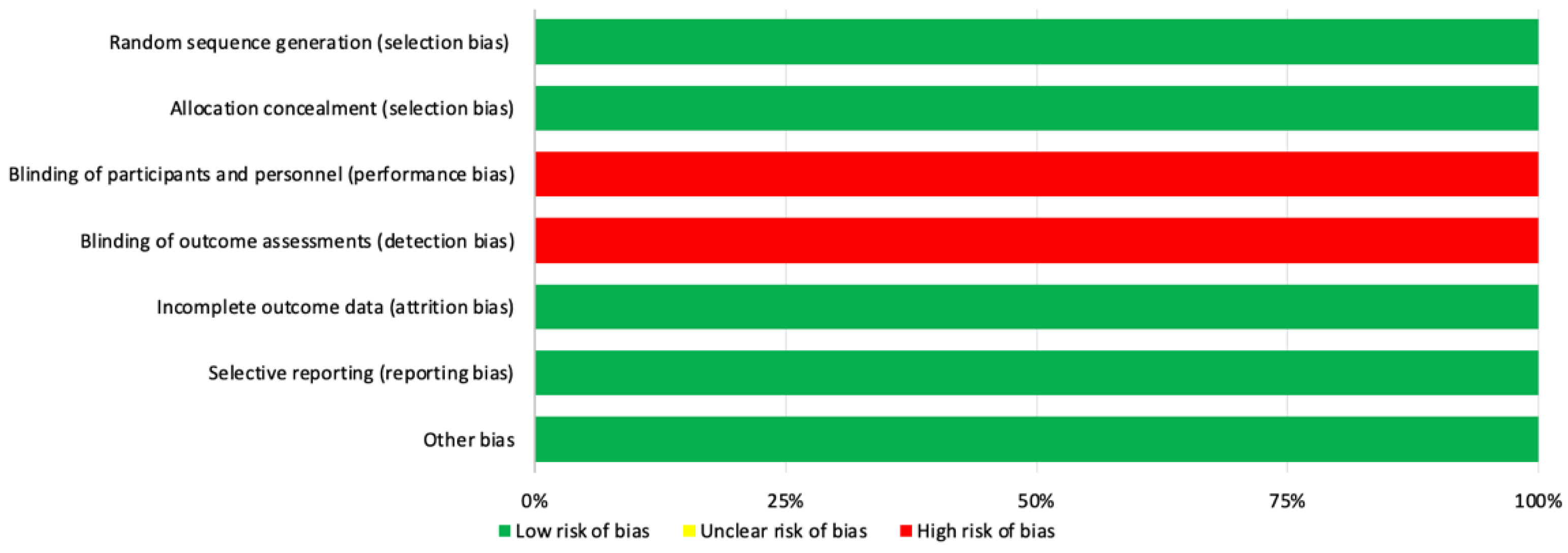
3.1. Methodological Quality and Risk of Bias of Included Studies
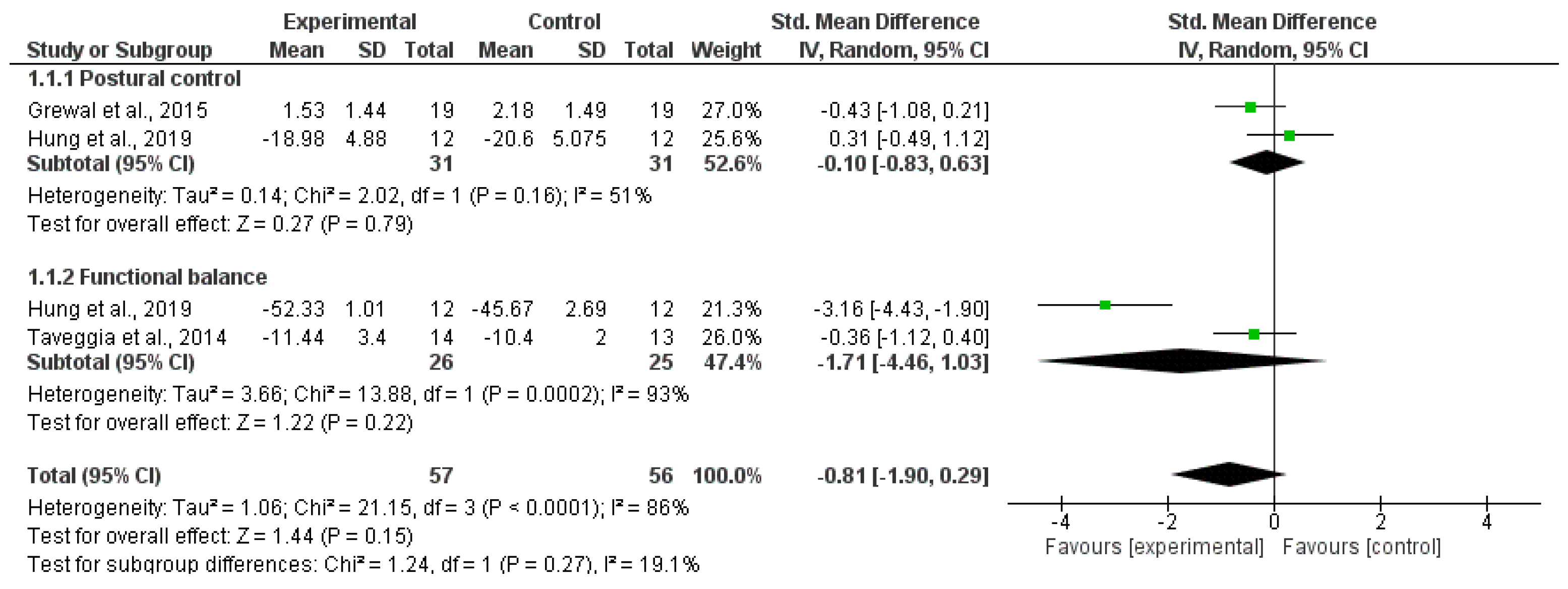
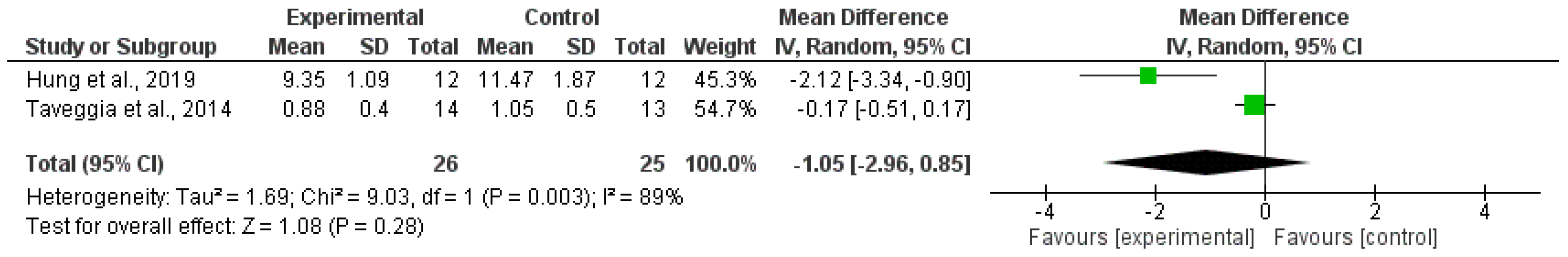
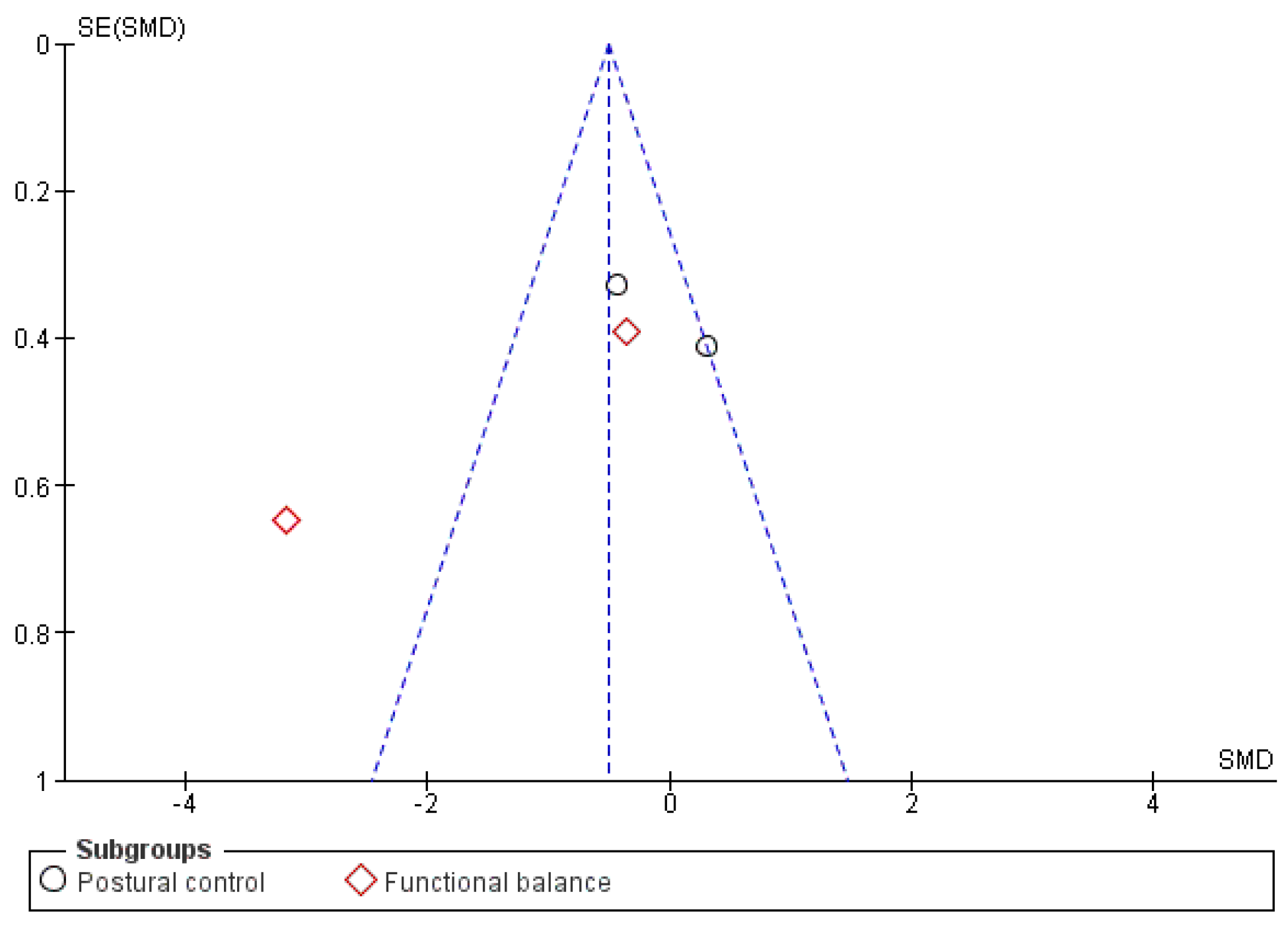
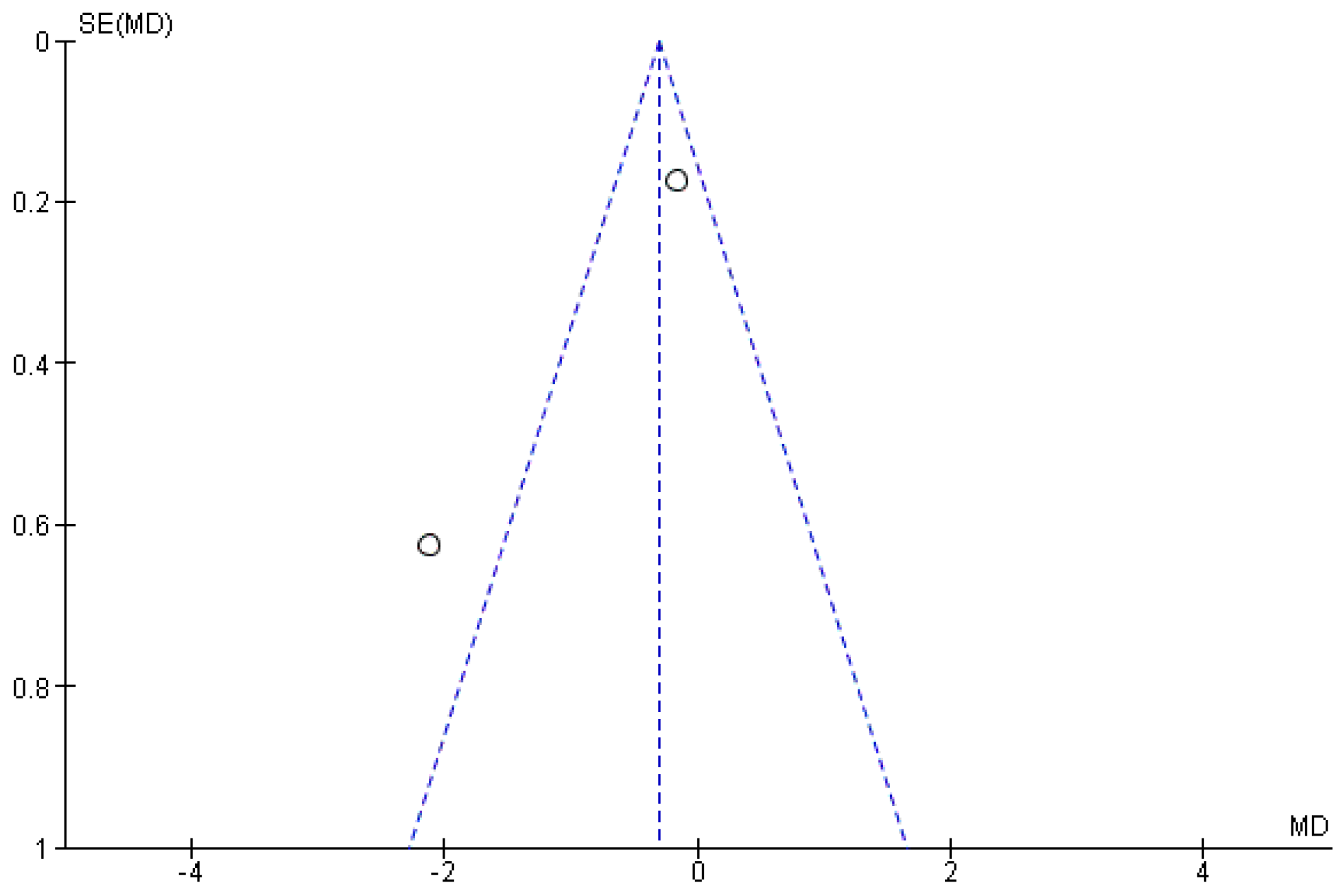
3.2. Synthesis of Results
3.3. Participant and Intervention Characteristics
3.3.1. Classification of Participants According to Peripheral Neuropathy
3.3.2. Training Sessions
3.4. Study Groups Included in the Statistical Analysis
4. Discussion
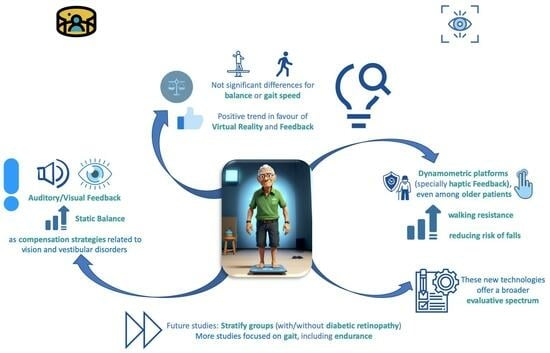
4.1. Balance
4.2. Gait
4.3. Risk of Falls
4.4. Study Limitations and Future Research Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cao, W.; Zhang, J.; Wang, J.; Liu, X.; Wu, Q.; Lin, Q. Determinants of Diabetic Peripheral Neuropathy and Their Clinical Significance: A Retrospective Cohort Study. Front. Endocrinol. 2022, 13, 934020. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, W.; Ghirlanda, G.; Cercone, S.; Pitocco, D.; Soponara, C.; Cosenza, A.; Paludetti, G.; Di Leo, M.A.S.; Galli, I. The use of dynamic posturography to detect neurosensorial disorder in IDDM without clinical neuropathy. J. Diabetes Complicat. 1999, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hohne, A.; Ali, S.; Stark, C.; Bruggemann, G.P. Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. Eur. J. Appl. Physiol. 2012, 112, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.I.; Chen, Y.R.; Liao, C.F.; Chou, C.W. Association between sensorimotor function and forward reach in patients with diabetes. Gait Posture 2010, 32, 581–585. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yahya, A.; Kluding, P.; Pasnoor, M.; Wick, J.; Liu, W.; dos Santos, M. The impact of diabetic peripheral neuropathy on pinch proprioception. Exp. Brain Res. 2019, 237, 3165–3174. [Google Scholar] [CrossRef]
- Mustapa, A.; Justine, M.; Mohd Mustafah, N.; Jamil, N.; Manaf, H. Postural Control and Gait Performance in the Diabetic Peripheral Neuropathy: A Systematic Review. Biomed Res. Int. 2016, 2016, 9305025. [Google Scholar] [CrossRef]
- Salsabili, H.; Bahrpeyma, F.; Forogh, B.; Rajabali, S. Dynamic stability training improves standing balance control in neuropathic patients with type 2 diabetes. J. Rehabil. Res. Dev. 2011, 48, 775–786. [Google Scholar] [CrossRef]
- Taveggia, G.; Villafañe, J.H.; Vavassori, F.; Lecchi, C.; Borboni, A.; Negrini, S. Multimodal treatment of distal sensorimotor polyneuropathy in diabetic patients: A randomized clinical trial. J. Manip. Physiol. Ther. 2014, 37, 242–252. [Google Scholar] [CrossRef]
- Nardone, A.; Grasso, M.; Schieppati, M. Balance control in peripheral neuropathy: Are patients equally unstable under static and dynamic conditions? Gait Posture 2006, 23, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Lesinski, M.; Hortobágyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-analysis. Sports Med. 2015, 45, 1721–1738. [Google Scholar] [CrossRef]
- Viseux, F.J.F. The sensory role of the sole of the foot: Review and update on clinical perspectives. Neurophysiol. Clin. 2020, 50, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.H.; Antigual, D.U.; Martínez, S.F.; Monrroy, M.A.; Gajardo, R.E. Static balance in patients presenting diabetes mellitus type 2 with and without diabetic polyneuropathy. Arq. Bras. Endocrinol. Metabol. 2013, 57, 722–726. [Google Scholar] [CrossRef]
- Ahmad, I.; Noohu, M.M.; Verma, S.; Singla, D.; Hussain, M.E. Effect of sensorimotor training on balance measures and proprioception among middle and older age adults with diabetic peripheral neuropathy. Gait Posture 2019, 74, 114–120. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. A tactile stimulus applied to the leg improves postural stability in young, old and neuropathic subjects. Neurosci. Lett. 2006, 406, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2015, 61, 567–574. [Google Scholar] [CrossRef]
- Metz, V.R.; Andrade, R.M.; Machado-Lima, A.; Amadio, A.C.; Serrão, J.C.; Greve, J.M.D.; Alonso, A.C. Influence of diabetic neuropathy on gait complexity. Rev. Bras. Med. Esporte 2020, 26, 431–435. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS ONE 2015, 10, e0135255. [Google Scholar] [CrossRef]
- van Schie, C.H.M. Neuropathy: Mobility and quality of life. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. S1), S45–S51. [Google Scholar] [CrossRef]
- Herrera-Rangel, A.; Aranda-Moreno, C.; Mantilla-Ochoa, T.; Zainos-Saucedo, L.; Jáuregui-Renaud, K. The Influence of Peripheral Neuropathy, Gender, and Obesity on the Postural Stability of Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 787202. [Google Scholar] [CrossRef]
- Cai, K.; Liu, Y.; Wang, D. Prevalence of diabetic retinopathy in patients with newly diagnosed type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2023, 39, e3586. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor-Moreno, J.; Aranda-Moreno, C.; Figueroa-Padilla, I.; Giraldez-Fernández, M.; Gresty, M.; Jáuregui-Renaud, K. Individual Cofactors and Multisensory Contributions to the Postural Sway of Adults with Diabetes. Brain Sci. 2022, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, L.J.; Lin, J.; Staecker, H.; Whitney, S.L.; Kluding, P.M. Impact of Diabetic Complications on Balance and Falls: Contribution of the Vestibular System. Phys. Ther. 2016, 96, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Deng, F.; Rui, S.; Ma, Y.; Wang, M.; Deng, B.; Wang, H.; Du, C.; Chen, B.; Yang, X.; et al. The Evaluation of Gait and Balance for Patients with Early Diabetic Peripheral Neuropathy: A Cross-Sectional Study. Risk Manag. Healthc. Policy 2022, 15, 543–552. [Google Scholar] [CrossRef]
- Chatwin, K.E.; Abbott, C.A.; Boulton, A.J.M.; Bowling, F.L.; Reeves, N.D. The role of foot pressure measurement in the prediction and prevention of diabetic foot ulceration-A comprehensive review. Diabetes Metab. Res. Rev. 2020, 36, e3258. [Google Scholar] [CrossRef]
- Carroll, K.E.; Galles, D.J. Case study using virtual rehabilitation for a patient with fear of falling due to diabetic peripheral neuropathy. Int. J. Child Health Hum. Dev. 2018, 11, 257–261. [Google Scholar]
- Tang, U.H.; Zügner, R.; Lisovskaja, V.; Karlsson, J.; Hagberg, K.; Tranberg, R. Foot deformities, function in the lower extremities, and plantar pressure in patients with diabetes at high risk to develop foot ulcers. Diabet. Foot Ankle 2015, 6, 27593. [Google Scholar] [CrossRef]
- Francia, P.; Gulisano, M.; Anichini, R.; Seghieri, G. Diabetic Foot and Exercise Therapy: Step by Step the Role of Rigid Posture and Biomechanics Treatment. Curr. Diabetes Rev. 2014, 10, 86–99. [Google Scholar] [CrossRef]
- Alam, U.; Riley, D.R.; Jugdey, R.S.; Azmi, S.; Rajbhandari, S.; D’Août, K.; Malik, R.A. Diabetic Neuropathy and Gait: A Review. Diabetes Ther. 2017, 8, 1253–1264. [Google Scholar] [CrossRef]
- Ramdharry, G. Peripheral nerve disease. Handb. Clin. Neurol. 2018, 159, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Eibel, B.; Sbruzzi, G.; Lago, P.D.; Plentz, R.D.M. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: Systematic review and meta-analysis. Braz. J. Phys. Ther. 2013, 17, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.; Armour, M.; Penkala, S. Acupuncture for the treatment of lower limb diabetic peripheral neuropathy: A systematic review. Acupunct. Med. 2019, 37, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kristianto, H.; Waluyo, A.; Gayatri, D.; Yunir, E.; Blow, D. Neuromuscular taping treatment of diabetic foot: A concept analysis. Clin. Ter. 2021, 172, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Horstink, K.A.; van der Woude, L.H.V.; Hijmans, J.M. Effects of offloading devices on static and dynamic balance in patients with diabetic peripheral neuropathy: A systematic review. Rev. Endocr. Metab. Disord. 2021, 22, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, I.J.M.; van de Port, I.G.L.; Meijer, J.W.G. Effect of virtual reality training on balance and gait ability in patients with stroke: Systematic review and Meta-Analysis. Phys. Ther. 2016, 96, 1905–1918. [Google Scholar] [CrossRef]
- Booth, A.T.C.; Buizer, A.I.; Meyns, P.; Oude Lansink, I.L.B.; Steenbrink, F.; van der Krogt, M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Sveistrup, H. Motor rehabilitation using virtual reality. J. Neuroeng. Rehabil. 2004, 1, 10. [Google Scholar] [CrossRef]
- Tuena, C.; Pedroli, E.; Trimarchi, P.D.; Gallucci, A.; Chiappini, M.; Goulene, K.; Gaggioli, A.; Riva, G.; Lattanzio, F.; Giunco, F.; et al. Usability Issues of Clinical and Research Applications of Virtual Reality in Older People: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, R.; Warren, J.; Lange, B.; Chang, C.-Y. Safety and Feasibility of a First-Person View, Full-Body Interaction Game for Telerehabilitation Post-Stroke. Int. J. Telerehabilitation 2018, 10, 29–36. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Cano-Bravo, F.; Kiper, P.; Solís-Marcos, I.; Moral-Munoz, J.A.; Agostini, M.; Oliva-Pascual-Vaca, Á.; Turolla, A. Reinforced Feedback in Virtual Environment for Plantar Flexor Poststroke Spasticity Reduction and Gait Function Improvement. Biomed Res. Int. 2019, 2019, 6295263. [Google Scholar] [CrossRef]
- Chamorro-Moriana, G.; Moreno, A.J.; Sevillano, J.L. Technology-Based Feedback and Its Efficacy in Improving Gait Parameters in Patients with Abnormal Gait: A Systematic Review. Sensors 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Abibullaev, B.; An, J.; Lee, S.H.; Moon, J. Il Design and evaluation of action observation and motor imagery based BCIs using Near-Infrared Spectroscopy. Meas. J. Int. Meas. Confed. 2017, 98, 250–261. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Oliva-Pascual-Vaca, A.; Kiper, P.; Rodríguez-Blanco, C.; Agostini, M.; Turolla, A. Virtual Reality to Assess and Treat Lower Extremity Disorders in Post-stroke Patients. Methods Inf. Med. 2016, 55, 89–92. [Google Scholar] [CrossRef]
- Elaraby, A.E.R.; Shahien, M.; Jahan, A.M.; Etoom, M.; Bekhet, A.H. The Efficacy of Virtual Reality Training in the Rehabilitation of Orthopedic Ankle Injuries: A Systematic Review and Meta-analysis. Adv. Rehabil. Sci. Pract. 2023, 12, 117957272311516. [Google Scholar] [CrossRef]
- Khdour, M.R. Treatment of diabetic peripheral neuropathy: A review. J. Pharm. Pharmacol. 2020, 72, 863–872. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Santos, C.M.d.C.; Pimenta, C.A.d.M.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke rehabilitation evidence-based review: Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, R.; Wang, H.; Dionne, C.; James, S.; Burzycki, J. Wearable Focal Muscle Vibration on Pain, Balance, Mobility, and Sensation in Individuals with Diabetic Peripheral Neuropathy: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 2415. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, A.; Jarzemski, I.; Maciąg, B.M.; Radzimowski, K.; Świercz, M.; Stolarczyk, M. Balance and motion coordination parameters can be improved in patients with type 2 diabetes with physical balance training: Non-randomized controlled trial. BMC Endocr. Disord. 2021, 21, 143. [Google Scholar] [CrossRef]
- Hung, E.S.W.; Chen, S.C.; Chang, F.C.; Shiao, Y.; Peng, C.W.; Lai, C.H. Effects of Interactive Video Game-Based Exercise on Balance in Diabetic Patients with Peripheral Neuropathy: An Open-Level, Crossover Pilot Study. Evid. Based Complement. Alternat. Med. 2019, 2019, 4540709. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Mosalem, D.M.; Tarshouby, W.A.; Alfeeli, A.K.; Baqer, A.B.; Mohamed, M.H. Computerized dynamic posturography in patients with diabetic peripheral neuropathy and visual feedback-based balance training effects. Maced. J. Med. Sci. 2014, 7, 267–272. [Google Scholar] [CrossRef]
- Grewal, G.S.; Sayeed, R.; Schwenk, M.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Balance rehabilitation: Promoting the role of virtual reality in patients with diabetic peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 498–507. [Google Scholar] [CrossRef]
- Salsabili, H.; Bahrpeyma, F.; Esteki, A.; Karimzadeh, M.; Ghomashchi, H. Spectral characteristics of postural sway in diabetic neuropathy patients participating in balance training. J. Diabetes Metab. Disord. 2013, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Fedele, D.; Comi, G.; Coscelli, C.; Cucinotta, D.; Feldman, E.L.; Ghirlanda, G.; Greene, D.A.; Negrin, P.; Santeusanio, F. A multicenter study on the prevalence of diabetic neuropathy in Italy. Italian Diabetic Neuropathy Committee. Diabetes Care 1997, 20, 836–843. [Google Scholar] [CrossRef]
- Dyck, P.J.; Melton, L.J.; O’Brien, P.C.; Service, F.J. Approaches to improve epidemiological studies of diabetic neuropathy: Insights from the Rochester Diabetic Neuropathy Study. Diabetes 1997, 46 (Suppl. S2), S5–S8. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4 (Updated August 2023); Cochrane: Oxford, UK, 2023. [Google Scholar]
- Dixon, C.J.; Knight, T.; Binns, E.; Ihaka, B.; O’Brien, D. Clinical measures of balance in people with type two diabetes: A systematic literature review. Gait Posture 2017, 58, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Alaee, S.J.; Barati, K.; Hajiaghaei, B.; Ghomian, B.; Moradi, S.; Poorpirali, M. Immediate effect of textured insoles on the balance in patients with diabetic neuropathy. J. Diabetes Investig. 2023, 14, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, G.; Thoumie, P.; Do, M.-C.; Schieppati, M. Cutaneous and muscular afferents from the foot and sensory fusion processing: Physiology and pathology in neuropathies. J. Peripher. Nerv. Syst. 2021, 26, 17–34. [Google Scholar] [CrossRef]
- Viseux, F.J.F.; Simoneau, M.; Billot, M. A Comprehensive Review of Pain Interference on Postural Control: From Experimental to Chronic Pain. Medicina 2022, 58, 812. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef]
- Venkataraman, K.; Tai, B.C.; Khoo, E.Y.H.; Tavintharan, S.; Chandran, K.; Hwang, S.W.; Phua, M.S.L.A.; Wee, H.L.; Koh, G.C.H.; Tai, E.S. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: A randomised controlled trial. Diabetologia 2019, 62, 2200–2210. [Google Scholar] [CrossRef]
- Melese, H.; Alamer, A.; Temesgen, M.H.; Kahsay, G. Effectiveness of Exercise Therapy on Gait Function in Diabetic Peripheral Neuropathy Patients: A Systematic Review of Randomized Controlled Trials. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2753. [Google Scholar] [CrossRef]
- De León Rodriguez, D.; Allet, L.; Golay, A.; Philippe, J.; Assal, J.P.; Hauert, C.A.; Pataky, Z. Biofeedback can reduce foot pressure to a safe level and without causing new at-risk zones in patients with diabetes and peripheral neuropathy. Diabetes Metab. Res. Rev. 2013, 29, 139–144. [Google Scholar] [CrossRef]
- Van Deursen, R.W.M.; Cavanagh, P.R.; Van Ingen Schenau, G.J.; Becker, M.B.; Ulbrecht, J.S. The role of cutaneous information in a contact control task of the leg in humans. Hum. Mov. Sci. 1998, 17, 95–120. [Google Scholar] [CrossRef]
- Femery, V.G.; Moretto, P.G.; Hespel, J.M.G.; Thévenon, A.; Lensel, G. A real-time plantar pressure feedback device for foot unloading. Arch. Phys. Med. Rehabil. 2004, 85, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.C. Sensory substitution in the diabetic neuropathic foot. J. Am. Podiatr. Med. Assoc. 1997, 87, 338. [Google Scholar] [CrossRef] [PubMed]
- York, R.M.; Perell-Gerson, K.L.; Barr, M.; Durham, J.; Roper, J.M. Motor learning of a gait pattern to reduce forefoot plantar pressures in individuals with diabetic peripheral neuropathy. PM R 2009, 1, 434–441. [Google Scholar] [CrossRef]
| Databases | Search |
|---|---|
| Medline/PubMed Scopus | (“virtual reality” OR feedback OR biofeedback OR wii OR game OR “augmented reality” OR kinect OR videogam* OR “virtual training” OR “immersive training”) AND (“diabetic neuropath*” OR “diabetic polineuropath*”) AND (gait OR balance OR walking OR “risk of fall”) |
| EMBASE | (‘virtual reality’/exp OR ‘virtual reality’ OR ‘virtual reality exposure therapy’/exp OR ‘virtual reality exposure therapy’ OR ‘video game’/exp OR ‘video game’ OR ‘feedback system’/exp OR ‘feedback system’) AND (‘diabetic neuropathy’/exp OR ‘diabetic neuropathy’) AND (‘gait’/exp OR gait OR ‘balance’/exp OR balance OR ‘falls’/exp OR falls) |
| Study | Total | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grewal et al., 2015 [17] | 7/10 | - | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Taveggia et al., 2014 [10] | 7/10 | - | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Study of Methodological Quality (Checklist for Measuring Quality), and Grade of Recommendation (Guidelines of the Oxford Centre for Evidence-Based Medicine). | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chadrashekhar et al., 2021 [55] | Stolarczyk et al., 2021 [56] | Hung et al., 2019 [57] | Carroll & Galles, 2018 [27] | Grewal et al., 2015 [17] | Mohieldin et al., 2014 [58] | Taveggia et al., 2014 [10] | Grewal et al., 2013 [59] | Salsabili et al., 2013 [60] | Salsabili et al., 2011 [9] | |
| Checklist items for Measuring Quality | ||||||||||
| 1. Study quality | ||||||||||
| Hypothesis/aim | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Eligibility criteria | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Interventions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Confounders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Findings | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Random variability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse events | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Lost to follow-up | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Probability values | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2. External validity | ||||||||||
| Source population | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Illustrative sample | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Illustrative treatment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Internal validity (study bias) | ||||||||||
| Blinding of subjects | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blinding | 0 | 0 | 0 | 0 | 0 | 0 | 1 0 | 0 | 0 | 0 |
| “Data extraction” | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-up adjustments | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Statistical tests | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Compliance | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4. Internal validity (confounding and selection bias) | 1 | |||||||||
| Source of patients | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Recruitment period | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Randomization | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Concealment | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Analysis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Loss to follow-up | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 5. Power | ||||||||||
| Effect | 2 | 5 | 2 | 0 | 5 | 5 | 5 | 2 | 2 | 2 |
| Total score | 16 | 20 | 19 | 5 | 19 | 19 | 22 | 18 | 14 | 14 |
| Percentage (%) | 50 | 62 | 59 | 16 | 59 | 59 | 69 | 56 | 44 | 44 |
| Authors (Year) Design | Sample | Age (Average, Standard Dev.) | Intervention | N Ses | Performance of Measurement | VR | FB | Results |
|---|---|---|---|---|---|---|---|---|
| Chandrashekhar et al., (2021) [55] PS | N = 23 | 66.6 ± 9.9 | Vibration wearables (Myovolt) | 10 min 3 ses/week 4 weeks | Static and dynamic Balance (BSS, TUG) Proactive Balance (TUG-Cognitive)Pain (BPI-DPN) | - | Haptic | ↑ TUG y TUG-Cognitive |
| Stolarczyk et al., (2021) [56] N-RCT | N = 77 | 73.22 ± 7.57 | BSSys | 30 min 5 ses/week 12 weeks | Static and Dynamic Balance (2 BSSys tests EO and EC: general data, AP, and ML) Risk of Fall (BSSys) Motor coordination (BSSys) | - | Visual and Haptic | ↑ static balance EO and EC, all parameters ↓ risk of fall All parameters were significantly worse in EG (DPN patients) than CG (healthy participants) |
| Hung et al., (2019) [57] CCT | N = 24 A = 12 B = 12 | 49.9 ± 12 | Interactive game + mat (XavixPORT) | 30 min 3 ses/week 6 weeks | Static and Dynamic Balance (TUG, BSS) Risk of Fall (MFES) Monopodial Balance (UST) | Semi- immersive | Visual and auditive | ↑ BSS, MFES y TUG. = UST |
| Carroll & Galles (2018) [27] CC | N = 1 | 60 | 3 interactive games (OGRE) | 45 min 6 ses | Balance (TUG, 2° session) Security (ABC) | Semi-immersive | Visual and auditive | ↑ TUG ↑ ABC Plus positive verbal feedback |
| Grewal et al., (2015) [17] RCT | N = 35 CG = 20 EG = 19 | 63.7 ± 8.2 | CG: standard care EG: interactive game (MATLAB), 5 sensors (LegSys) hips and lower limbs. | 45 min 2 ses/week 4 weeks | Risk of Fall (FES-I) Quality of life (SF-12) Balance (double stance, CoM AP sway, LM sway and total sway) Daily physical activity (PAMSys) | Non- immersive | Visual and auditive | ↓ CoM total sway and ML sway EO ↓ sway hips–ankles ↑ mental punctuation SF-12 |
| Mohieldin et al., (2014) [58] N-RCT | N = 87 CG = 30 EG = 57 | 49.9 ± 12 | CG: standard care EG: Dynamic posturography (SMART Balance Master) | CG: 3 ses/week, 12 weeks EG: 2 ses/week, 12 weeks | Static balance: visual and vestibular proportion EO and EC (SMART Balance Master) | - | Visual | ↓ compost balance and SMTS relation DPN light and CG. ↓ compost balance and SMTS relation DPN severe and CG. ↑ pre-post SMTS relation DPN light. |
| Taveggia et al., (2014) [10] RCT | N = 27 CG = 14 EG = 13 | 72 ± 9 | CG: standar care EG: Biodex protocol | 60 min 5 ses/week 4 weeks | Gait (6MWT, 10MWT and Tinetti) Function (FIM and Tinetti) Balance (Tinetti) | - | Visual and haptic. | ↑ walking resistance EG ↑ functionality (FIM) both groups |
| Grewal et al., (2013) [59] PCS | N = 29 | 57 ± 10 | Interactive gam (MATLAB) 6 inertial sensors (BalanSens) ankles and wrist. | 10–15 min 1 ses | Balance (CoM sway = BalanSens) Coordination between upper and lower limbs AP and ML (RCI) | Non- immersive | Visual and auditive | ↓ CoM hips-ankles sway, EC. ↓ CoM Sway, EO. ↑ coordination AP EO. |
| Salsabili et al., (2013) [60] T-SS | N = 19 | 56 ± 8.96 | BSSys | 30 min 10 ses/week 3 weeks | Static Balance (torque platform) DPN severity (Valk score) | - | Visual | = Valk Score: no ↓ DPN severity. ↓ CoM sway ML low-medium frequencies OE. |
| Salsabili et al., (2011) [9] T-SS | N = 19 | 56 ± 8.96 | BSSys | 30 min 10 ses/week 3 weeks | DPN severity (Valk score) Balance (CoM fluctuation = BSSys, 3 trials EO and 3 EC) | - | Visual | = Valk Score: no ↓ DPN severity. ↑ stability total, AP, and ML EO. ↑ stability total and AP EC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Enríquez, L.; Gómez-Cuaresma, L.; Billot, M.; Garcia-Bernal, M.I.; Benitez-Lugo, M.L.; Casuso-Holgado, M.J.; Luque-Moreno, C. Effectiveness of Virtual Reality and Feedback to Improve Gait and Balance in Patients with Diabetic Peripheral Neuropathies: Systematic Review and Meta-Analysis. Healthcare 2023, 11, 3037. https://doi.org/10.3390/healthcare11233037
Alonso-Enríquez L, Gómez-Cuaresma L, Billot M, Garcia-Bernal MI, Benitez-Lugo ML, Casuso-Holgado MJ, Luque-Moreno C. Effectiveness of Virtual Reality and Feedback to Improve Gait and Balance in Patients with Diabetic Peripheral Neuropathies: Systematic Review and Meta-Analysis. Healthcare. 2023; 11(23):3037. https://doi.org/10.3390/healthcare11233037
Chicago/Turabian StyleAlonso-Enríquez, Laura, Laura Gómez-Cuaresma, Maxime Billot, Maria Isabel Garcia-Bernal, Maria Luisa Benitez-Lugo, María Jesús Casuso-Holgado, and Carlos Luque-Moreno. 2023. "Effectiveness of Virtual Reality and Feedback to Improve Gait and Balance in Patients with Diabetic Peripheral Neuropathies: Systematic Review and Meta-Analysis" Healthcare 11, no. 23: 3037. https://doi.org/10.3390/healthcare11233037
APA StyleAlonso-Enríquez, L., Gómez-Cuaresma, L., Billot, M., Garcia-Bernal, M. I., Benitez-Lugo, M. L., Casuso-Holgado, M. J., & Luque-Moreno, C. (2023). Effectiveness of Virtual Reality and Feedback to Improve Gait and Balance in Patients with Diabetic Peripheral Neuropathies: Systematic Review and Meta-Analysis. Healthcare, 11(23), 3037. https://doi.org/10.3390/healthcare11233037