Microfracture- and Xeno-Matrix-Induced Chondrogenesis for Treatment of Focal Traumatic Cartilage Defects of the Knee: Age-Based Mid-Term Results
Abstract
:1. Introduction
2. Materials and Methods
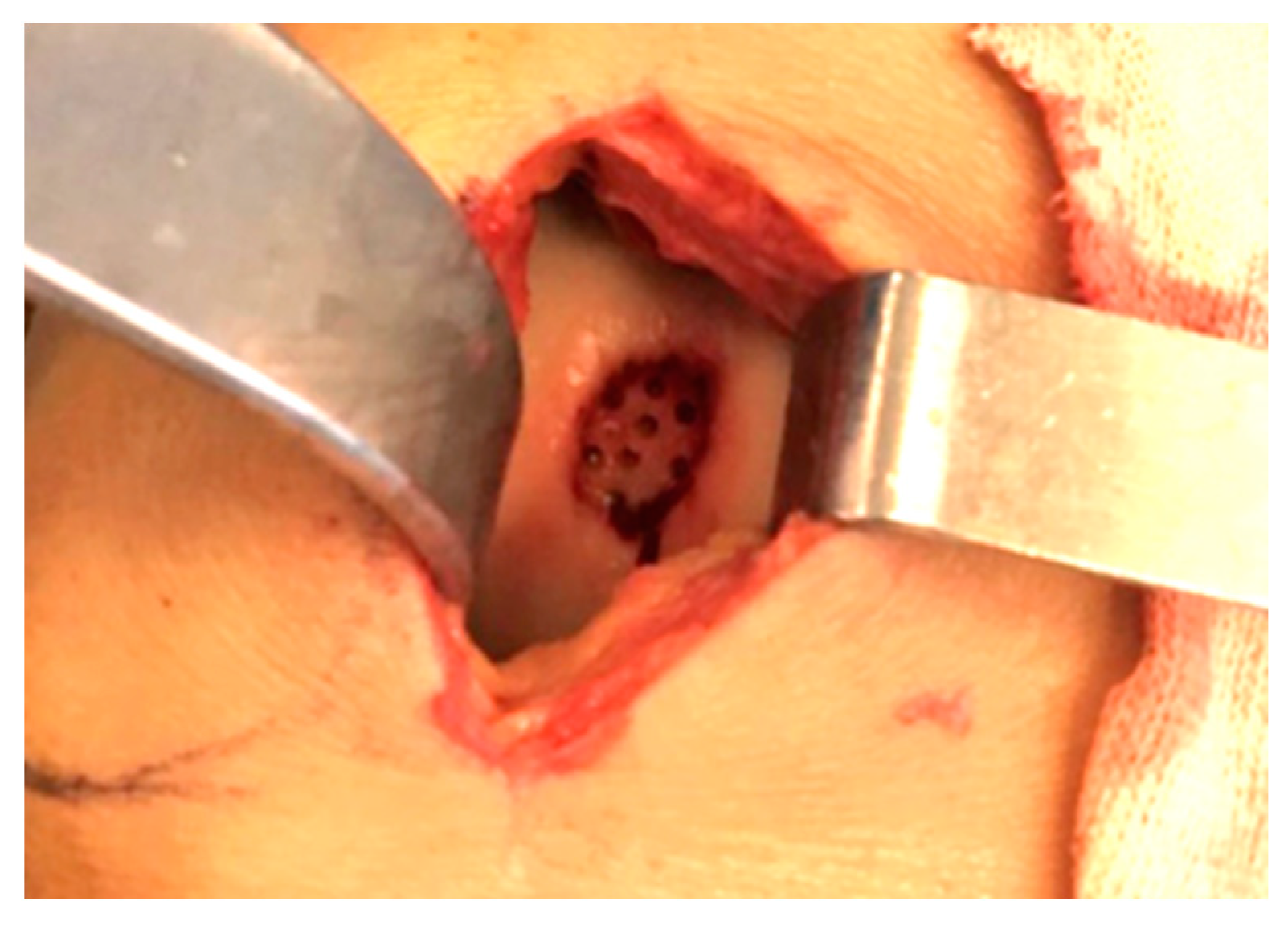
2.1. Surgical Procedure
2.2. Rehabilitation Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alford, J.W.; Cole, B.J. Cartilage restoration, part 1: Basic science, historical perspective, patient evaluation, and treatment options. Am. J. Sports Med. 2005, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kril, M.; Early, N.; Everhart, J.S.; Flanigan, D.C. Autologous Chondrocyte Implantation (ACI) for Knee Cartilage Defects: A Review of Indications, Technique, and Outcome. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous Chondrocyte Implantation: A Systematic Review. JBJS 2010, 92, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture to treat full-thickness chondral defects: Surgical technique, rehabilitation, and outcomes. J. Knee Surg. 2002, 15, 170–176. [Google Scholar]
- Hinckel, B.B.; Thomas, D.; Vellios, E.E.; Hancock, K.J.; Calcei, J.G.; Sherman, S.L.; Eliasberg, C.D.; Fernandes, T.L.; Farr, J.; Lattermann, C.; et al. Algorithm for Treatment of focal Cartilage Defects oh the knee: Classic and New Procedures. Cartilage 2021, 13 (Suppl. 1), 473S–495S. [Google Scholar] [CrossRef]
- Hangody, L.; Balò, E.; Panics, G.; Hangody, L.R.; Berkes, I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: A 17-year prospective multicenter study. Am. J. Sports Med. 2010, 38, 1125–1133. [Google Scholar] [CrossRef]
- Volz, M.; Schaumburger, M.; Frick, H.; Grifka, J.; Anders, S. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int. Orthop. 2017, 41, 797–804. [Google Scholar] [CrossRef]
- Moseley, J.B.; Anderson, A.F.; Browne, J.E.; Mandelbaum, B.R.; Micheli, L.J.; Fu, F.; Erggelet, C. Long-term durability of autologous chondrocyte implantation: A multicenter, observational study in US patients. Am. J. Sports Med. 2010, 38, 238–246. [Google Scholar] [CrossRef]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef]
- Colombini, A.; Libonati, F.; Lopa, S.; Peretti, G.M.; Moretti, M.; de Girolamo, L. Autologous chondrocyte implantation provides good long-term clinical results in the treatment of knee osteoarthritis: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2338–2348. [Google Scholar] [CrossRef]
- Dhillon, J.; Decilveo, A.P.; Kraeutler, M.J.; Belk, J.W.; McCulloch, P.C.; Scillia, A.J. Third-Generation Autologous Chondrocyte Implantation (Cells Cultured Within Collagen Membrane) Is Superior to Microfracture for Focal Chondral Defects of the Knee Joint: Sistematic Review and Meta-analysis. Arthroscopy 2022, 38, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Götze, C.; Hildebrand, F.; Betsch, M. Prognostic factors for the management of chondral defects of the knee and ankle joint: A systematic review. Eur. J. Trauma Emerg. Surg. 2023, 49, 723–745. [Google Scholar] [CrossRef]
- Migliorini, F.; Eschweiler, J.; Götze, C.; Driessen, A.; Tingart, M.; Maffulli, N. Matrix-induced autologous chondrocyte implantation (mACI) versus autologous matrix-induced chondrogenesis (AMIC) for chondral defects of the knee: A systematic review. Br. Med. Bull. 2022, 141, 47–59. [Google Scholar] [CrossRef]
- Steinwachs, M.R.; Gille, J.; Volz, M.; Anders, S.; Jakob, R.; De Girolamo, L.; Volpi, P.; Schiavone-Panni, A.; Scheffler, S.; Reiss, E.; et al. Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee. Cartilage 2021, 13 (Suppl. 1), 42S–56S. [Google Scholar] [CrossRef]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L. Responsiveness of the international knee documentation committee subjective knee form. Am. J. Sports Med. 2006, 34, 1567–1573. [Google Scholar] [CrossRef]
- Briggs, K.K.; Steadman, J.R.; Hay, C.J.; Hines, S.L. Lysholm score and Tegner activity level in individuals with normal knees. Am. J. Sports Med. 2009, 37, 898–901. [Google Scholar] [CrossRef]
- Heller, G.Z.; Manuguerra, M.; Chow, R. How to analyze the Visual Analogue Scale: Myths, truths and clinical relevance. Scand. J. Pain 2016, 13, 67–75. [Google Scholar] [CrossRef]
- Marlovits, S.; Striessnig, G.; Resinger, C.T.; Aldrian, S.M.; Vecsei, V.; Siegfried, H.I. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur. J. Radiol. 2004, 52, 310–319. [Google Scholar] [CrossRef]
- De Girolamo, L.; Quaglia, A.; Bait, C.; Cervellin, M.; Propsero, E.; Volpi, P. Modified autologous matrix induced chondrogenesis (AMIC) for the treatment of a large osteochondral defect in a varus knee: A case report. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2287–2290. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.I.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfractures for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Espregueira-Mendes, J.; Lane, J.G.; Karahan, M. Bio-Orthopaedics: A New Approach; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-54181-4. [Google Scholar]
- Bolignano, D.; Mattace-Raso, F.; Torino, C.; D’Arrigo, G.; Abd ElHafeez, S.; Provenzano, F.; Zoccali, C.; Tripepi, G. The quality of reporting in clinical research: The CONSORT and STROBE initiatives. Aging Clin. Exp. Res. 2013, 25, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.J.; Aman, Z.S.; DePhillipo, N.N.; Dickens, J.F.; Anz, A.W.; LaPrade, R.F. Chondral Lesions of the Knee: An Evidence-Based Approach. J. Bone Jt. Surg. Am. 2021, 103, 629–645. [Google Scholar] [CrossRef]
- Migliorini, F.; Eschweiler, J.; Schenker, H.; Baroncini, A.; Tingart, M.; Maffulli, N. Surgical management of focal chondral defects of the knee: A Bayesian network meta-analysis. J. Orthop. Surg. Res. 2021, 16, 543. [Google Scholar] [CrossRef]
- Migliorini, F.; Baroncini, A.; Bell, A.; Weber, C.; Hildebrand, F.; Maffulli, N. Surgical strategies for chondral defects of the patellofemoral joint: A systematic review. J. Orthop. Surg. Res. 2022, 17, 524. [Google Scholar] [CrossRef]
- Perea, G.A.; Vergara, M.G.; Rodriguez Muñoz, L.F.; Arias Perez, R.D.; Piovesan, N.O.; Muñoz Salamanca, J.A. Satisfactory clinical outcomes with autologous matrix-induced chondrogenesis in the treatment of grade IV chondral injuries of the knee. J. ISAKOS 2023, 8, 86–93. [Google Scholar]
- Snow, M.; Middleton, L.; Mehta, S.; Roberts, A.; Gray, R.; Richardson, J.; Kuiper, J.H.; ACTIVE Consortium; Smith, A.; White, S.; et al. A Randomized Trial of Autologous Chondrocyte Implantation Versus Alternative Forms of Surgical Cartilage Management in Patients With a Failed Primary Treatment for Chondral or Osteochondral Defects in the Knee. Am. J. Sports Med. 2023, 51, 367–378. [Google Scholar] [CrossRef]
- Kusano, T.; Jakob, R.P.; Gautier, E.; Magnussen, R.A.; Hoogewoud, H.; Jacobi, M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2109–2115. [Google Scholar] [CrossRef]
- Schiavone-Panni, S.A.; Cerciello, S.; Vasso, M. The management of knee cartilage defects with modified AMIC technique: Preliminary results. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 149–152. [Google Scholar] [CrossRef]
- Pascarella, A.; Ciatti, R.; Pascarella, F.; Latte, C.; Di Salvatore, M.G.; Liguori, L. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.; Behrens, P.; Volpi, P.; De Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of Autologous Matrix Induced Chondrogenesis (AMIC) in cartilage knee surgery: Data of the AMIC Registry. Arch. Orthop. Trauma Surg. 2013, 133, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral lesions of the knee: A new one step repair technique with bone marrow derived cells. J. Bone Jt. Surg. Am. 2010, 92 (Suppl. 2), 2–11. [Google Scholar] [CrossRef] [PubMed]
- Blackman, A.J.; Smith, M.V.; Flanigan, D.C.; Matava, M.J.; Wright, R.W.; Brophy, R.H. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: A systematic review and meta-analysis. Am. J. Sports Med. 2013, 41, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- De Windt, T.S.; Welsch, G.H.; Brittberg, M.; Vonk, L.A.; Marlovits, S.; Trattnig, S. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am. J. Sports Med. 2013, 41, 1695–1702. [Google Scholar] [CrossRef]
- Gille, J.; Schuseil, E.; Wimmer, J.; Gellissen, J.; Schulz, A.P.; Behrens, P. Mid-term results of autologous matrix induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1456–1464. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Andriolo, L.; Di Matteo, B.; Balboni, F.; Marcacci, M. Clinical Profiling in Cartilage Regeneration: Prognostic Factors for Midterm Results of Matrix-Assisted Autologous Chondrocyte Transplantation. Am. J. Sports Med. 2014, 42, 898. [Google Scholar] [CrossRef]
- Schagemann, J.; Behrens, J.; Paech, A.; Riepenhof, H.; Kienast, B.; Mittelstadt, H.; Gille, J. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch. Orthop. Trauma Surg. 2018, 138, 819–825. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Condello, V. Second-generation autologous chondrocyte implantation: Results in patients older than 40 years. Am. J. Sports Med. 2011, 39, 1668–1675. [Google Scholar] [CrossRef]
- Tan, C.H.B.; Huang, X.Y.O.; Tay, Z.Q.; Bin Abd Razak, H.R. Arthroscopic and open approach for autologous matrix induced chondrogenesis (AMIC®) repair of the knee have similar results: A meta-analysis. J ISAKOS 2023, in press. [CrossRef]
- Hunziker, E.B.; Stahli, A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr. Cartil. 2008, 16, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Hoffman, L.; Zak, L.; Tiefenboeck, T.; Aldrian, S.; Albrecht, C. Clinical evaluation after matrix-associated autologous chondrocyte transplantation: A comparison of four different graft types. Bone Jt. Res. 2021, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H.S.; Danielson, B.; Ljungberg, M.; McKeon, B.; Lindahl, A.; Peterson, L. Autologous chondrocyte implantation in cartilage lesions of the knee: Long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am. J. Sports Med. 2010, 38, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Dickhut, A.; Dexheimer, V.; Martin, K.; Lauinger, R.; Heisel, C.; Richter, W. Chondrogenesis of human mes-enchymal stem cells by local transforming growth factor-beta delivery in a biphasic resorbable carrier. Tissue Eng. Part A 2010, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- De Windt, T.S.; Bekkers, J.E.; Creemers, L.B. Patient profiling in cartilage regeneration: Prognostic factors determining success of treatment for cartilage defects. Am. J. Sports Med. 2009, 37, 58–62. [Google Scholar] [CrossRef]


| n | Gender | Mean Age (y.o.) | Mean Area of Defect (cm2) | |
|---|---|---|---|---|
| Group A<45 y.o. | 12 | 5 M; 7 F | 34 ± 7 | 2.7 ± 1.6 |
| Group B>45 y.o. | 13 | 8 M; 5 F | 53 ± 3.5 | 3.0 ± 1.2 |
| Pre-Operative | One Year Follow-Up | Two Years Follow-Up | |
|---|---|---|---|
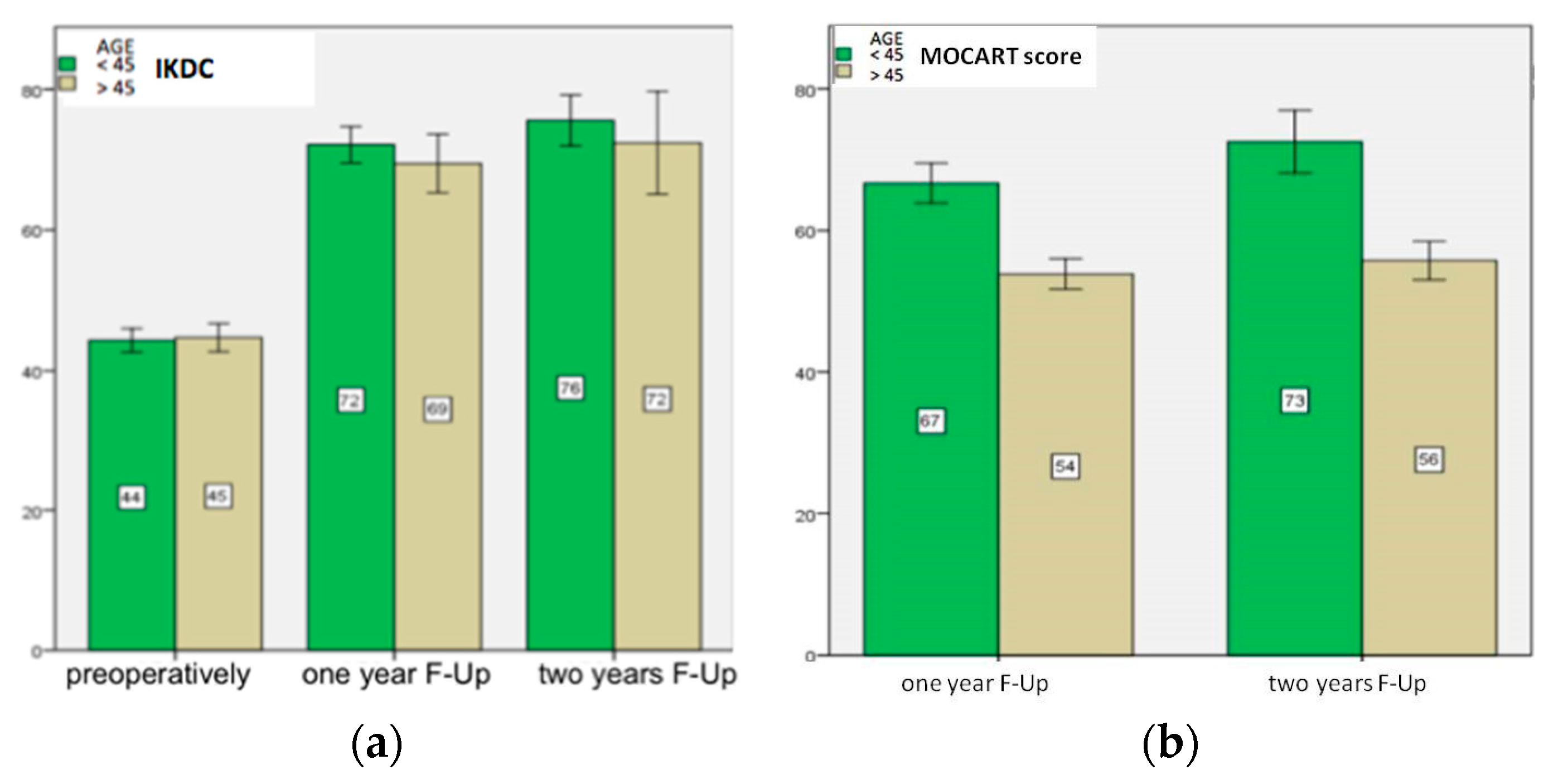
| IKDC Group A p-value Group B p-value | 44 ± 4 | 72 ± 2 <0.001 | 76 ± 2 =0.001 |
| 45 ± 4 | 69 ± 3 <0.001 | 72 ± 4 =0.001 | |
| Lysholm Group A p-value Group B p-value | 62 ± 3 | 90 ± 4 <0.001 | 96 ± 3 <0.001 |
| 64 ± 2 | 89 ± 3 <0.001 | 95 ± 4 =0.04 | |
| VAS Group A p-value Group B p-value | 4 ± 1 | 1 ± 1 <0.001 | 1 ± 1 - |
| 3 ± 1 | 2 ± 1 <0.001 | 1 ± 1 =0.2 | |
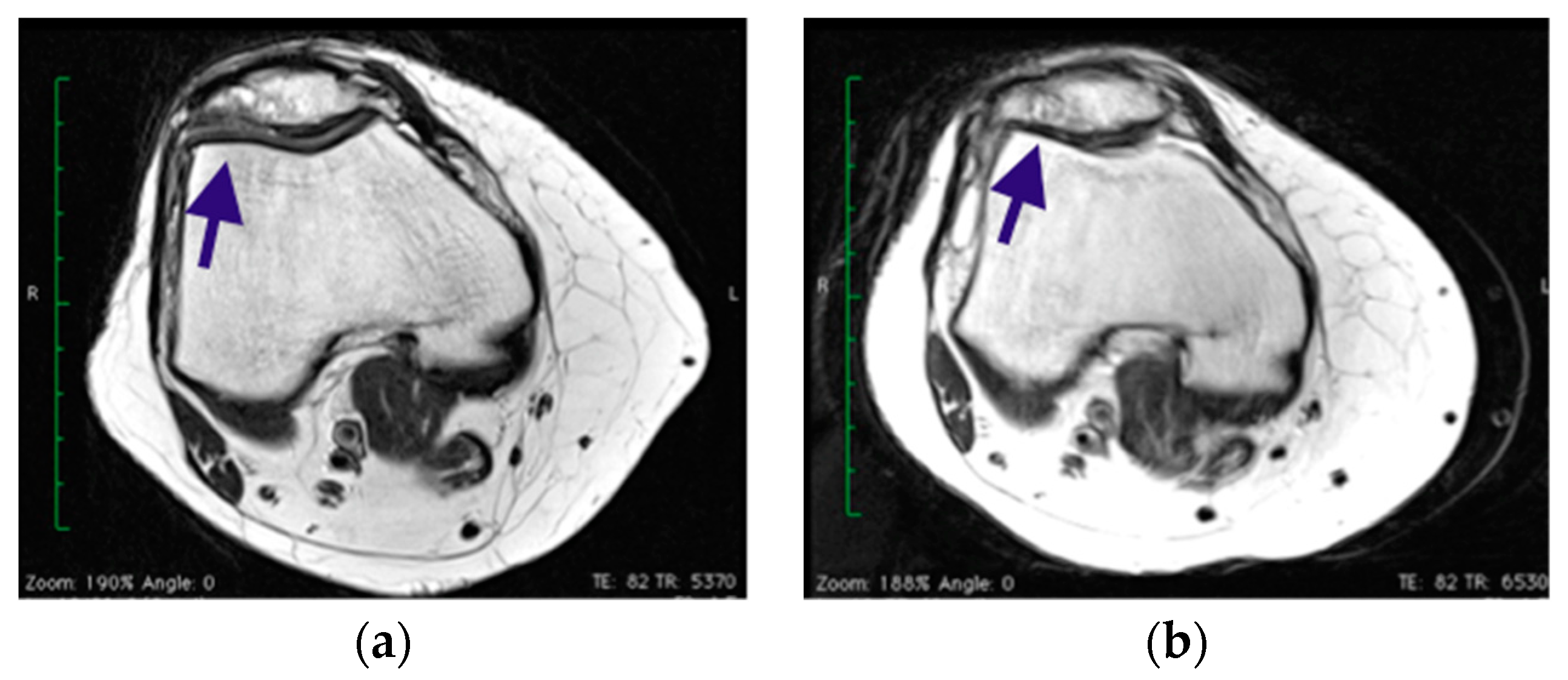
| MOCART Group A p-value Group B p-value | - | 67 ± 4 | 73 ± 7 =0.02 |
| - | 54 ± 4 | 56 ± 5 =0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, F.; Picchi, A.; Ratano, M.; Gumina, S.; Fidanza, A.; Logroscino, G. Microfracture- and Xeno-Matrix-Induced Chondrogenesis for Treatment of Focal Traumatic Cartilage Defects of the Knee: Age-Based Mid-Term Results. Healthcare 2023, 11, 2995. https://doi.org/10.3390/healthcare11222995
Allegra F, Picchi A, Ratano M, Gumina S, Fidanza A, Logroscino G. Microfracture- and Xeno-Matrix-Induced Chondrogenesis for Treatment of Focal Traumatic Cartilage Defects of the Knee: Age-Based Mid-Term Results. Healthcare. 2023; 11(22):2995. https://doi.org/10.3390/healthcare11222995
Chicago/Turabian StyleAllegra, Francesco, Aurelio Picchi, Marco Ratano, Stefano Gumina, Andrea Fidanza, and Giandomenico Logroscino. 2023. "Microfracture- and Xeno-Matrix-Induced Chondrogenesis for Treatment of Focal Traumatic Cartilage Defects of the Knee: Age-Based Mid-Term Results" Healthcare 11, no. 22: 2995. https://doi.org/10.3390/healthcare11222995
APA StyleAllegra, F., Picchi, A., Ratano, M., Gumina, S., Fidanza, A., & Logroscino, G. (2023). Microfracture- and Xeno-Matrix-Induced Chondrogenesis for Treatment of Focal Traumatic Cartilage Defects of the Knee: Age-Based Mid-Term Results. Healthcare, 11(22), 2995. https://doi.org/10.3390/healthcare11222995







