The RODI mHealth app Insight: Machine-Learning-Driven Identification of Digital Indicators for Neurodegenerative Disorder Detection
Abstract
:1. Introduction
2. Recent Developments in Mobile Platforms for Digital Evolution of Cognitive Assessment
3. Materials and Methods
3.1. Case Study Description
3.2. Participants
3.3. RODI App Execution
3.4. Methodology
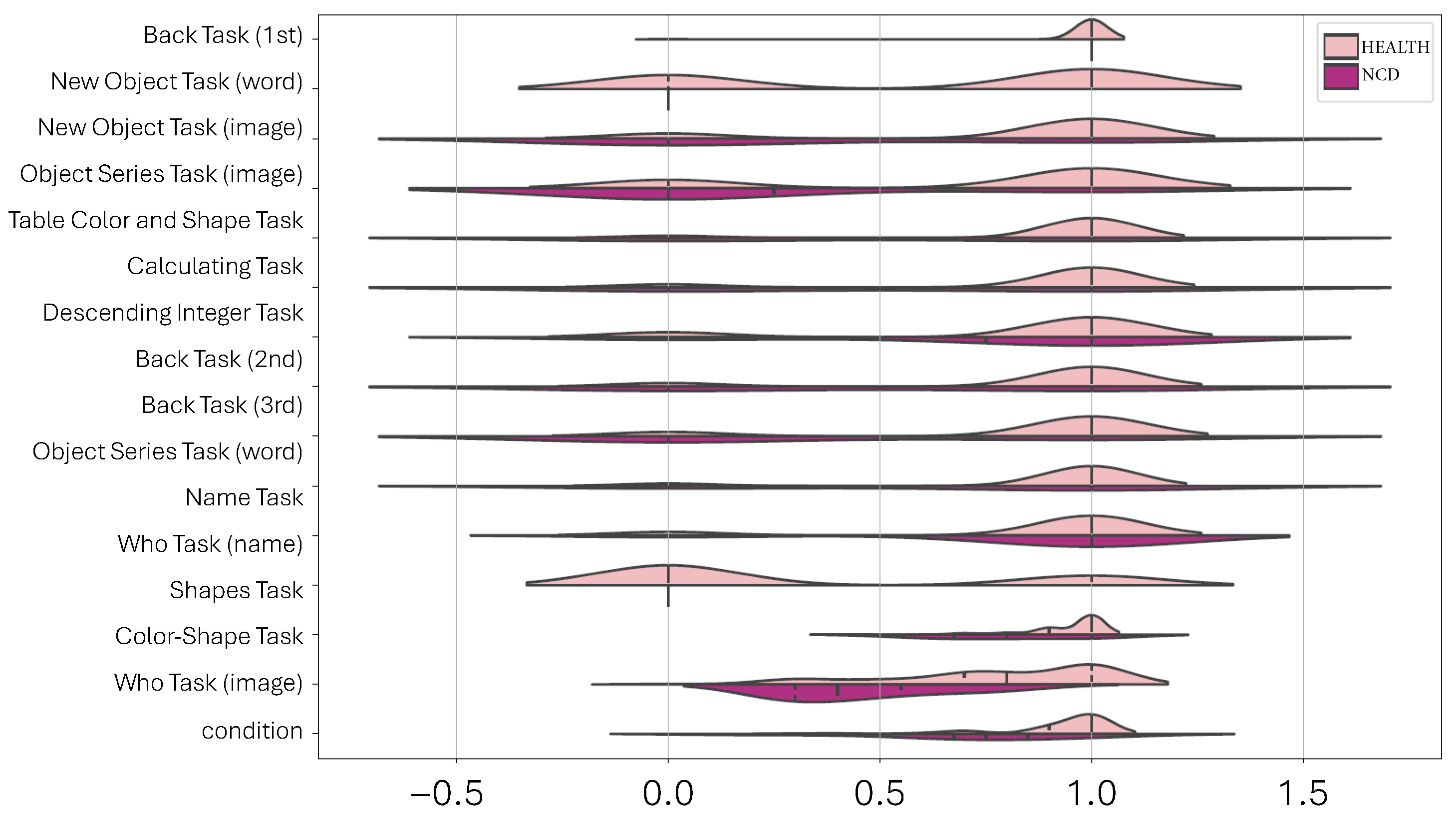
3.4.1. Dimensionality Reduction Techniques for 2D Data Visualization
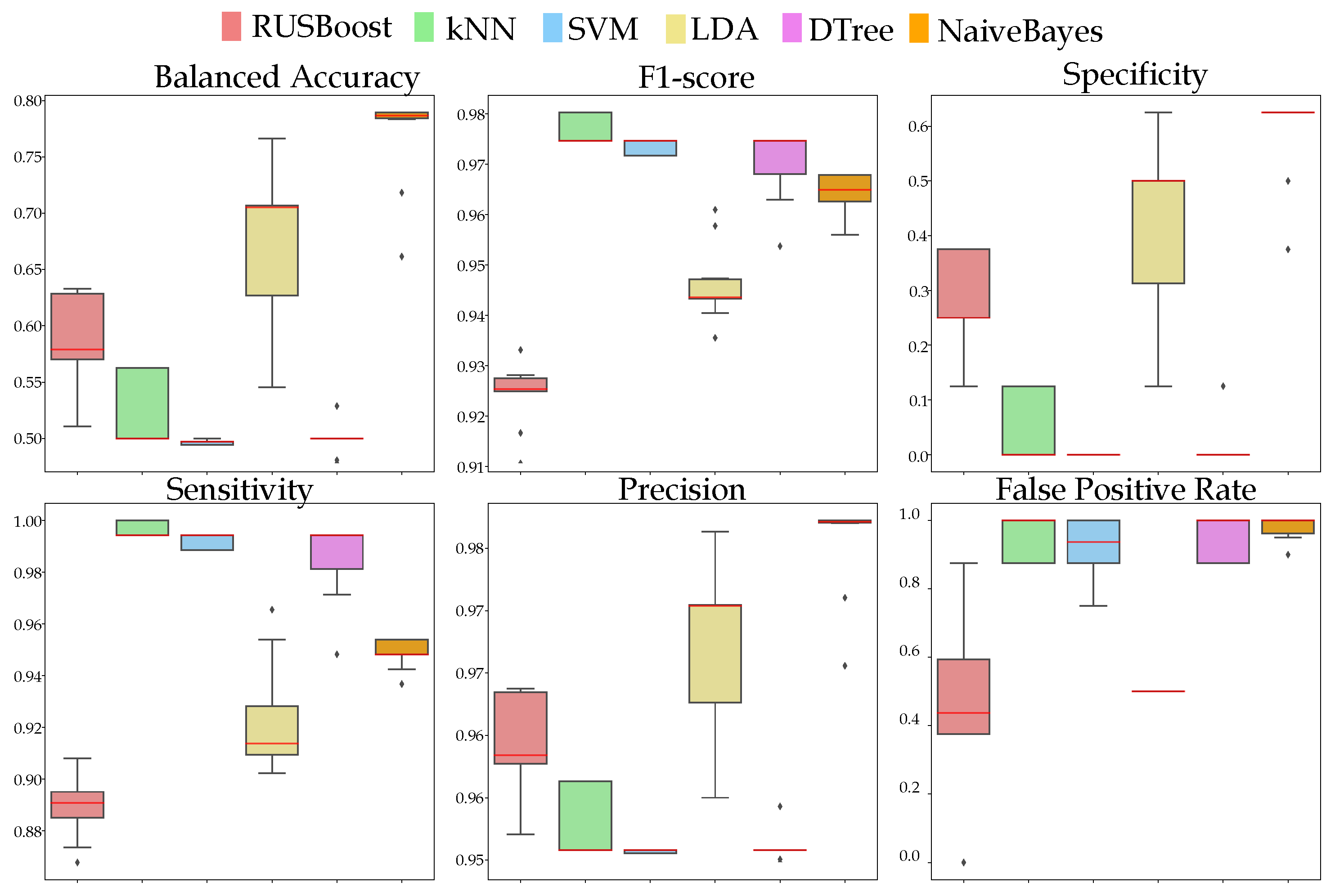
3.4.2. NCD Prediction Performance
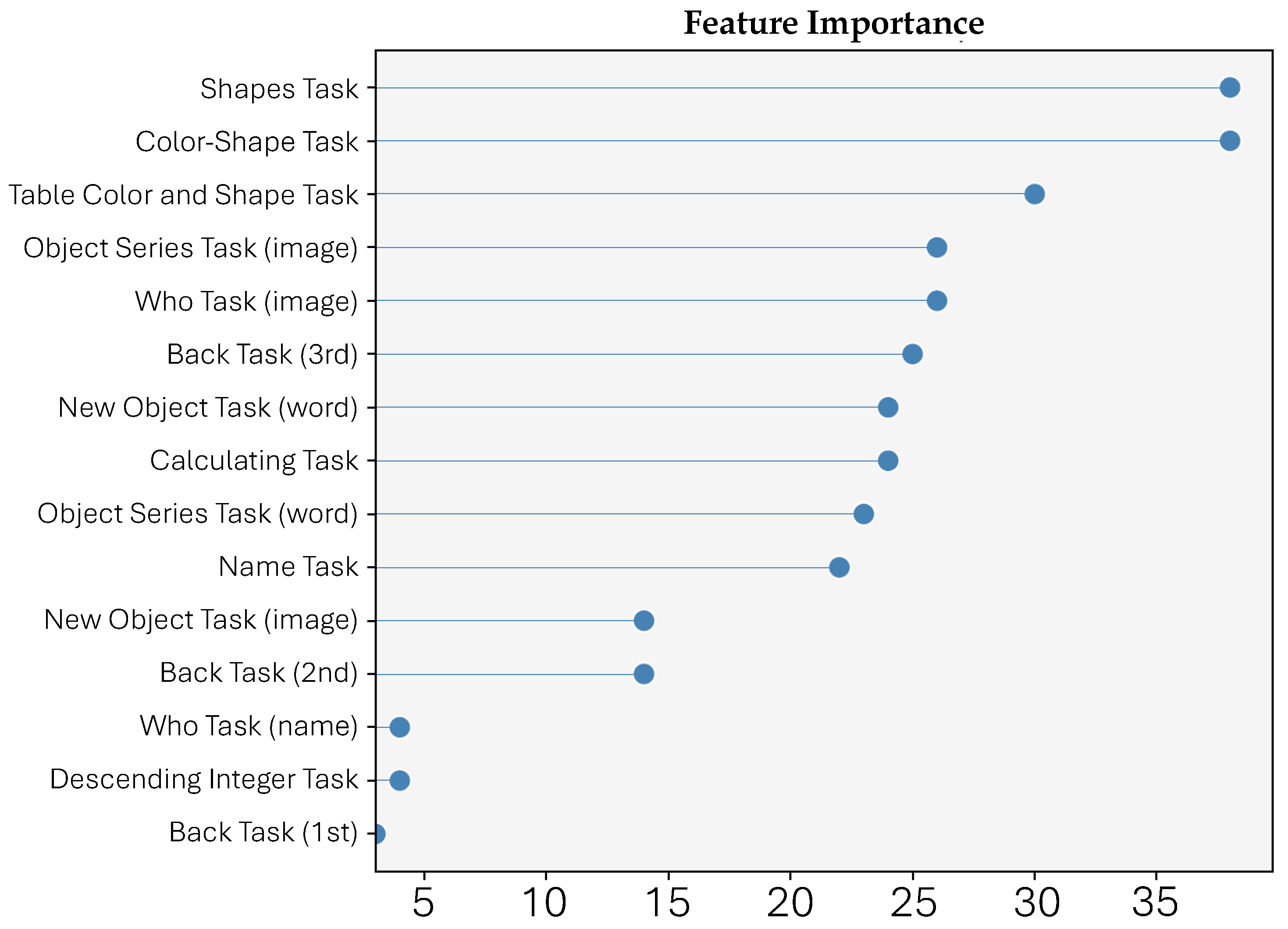
3.4.3. Feature Importance
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, Z.; Babulal, G.M. Attitudinal adjustment about dementia awareness and assessment: Finetuning inclusion, diversity, and measurement of behavioral and psychological symptoms. Int. Psychogeriatr. 2023, 35, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimer’s Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef]
- World Health Organization. Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity; World Health Organization: Geneva, Switerland, 2017; Section ix; 46p. [Google Scholar]
- Katsuno, M.; Sahashi, K.; Iguchi, Y.; Hashizume, A. Preclinical progression of neurodegenerative diseases. Nagoya J. Med. Sci. 2018, 80, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Koen, J.D.; Rugg, M.D. Neural Dedifferentiation in the Aging Brain. Trends Cogn. Sci. 2019, 23, 547–559. [Google Scholar] [CrossRef]
- Elliott, M.L.; Belsky, D.W.; Knodt, A.R.; Ireland, D.; Melzer, T.R.; Poulton, R.; Ramrakha, S.; Caspi, A.; Moffitt, T.E.; Hariri, A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry 2021, 26, 3829–3838. [Google Scholar] [CrossRef]
- Aron, L.; Zullo, J.; Yankner, B.A. The adaptive aging brain. Curr. Opin. Neurobiol. 2022, 72, 91–100. [Google Scholar] [CrossRef]
- Stern, Y.; Albert, M.; Barnes, C.A.; Cabeza, R.; Pascual-Leone, A.; Rapp, P.R. A framework for concepts of reserve and resilience in aging. Neurobiol. Aging 2023, 124, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, N.; Armstrong, N. Understanding and managing uncertainty in health care: Revisiting and advancing sociological contributions. Sociol. Health Illn. 2020, 42, 1–20. [Google Scholar] [CrossRef]
- Zhang, M.W.; Ho, R.C. M-health and smartphone technologies and their impact on patient care and empowerment. In The Digitization of Healthcare: New Challenges and Opportunities; Palgrave Macmillan: London, UK, 2017; ISBN-10: 1349951722. [Google Scholar]
- Zakerabasali, S.; Ayyoubzadeh, S.M.; Baniasadi, T.; Yazdani, A.; Abhari, S. Mobile health technology and healthcare providers: Systemic barriers to adoption. Healthc. Informat. Res. 2021, 27, 267–278. [Google Scholar] [CrossRef]
- Marsch, L.A. Digital health data-driven approaches to understand human behavior. Neuropsychopharmacology 2021, 46, 191–196. [Google Scholar] [CrossRef]
- Thabtah, F.; Peebles, D.; Retzler, J.; Hathurusingha, C. Dementia medical screening using mobile applications: A systematic review with a new mapping model. J. Biomed. Informat. 2020, 111, 103573. [Google Scholar] [CrossRef] [PubMed]
- Jakob, R.; Harperink, S.; Rudolf, A.M.; Fleisch, E.; Haug, S.; Mair, J.L.; Salamanca-Sanabria, A.; Kowatsch, T. Factors influencing adherence to mHealth apps for prevention or management of noncommunicable diseases: Systematic review. J. Med. Internet Res. 2022, 24, e35371. [Google Scholar] [CrossRef]
- Rollo, M.E.; Ash, S.; Lyons-Wall, P.; Russell, A. Trial of a mobile phone method for recording dietary intake in adults with type 2 diabetes: Evaluation and implications for future applications. J. Telemed. Telecare 2011, 17, 318–323. [Google Scholar] [CrossRef]
- Joe, J.; Demiris, G. Older adults and mobile phones for health: A review. J. Biomed. Informat. 2013, 46, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Morii, M.; Asai, Y.; Sakane, N. Application of mobile-phone cameras to home health care and welfare in the elderly: Experience in a rural practice. Aust. J. Rural Health 2005, 13, 193–194. [Google Scholar] [CrossRef]
- Lee, R.Y.; Carlisle, A.J. Detection of falls using accelerometers and mobile phone technology. Age Ageing 2011, 40, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Gill, D.P.; Wolpin, S.; Steele, B.G.; Benditt, J.O. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int. J. Chron. Obstr. Pulmonary Dis. 2009, 4, 301–313. [Google Scholar] [CrossRef]
- Josephine, K.; Josefine, L.; Philipp, D.; David, E.; Harald, B. Internet-and mobile-based depression interventions for people with diagnosed depression: A systematic review and meta-analysis. J. Affect. Disord. 2017, 223, 28–40. [Google Scholar] [CrossRef]
- Richards, D.; Richardson, T. Computer-based psychological treatments for depression: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 329–342. [Google Scholar] [CrossRef]
- Watts, S.; Mackenzie, A.; Thomas, C.; Griskaitis, A.; Mewton, L.; Williams, A.; Andrews, G. CBT for depression: A pilot RCT comparing mobile phone vs. computer. BMC Psychiatry 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Burns, M.N.; Begale, M.; Duffecy, J.; Gergle, D.; Karr, C.J.; Giangrande, E.; Mohr, D.C. Harnessing context sensing to develop a mobile intervention for depression. J. Med. Internet Res. 2011, 13, e55. [Google Scholar] [CrossRef]
- Berg, J.L.; Durant, J.; Léger, G.C.; Cummings, J.L.; Nasreddine, Z.; Miller, J.B. Comparing the Electronic and Standard Versions of the Montreal Cognitive Assessment in an Outpatient Memory Disorders Clinic: A Validation Study. J. Alzheimer’s Dis. 2018, 62, 93–97. [Google Scholar] [CrossRef]
- Scharre, D.W.; Chang, S.I.; Nagaraja, H.N.; Vrettos, N.E.; Bornstein, R.A. Digitally translated Self-Administered Gerocognitive Examination (eSAGE): Relationship with its validated paper version, neuropsychological evaluations, and clinical assessments. Alzheimer’s Res. Ther. 2017, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Vidal, J.S.; De Rotrou, J.; Sikkes, S.A.; Rigaud, A.S.; Plichart, M. A Tablet-PC-Based Cancellation Test Assessing Executive Functions in Older Adults. Am. J. Geriatr. Psychiatry 2015, 23, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Vidal, J.S.; De Rotrou, J.; Sikkes, S.A.M.; Rigaud, A.S.; Plichart, M. Can a tablet-based cancellation test identify cognitive impairment in older adults? PLoS ONE 2017, 12, e0181809. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, K.; Maguire, Á.; Andrews, J.L.; Martin, E.; Menon, S. Are we there yet? Exploring the impact of translating cognitive tests for dementia using mobile technology in an aging population. Front. Aging Neurosci. 2016, 8, 21. [Google Scholar] [CrossRef]
- Mielke, M.M.; Machulda, M.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Pankratz, V.S.; Knopman, D.S.; Jack, C.R.; Petersen, R.C. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimer’s Dement. 2015, 11, 1367–1376. [Google Scholar] [CrossRef]
- Suzumura, S.; Osawa, A.; Maeda, N.; Sano, Y.; Kandori, A.; Mizuguchi, T.; Yin, Y.; Kondo, I. Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity: Finger skills of AD and MCI patients. Geriatr. Gerontol. Int. 2018, 18, 907–914. [Google Scholar] [CrossRef]
- Tong, T.; Chignell, M.; Tierney, M.C.; Lee, J. A Serious Game for Clinical Assessment of Cognitive Status: Validation Study. JMIR Serious Games 2016, 4, e7. [Google Scholar] [CrossRef]
- Zygouris, S.; Ntovas, K.; Giakoumis, D.; Votis, K.; Doumpoulakis, S.; Segkouli, S.; Karagiannidis, C.; Tzovaras, D.; Tsolaki, M. A Preliminary Study on the Feasibility of Using a Virtual Reality Cognitive Training Application for Remote Detection of Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 56, 619–627. [Google Scholar] [CrossRef]
- Kokubo, N.; Yokoi, Y.; Saitoh, Y.; Murata, M.; Maruo, K.; Takebayashi, Y.; Shinmei, I.; Yoshimoto, S.; Horikoshi, M. A new device-aided cognitive function test, User eXperience-Trail Making Test (UX-TMT), sensitively detects neuropsychological performance in patients with dementia and Parkinson’s disease. BMC Psychiatry 2018, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Possin, K.L.; Moskowitz, T.; Erlhoff, S.J.; Rogers, K.M.; Johnson, E.T.; Steele, N.Z.R.; Higgins, J.J.; Stiver, J.; Alioto, A.G.; Farias, S.T.; et al. The Brain Health Assessment for Detecting and Diagnosing Neurocognitive Disorders. J. Am. Geriatr. Soc. 2018, 66, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zorluoglu, G.; Kamasak, M.E.; Tavacioglu, L.; Ozanar, P.O. A mobile application for cognitive screening of dementia. Comput. Methods Programs Biomed. 2015, 118, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.; Husky, M.; Catheline, G.; Pelletier, A.; Dilharreguy, B.; Amieva, H.; Pérès, K.; Foubert-Samier, A.; Dartigues, J.F.; Swendsen, J. Mobile Technologies in the Early Detection of Cognitive Decline. PLoS ONE 2014, 9, e112197. [Google Scholar] [CrossRef] [PubMed]
- Bissig, D.; Kaye, J.; Erten-Lyons, D. Validation of SATURN, a free, electronic, self-administered cognitive screening test. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, R.M.; Foil, H.; Fontenot, S.; Correro, A.; Allen, R.; Martin, C.K.; Bruce-Keller, A.J.; Keller, J.N. Feasibility, Reliability, and Validity of a Smartphone Based Application for the Assessment of Cognitive Function in the Elderly. PLoS ONE 2013, 8, e65925. [Google Scholar] [CrossRef]
- Scanlon, L.; O’Shea, E.; O’Caoimh, R.; Timmons, S. Usability and Validity of a Battery of Computerised Cognitive Screening Tests for Detecting Cognitive Impairment. Gerontology 2016, 62, 247–252. [Google Scholar] [CrossRef]
- Molony, S.L.; Kolanowski, A.; Van Haitsma, K.; Rooney, K.E. Person-Centered Assessment and Care Planning. Gerontology 2018, 58, S32–S47. [Google Scholar] [CrossRef]
- Jongstra, S.; Wijsman, L.W.; Cachucho, R.; Hoevenaar-Blom, M.P.; Mooijaart, S.P.; Richard, E. Cognitive Testing in People at Increased Risk of Dementia Using a Smartphone App: The iVitality Proof-of-Principle Study. JMIR MHealth UHealth 2017, 5, e68. [Google Scholar] [CrossRef]
- Lange, S.; Süß, H.M. Measuring slips and lapses when they occur – Ambulatory assessment in application to cognitive failures. Conscious. Cogn. 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Giannopoulou, P.; Vlamos, P. Analysis and design of an information system for cognitive training of patients with mild cognitive impairment using mobile devices. In Proceedings of the 2020 5th South-East Europe Design Automation, Computer Engineering, Computer Networks and Social Media Conference (SEEDA-CECNSM), Corfu, Greece, 25–27 September 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Giannopoulou, P.; Vlamos, P.; Papalaskari, M.A. Evaluation of a Mobile Application for Cognitive Training in Healthy Adults. Int. J. Interact. Mob. Technol. (iJIM) 2023, 17, 84–102. [Google Scholar] [CrossRef]
- Association, A.P.; Association, A.P. (Eds.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Maaten, L.V.D. Accelerating t-SNE using Tree-Based Algorithms. J. Mach. Learn. Res. 2014, 15, 3221–3245. [Google Scholar]
- McInnes, L.; Healy, J.; Saul, N.; Großberger, L. UMAP: Uniform Manifold Approximation and Projection. J. Open Source Softw. 2018, 3, 861. [Google Scholar] [CrossRef]
- Kurita, T. Principal Component Analysis (PCA). In Computer Vision; Springer International Publishing: Cham, Switerland, 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Seiffert, C.; Khoshgoftaar, T.M.; Van Hulse, J.; Napolitano, A. RUSBoost: A Hybrid Approach to Alleviating Class Imbalance. IEEE Trans. Syst. Man Cybern.—Part A Syst. Hum. 2010, 40, 185–197. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the Advances in Neural Information Processing Systems; Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; Volume 30. [Google Scholar]
- Hancock, J.T.; Khoshgoftaar, T.M. CatBoost for big data: An interdisciplinary review. J. Big Data 2020, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Paplomatas, P.; Krokidis, M.G.; Vlamos, P.; Vrahatis, A.G. An Ensemble Feature Selection Approach for Analysis and Modeling of Transcriptome Data in Alzheimer’s Disease. Appl. Sci. 2023, 13, 2353. [Google Scholar] [CrossRef]
- Park, D.C.; Lautenschlager, G.; Hedden, T.; Davidson, N.S.; Smith, A.D.; Smith, P.K. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 2002, 17, 299–320. [Google Scholar] [CrossRef]
- Brockmole, J.R.; Logie, R.H. Age-Related Change in Visual Working Memory: A Study of 55,753 Participants Aged 8–75. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Iachini, T.; Ruotolo, F.; Rapuano, M.; Sbordone, F.L.; Ruggiero, G. The Role of Temporal Order in Egocentric and Allocentric Spatial Representations. J. Clin. Med. 2023, 12, 1132. [Google Scholar] [CrossRef]
- Kosslyn, S.M. Image and Brain: The Resolution of the Imagery Debate; MIT Press: Cambridge, MA, USA, 1994. [Google Scholar]
- De Wit, M.M.; Van Der Kamp, J.; Masters, R.S. Distinct task-independent visual thresholds for egocentric and allocentric information pick up. Conscious. Cogn. 2012, 21, 1410–1418. [Google Scholar] [CrossRef]
- Iachini, T.; Iavarone, A.; Senese, V.; Ruotolo, F.; Ruggiero, G. Visuospatial Memory in Healthy Elderly, AD and MCI: A Review. Curr. Aging Sci. 2009, 2, 43–59. [Google Scholar] [CrossRef]
- Puthusseryppady, V.; Emrich-Mills, L.; Lowry, E.; Patel, M.; Hornberger, M. Spatial Disorientation in Alzheimer’s Disease: The Missing Path From Virtual Reality to Real World. Front. Aging Neurosci. 2020, 12, 550514. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Wong, S.; Hodges, J.R.; Irish, M.; Piguet, O.; Hornberger, M. Lost in spatial translation – A novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex 2015, 67, 83–94. [Google Scholar] [CrossRef]
- Forno, G.; Lladó, A.; Hornberger, M. Going round in circles—The Papez circuit in Alzheimer’s disease. Eur. J. Neurosci. 2021, 54, 7668–7687. [Google Scholar] [CrossRef]
- Rizzo, M.; Anderson, S.; Dawson, J.; Myers, R.; Ball, K. Visual attention impairments in Alzheimer’s disease. Neurology 2000, 54, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Alescio-Lautier, B.; Michel, B.; Herrera, C.; Elahmadi, A.; Chambon, C.; Touzet, C.; Paban, V. Visual and visuospatial short-term memory in mild cognitive impairment and Alzheimer disease: Role of attention. Neuropsychologia 2007, 45, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Laczó, J.; Vyhnálek, M.; Bojar, M.; Bureš, J.; Vlček, K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc. Natl. Acad. Sci. USA 2007, 104, 4042–4047. [Google Scholar] [CrossRef]
- Elamin, M.; Holloway, G.; Bak, T.H.; Pal, S. The Utility of the Addenbrooke’s Cognitive Examination Version Three in Early-Onset Dementia. Dement. Geriatr. Cogn. Disord. 2016, 41, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- NEURONORMA Study Team; Sánchez-Benavides, G.; Peña-Casanova, J.; Casals-Coll, M.; Gramunt, N.; Molinuevo, J.L.; Gómez-Ansón, B.; Aguilar, M.; Robles, A.; Antúnez, C.; et al. Cognitive and Neuroimaging Profiles in Mild Cognitive Impairment and Alzheimer’s Disease: Data from the Spanish Multicenter Normative Studies (NEURONORMA Project). J. Alzheimer’s Dis. 2014, 41, 887–901. [Google Scholar] [CrossRef]
- Huang, S.F.; Liu, C.K.; Chang, C.C.; Su, C.Y. Sensitivity and specificity of executive function tests for Alzheimer’s disease. Appl. Neuropsychol. Adult 2017, 24, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.J.; Chen, Y.F.; Chen, T.F.; Yang, S.Y.; Yang, F.P.G.; Tseng, T.W.; Chieh, J.J.; Chen, J.C.R.; Tzen, K.Y.; Hua, M.S.; et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early alzheimer’s disease: Plasma Tau in MCI and Early AD. Hum. Brain Map. 2014, 35, 3132–3142. [Google Scholar] [CrossRef]
- Stokholm, J.; Vogel, A.; Gade, A.; Waldemar, G. Heterogeneity in Executive Impairment in Patients with Very Mild Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2006, 22, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Collette, F.; Van Der Linden, M.; Salmon, E. Executive Dysfunction in Alzheimer’s Disease. Cortex 1999, 35, 57–72. [Google Scholar] [CrossRef]
- Huntley, J.D.; Howard, R.J. Working memory in early Alzheimer’s disease: A neuropsychological review. Int. J. Geriatr. Psychiatry 2010, 25, 121–132. [Google Scholar] [CrossRef]
- Adler, G. Das EEG als Indikator des cholinergen Defizits bei der Alzheimerschen Krankheit. Fortschritte Der Neurol. Psychiatr. 2000, 68, 352–356. [Google Scholar] [CrossRef]
- Postle, B.R.; Berger, J.S.; D’Esposito, M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc. Natl. Acad. Sci. USA 1999, 96, 12959–12964. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B: Imaging Amyloid in AD with PIB. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.C.; Ng, S.; Ackermann, U.; Gong, S.J.; Pike, K.; Savage, G.; Cowie, T.F.; Dickinson, K.L.; Maruff, P.; Darby, D.; et al. Imaging -amyloid burden in aging and dementia. Neurology 2007, 68, 1718–1725. [Google Scholar] [CrossRef]
- Van Der Hiele, K.; Vein, A.; Kramer, C.; Reijntjes, R.; Van Buchem, M.; Westendorp, R.; Bollen, E.; Van Dijk, J.; Middelkoop, H. Memory activation enhances EEG abnormality in mild cognitive impairment. Neurobiol. Aging 2007, 28, 85–90. [Google Scholar] [CrossRef]
- Gurja, J.P.K.; Muthukrishnan, S.P.; Tripathi, M.; Mehta, N.; Sharma, R. Multi-domain Cognitive Testing: A Biomarker for Classifying the Cognitive Status of Mild Cognitive Impairment and Alzheimer’s Disease. Neurol. India 2022, 70, 1057–1063. [Google Scholar] [CrossRef]
- Leirer, V.O.; Morrow, D.G.; Sheikh, J.I.; Pariante, G.M. Memory skills elders want to improve. Exp. Aging Res. 1990, 16, 155–158. [Google Scholar] [CrossRef]
- Werheid, K.; Clare, L. Are Faces Special in Alzheimer’s Disease? Cognitive Conceptualisation, Neural Correlates, and Diagnostic Relevance of Impaired Memory for Faces and Names. Cortex 2007, 43, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Celone, K.; DePeau, K.; Diamond, E.; Dickerson, B.C.; Rentz, D.; Pihlajamäki, M.; Sperling, R.A. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl. Acad. Sci. USA 2008, 105, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, J.; Andrés, P. The Face-Name Associative Memory Test as a Tool for Early Diagnosis of Alzheimer’s Disease. Front. Psychol. 2018, 9, 1464. [Google Scholar] [CrossRef]
- Alegret, M.; Sotolongo-Grau, O.; De Antonio, E.E.; Pérez-Cordón, A.; Orellana, A.; Espinosa, A.; Gil, S.; Jiménez, D.; Ortega, G.; Sanabria, A.; et al. Automatized FACEmemory® scoring is related to Alzheimer’s disease phenotype and biomarkers in early-onset mild cognitive impairment: The BIOFACE cohort. Alzheimer’s Res. Ther. 2022, 14, 43. [Google Scholar] [CrossRef]
- Frasson, P.; Ghiretti, R.; Catricalà, E.; Pomati, S.; Marcone, A.; Parisi, L.; Rossini, P.M.; Cappa, S.F.; Mariani, C.; Vanacore, N.; et al. Free and cued selective reminding test: An Italian normative study. Neurol. Sci. 2011, 32, 1057–1062. [Google Scholar] [CrossRef]
- Clarke, A.; Ashe, C.; Jenkinson, J.; Rowe, O.; Hyland, P.; Commins, S. Predicting conversion of patients with Mild Cognitive Impairment to Alzheimer’s disease using bedside cognitive assessments. J. Clin. Exp. Neuropsychol. 2022, 44, 703–712. [Google Scholar] [CrossRef]
- Gainotti, G.; Quaranta, D.; Vita, M.G.; Marra, C. Neuropsychological Predictors of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 38, 481–495. [Google Scholar] [CrossRef]
- Sano, M.; Raman, R.; Emond, J.; Thomas, R.G.; Petersen, R.; Schneider, L.S.; Aisen, P.S. Adding Delayed Recall to the Alzheimer Disease Assessment Scale is Useful in Studies of Mild Cognitive Impairment But Not Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Varela, S.; Burgio, F.; Meneghello, F.; De Marco, M.; Arcara, G.; Rigon, J.; Pilosio, C.; Butterworth, B.; Venneri, A.; Semenza, C. Anatomical substrates and neurocognitive predictors of daily numerical abilities in mild cognitive impairment. Cortex 2015, 71, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; Triebel, K.; Dreer, L.E.; Novack, T.A.; Turner, C.; Marson, D.C. Neurocognitive Predictors of Financial Capacity in Traumatic Brain Injury. J. Head Trauma Rehabil. 2012, 27, E81–E90. [Google Scholar] [CrossRef] [PubMed]
- Marson, D.C. Clinical and Ethical Aspects of Financial Capacity in Dementia: A Commentary. Am. J. Geriatr. Psychiatry 2013, 21, 382–390. [Google Scholar] [CrossRef]
- Sherod, M.G.; Griffith, H.R.; Copeland, J.; Belue, K.; Krzywanski, S.; Zamrini, E.Y.; Harrell, L.E.; Clark, D.G.; Brockington, J.C.; Powers, R.E.; et al. Neurocognitive predictors of financial capacity across the dementia spectrum: Normal aging, mild cognitive impairment, and Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2009, 15, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Zamarian, L.; Semenza, C.; Domahs, F.; Benke, T.; Delazer, M. Alzheimer’s disease and mild cognitive impairment: Effects of shifting and interference in simple arithmetic. J. Neurol. Sci. 2007, 263, 79–88. [Google Scholar] [CrossRef]
- Doukakis, S.; Vrahatis, A.G.; Exarchos, T.; Hadjinicolaou, M.; Vlamos, P.; Mouza, C. Design, Implementation, and Evaluation of Online Bioinformatics and Neuroinformatics Labs. Int. J. Online Biomed. Eng. 2023, 19. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannopoulou, P.; Vrahatis, A.G.; Papalaskari, M.-A.; Vlamos, P. The RODI mHealth app Insight: Machine-Learning-Driven Identification of Digital Indicators for Neurodegenerative Disorder Detection. Healthcare 2023, 11, 2985. https://doi.org/10.3390/healthcare11222985
Giannopoulou P, Vrahatis AG, Papalaskari M-A, Vlamos P. The RODI mHealth app Insight: Machine-Learning-Driven Identification of Digital Indicators for Neurodegenerative Disorder Detection. Healthcare. 2023; 11(22):2985. https://doi.org/10.3390/healthcare11222985
Chicago/Turabian StyleGiannopoulou, Panagiota, Aristidis G. Vrahatis, Mary-Angela Papalaskari, and Panagiotis Vlamos. 2023. "The RODI mHealth app Insight: Machine-Learning-Driven Identification of Digital Indicators for Neurodegenerative Disorder Detection" Healthcare 11, no. 22: 2985. https://doi.org/10.3390/healthcare11222985
APA StyleGiannopoulou, P., Vrahatis, A. G., Papalaskari, M.-A., & Vlamos, P. (2023). The RODI mHealth app Insight: Machine-Learning-Driven Identification of Digital Indicators for Neurodegenerative Disorder Detection. Healthcare, 11(22), 2985. https://doi.org/10.3390/healthcare11222985







