Influence of Nutritional Status and Physical Fitness on Cognitive Domains among Older Adults: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
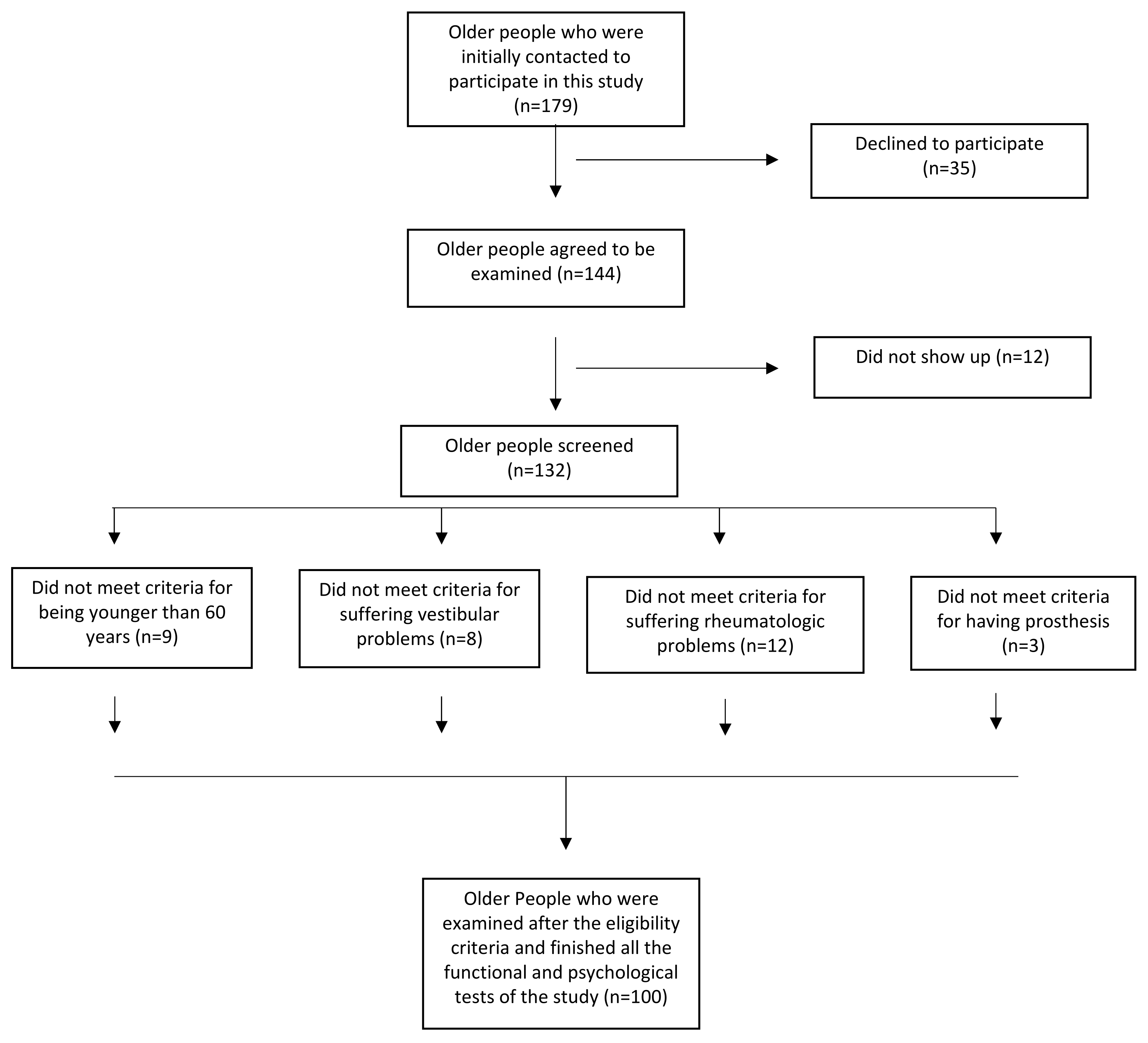
2.1. Study Design and Participants
2.2. Study Outcomes
2.2.1. Sociodemographic and Anthropometric Data
2.2.2. Nutritional Status
2.2.3. Physical Fitness
2.2.4. Cognitive Impairment
2.3. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcos-Pardo, P.J.; González-Gálvez, N.; López-Vivancos, A.; Espeso-García, A.; Martínez-Aranda, L.M.; Gea-García, G.M.; Orquín-Castrillón, F.J.; Carbonell-Baeza, A.; Jiménez-García, J.D.; Velázquez-Díaz, D.; et al. Sarcopenia, Diet, Physical Activity and Obesity in European Middle-Aged and Older Adults: The LifeAge Study. Nutrients 2020, 13, 8. [Google Scholar] [CrossRef]
- Alvarado García, A.M.; Salazar Maya, Á.M. Análisis del concepto de envejecimiento. Gerokomos 2014, 25, 57–62. [Google Scholar] [CrossRef]
- León-Caballero, M.P.; Alcolea-Martínez, E. Estado nutricional en personas mayores y su influencia sobre el deterioro cognitivo y la demencia. Psicogeriatría 2016, 6, 99–109. [Google Scholar]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Requejo, V. Nutrición y deterioro cognitivo. Nutr. Hosp. 2016, 33 (Suppl. S4), 49–52. [Google Scholar] [CrossRef]
- Haan, M.N.; Wallace, R. Can dementia be prevented? Brain aging in a population-based context. Annu. Rev. Public Health 2004, 25, 1–24. [Google Scholar] [CrossRef]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. Geriatr. J. 2015, 18, 231–245. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Barha, C.K.; Best, J.R. Physical activity for brain health in older adults. Appl. Physiol. Nutr. Metab. 2018, 43, 1105–1112. [Google Scholar] [CrossRef]
- Gerger, P.; Pai, R.K.; Stuckenschneider, T.; Falkenreck, J.; Weigert, H.; Stahl, W.; Weber, B.; Nelles, G.; Spazzafumo, L.; Schneider, S.; et al. Associations of Lipophilic Micronutrients with Physical and Cognitive Fitness in Persons with Mild Cognitive Impairment. Nutrients 2019, 11, 902. [Google Scholar] [CrossRef]
- Lee, K.S.; Cheong, H.K.; Kim, E.A.; Kim, K.R.; Oh, B.H.; Hong, C.H. Nutritional risk and cognitive impairment in the elderly. Arch. Gerontol. Geriatr. 2009, 48, 95–99. [Google Scholar] [CrossRef]
- Gimeno, E. Medidas empleadas para evaluar el estado nutricional. Offarm 2003, 22, 4. [Google Scholar]
- Saleedaeng, P.; Korwanich, N.; Muangpaisan, W.; Korwanich, K. Effect of Dysphagia on the Older Adults’ Nutritional Status and Meal Pattern. J. Prim. Care Community Health 2023, 14, 21501319231158280. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31, Erratum in Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Wiśniowska-Szurlej, A.; Ćwirlej-Sozańska, A.; Wołoszyn, N.; Sozański, B.; Wilmowska-Pietruszyńska, A. Association between Handgrip Strength, Mobility, Leg Strength, Flexibility, and Postural Balance in Older Adults under Long-Term Care Facilities. Biomed. Res. Int. 2019, 2019, 1042834. [Google Scholar] [CrossRef]
- Burgos, R.; Sarto, B.; Segurola, H.; Romagosa, A.; Puiggrós, C.; Vázquez, C.; Cárdenas, G.; Barcons, N.; Araujo, K.; Pérez-Portabella, C. Traducción y validación de la versión en español de la escala EAT-10 (Eating Assessment Tool-10) para el despistaje de la disfagia. Nutr. Hosp. 2012, 27, 2048–2054. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Ranhoff, A.H.; Gjøen, A.U.; Mowé, M. Screening for malnutrition in elderly acute medical patients: The usefulness of MNA-SF. J. Nutr. Health Aging 2005, 9, 221–225. [Google Scholar]
- Muñoz Díaz, B.; Molina-Recio, G.; Romero-Saldaña, M.; Redondo Sánchez, J.; Aguado Taberné, C.; Arias Blanco, C.; Molina-Luque, R.; Martínez De La Iglesia, J. Validation (in Spanish) of the Mini Nutritional Assessment survey to assess the nutritional status of patients over 65 years of age. Fam. Pract. 2019, 36, 172–178, Erratum in Fam. Pract. 2019, 36, 528. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Mesa, J.L.; Gutiérrez, A.; Castillo, M.J. Hand size influences optimal grip span in women but not in men. J. Hand Surg. Am. 2002, 27, 897–901. [Google Scholar] [CrossRef]
- Rydwik, E.; Bergland, A.; Forsén, L.; Frändin, K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: A systematic review. Physiother. Theory Pract. 2012, 28, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.P.; Ticinesi, A.; De Vita, F.; Gelmini, G.; Costantino, C.; Meschi, T.; Kressig, R.W.; Cesari, M.; Fabi, M.; et al. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals. PLoS ONE 2016, 11, e0153583. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; García-Rueda, L.; Salgueiro, C.; Pérez-Bellmunt, A.; Rodríguez-Sanz, J.; López-de-Celis, C. Assessment of the 4-meter walk test test-retest reliability and concurrent validity and its correlation with the five sit-to-stand test in chronic ambulatory stroke survivors. Gait Posture 2023, 101, 8–13. [Google Scholar] [CrossRef]
- Rodrigues, F.; Teixeira, J.E.; Forte, P. The Reliability of the Timed Up and Go Test among Portuguese Elderly. Healthcare 2023, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kaplan, E.F.; Goodglass, H.; Weintraub, S. The Boston Naming Test, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Mack, W.J.; Freed, D.M.; Williams, B.W.; Henderson, V.W. Boston Naming Test: Shortened versions for use in Alzheimer’s disease. J. Gerontol. 1992, 47, P154–P158. [Google Scholar] [CrossRef]
- Calero, M.D.; Arnedo, M.L.; Navarro, E.; Ruiz-Pedrosa, M.; Carnero, C. Usefulness of a 15-Item Version of the Boston Naming Test in Neuropsychological Assessment of Low-Educational Elders with Dementia. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, P187–P191. [Google Scholar] [CrossRef]
- Benton, A.; Hamsher, S.; Sivan, A. Controlled Oral Word Association Test (COWAT) [Database record]. APA PsycTests 1983. [Google Scholar] [CrossRef]
- Lim, K.-B.; Kim, J.; Lee, H.-J.; Yoo, J.; Kim, H.S.; Kim, C.; Lee, H. COWAT Performance of Persons with Alzheimer’s Dementia, Vascular Dementia, and Parkinson’s Disease Dementia According to Stage of Cognitive Impairment. PMR 2019, 11, 737–744. [Google Scholar] [CrossRef]
- Bank, A.L.; Yochim, B.P.; MacNeill, S.E.; Lichtenberg, P.A. Expanded normative data for the Mattis Dementia Rating Scale for use with urban, elderly medical patients. Clin. Neuropsychol. 2000, 14, 149–156. [Google Scholar] [CrossRef]
- O’Connell, M.E.; Tuokko, H. Age corrections and dementia classification accuracy. Arch. Clin. Neuropsychol. 2010, 25, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Loonstra, A.S.; Tarlow, A.R.; Sellers, A.H. COWAT metanorms across age, education, and gender. Appl. Neuropsychol. 2001, 8, 161–166. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; Kozak, J.; Rees, L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999, 14, 167–177. [Google Scholar] [PubMed]
- Leirós, M.; Amenedo, E.; Rodríguez, M.; Pazo-Álvarez, P.; Franco, L.; Leis, R.; Martínez-Olmos, M.Á.; Arce, C.; Rest of NUTRIAGE Study Researchers. Cognitive Status and Nutritional Markers in a Sample of Institutionalized Elderly People. Front. Aging Neurosci. 2022, 14, 880405. [Google Scholar] [CrossRef] [PubMed]
- Handing, E.P.; Leng, X.I.; Kritchevsky, S.B.; Craft, S. Association Between Physical Performance and Cognitive Function in Older Adults Across Multiple Studies: A Pooled Analysis Study. Innov. Aging 2020, 4, igaa050. [Google Scholar] [CrossRef]
- Dunn-Lewis, C.; Kraemer, W.J.; Kupchak, B.R.; Kelly, N.A.; Creighton, B.A.; Luk, H.Y.; Ballard, K.D.; Comstock, B.A.; Szivak, T.K.; Hooper, D.R.; et al. A multi-nutrient supplement reduced markers of inflammation and improved physical performance in active individuals of middle to older age: A randomized, double-blind, placebo-controlled study. Nutr. J. 2011, 10, 90. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhu, H.; Huang, D.; Li, W.; Wang, J.; Liu, Z. Association between grip strength and cognitive impairment in older American adults. Front. Mol. Neurosci. 2022, 15, 973700. [Google Scholar] [CrossRef]
- Alfaro-Acha, A.; Al Snih, S.; Raji, M.A.; Kuo, Y.F.; Markides, K.S.; Ottenbacher, K.J. Handgrip strength and cognitive decline in older Mexican Americans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 859–865. [Google Scholar] [CrossRef]
- Eggermont, L.H.; Gavett, B.E.; Volkers, K.M.; Blankevoort, C.G.; Scherder, E.J.; Jefferson, A.L.; Steinberg, E.; Nair, A.; Green, R.C.; Stern, R.A. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer’s disease. Arch. Phys. Med. Rehabil. 2010, 91, 584–588. [Google Scholar] [CrossRef]
- Ye, B.S.; Seo, S.W.; Cho, H.; Kim, S.Y.; Lee, J.S.; Kim, E.J.; Lee, Y.; Back, J.H.; Hong, C.H.; Choi, S.H.; et al. Effects of education on the progression of early- versus late-stage mild cognitive impairment. Int. Psychogeriatr. 2013, 25, 597–606. [Google Scholar] [CrossRef]
- Zec, R.F.; Burkett, N.R.; Markwell, S.J.; Larsen, D.L. Normative data stratified for age, education, and gender on the Boston Naming Test. Clin. Neuropsychol. 2007, 21, 617–637. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aranda, C.; Martinussen, M. Age-related differences in performance of phonemic verbal fluency measured by Controlled Oral Word Association Task (COWAT): A meta-analytic study. Dev. Neuropsychol. 2006, 30, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Sugimoto, T.; Kitamori, K.; Saji, N.; Niida, S.; Toba, K.; Sakurai, T. Malnutrition is Associated with Behavioral and Psychiatric Symptoms of Dementia in Older Women with Mild Cognitive Impairment and Early-Stage Alzheimer’s Disease. Nutrients 2019, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Seesen, M.; Sirikul, W.; Ruangsuriya, J.; Griffiths, J.; Siviroj, P. Cognitive Frailty in Thai Community-Dwelling Elderly: Prevalence and Its Association with Malnutrition. Nutrients 2021, 13, 4239. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Yorozu, A.; Adachi, D.; Takahashi, M.; Aoyama, T. Association between mild cognitive impairment and trajectory-based spatial parameters during timed up and go test using a laser range sensor. J. Neuroeng. Rehabil. 2017, 14, 78. [Google Scholar] [CrossRef]
| Characteristics | Values Total = 100 | Values | Men = 21 | Values | Women = 79 | ||
|---|---|---|---|---|---|---|---|
| FREQUENCY | % | FREQUENCY | % | FREQUENCY | % | ||
| Educational status, n (%) | No formal education | 14 | 14 | 2 | 9.52 | 12 | 15.18 |
| Primary education | 65 | 65 | 16 | 76.19 | 49 | 60.02 | |
| Secondary education | 17 | 17 | 2 | 9.52 | 15 | 19.98 | |
| University | 4 | 4 | 1 | 4.76 | 3 | 3.79 | |
| MEAN | SD | MEAN | SD | MEAN | SD | ||
| Age (years) | 67.5 | 5.67 | 64.80 | 8.59 | 69.56 | 7.89 | |
| BMI (kg/m2) | 29.70 | 4.91 | 29.63 | 4.18 | 29.46 | 5.60 | |
| EAT-10 (score) | 0.64 | 1.48 | 0.50 | 0.79 | 0.65 | 1.56 | |
| MNA-SF (score) | 12.28 | 1.80 | 12.25 | 1.95 | 12.28 | 1.80 | |
| Handgrip (kg) | 24.60 | 7.38 | 37.18 | 7.66 | 22.25 | 4.32 | |
| Gait speed (s) | 2.27 | 0.48 | 2.06 | 0.45 | 2.31 | 0.48 | |
| Dynamic balance (s) | 7.07 | 1.53 | 6.66 | 1.46 | 7.15 | 1.54 | |
| MMSE (score) | 25.96 | 3.20 | 26.66 | 2.47 | 25.83 | 5.05 | |
| BNT (score) | 10.27 | 2.93 | 12.00 | 2.51 | 9.95 | 2.91 | |
| P-COWAT (words) | 30.24 | 12.78 | 36.52 | 12.18 | 29.07 | 5.05 | |
| S-COWAT (words) | 31.93 | 9.85 | 34.76 | 13.30 | 31.43 | 9.04 | |
| MMSE (s) | BNT (Score) | P-COWA (Words) | S-COWA (Words) | |
|---|---|---|---|---|
| EAT-10 (score) | 0.019 | −0.037 | 0.049 | −0.089 |
| MNA-SF(score) | −0.099 | 0.004 | −0.219 1 | 0.064 |
| Handgrip (kg) | 0.156 | 0.273 2 | 0.271 1 | 0.142 |
| Gait Speed (s) | −0.206 1 | −0.331 2 | −0.121 | −0.358 2 |
| Dynamic Balance(s) | −0.272 2 | −0.240 2 | −0.139 | −0.304 2 |
| Sex | −0.096 | −0.255 2 | −0.213 1 | −0.124 |
| Educational Status | 0.341 2 | 0.523 2 | 0.555 2 | −0.324 2 |
| Age (years) | −0.214 1 | −0.404 2 | −0.411 2 | −0.154 |
| BMI (kg/m2) | −0.116 | −0.089 | −0.063 | −0.115 |
| Variable | B | β | t | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| MMSE (s) | Educational | 1.177 | 0.278 | 3.195 | 0.448 | 1.906 | 0.002 |
| Dynamic Balance | −0.422 | −0.203 | −2.439 | −0.765 | −0.080 | 0.016 | |
| Gait Speed | −1.353 | −0.206 | −2.409 | −2.465 | −0.242 | 0.017 | |
| BNT (score) | Educational | 1.597 | 0.410 | 4.990 | 0.964 | 2.231 | 0.000 |
| Age | −0.119 | −0.230 | −3.023 | −0.197 | −0.041 | 0.003 | |
| Gait Speed | −2.090 | −0.346 | −2.890 | −3.521 | −0.659 | 0.005 | |
| P-COWAT (words) | Educational | 8.366 | 0.500 | 5.012 | 5.052 | 11.679 | 0.000 |
| Age | −0.512 | −0.231 | −2.676 | −0.891 | −0.132 | 0.009 | |
| MNA-SF | −1.186 | −0.167 | −2.090 | −2.313 | −0.059 | 0.039 | |
| S-COWAT (words) | Educational | 3.455 | 0.265 | 3.283 | 1.373 | 5.536 | 0.001 |
| Gait Speed | −5.957 | −0.294 | −2.201 | −11.313 | −0.601 | 0.030 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boquete-Pumar, C.; Álvarez-Salvago, F.; Martínez-Amat, A.; Molina-García, C.; De Diego-Moreno, M.; Jiménez-García, J.D. Influence of Nutritional Status and Physical Fitness on Cognitive Domains among Older Adults: A Cross-Sectional Study. Healthcare 2023, 11, 2963. https://doi.org/10.3390/healthcare11222963
Boquete-Pumar C, Álvarez-Salvago F, Martínez-Amat A, Molina-García C, De Diego-Moreno M, Jiménez-García JD. Influence of Nutritional Status and Physical Fitness on Cognitive Domains among Older Adults: A Cross-Sectional Study. Healthcare. 2023; 11(22):2963. https://doi.org/10.3390/healthcare11222963
Chicago/Turabian StyleBoquete-Pumar, Carmen, Francisco Álvarez-Salvago, Antonio Martínez-Amat, Cristina Molina-García, Manuel De Diego-Moreno, and José Daniel Jiménez-García. 2023. "Influence of Nutritional Status and Physical Fitness on Cognitive Domains among Older Adults: A Cross-Sectional Study" Healthcare 11, no. 22: 2963. https://doi.org/10.3390/healthcare11222963
APA StyleBoquete-Pumar, C., Álvarez-Salvago, F., Martínez-Amat, A., Molina-García, C., De Diego-Moreno, M., & Jiménez-García, J. D. (2023). Influence of Nutritional Status and Physical Fitness on Cognitive Domains among Older Adults: A Cross-Sectional Study. Healthcare, 11(22), 2963. https://doi.org/10.3390/healthcare11222963









