Surgical Treatment for Early Cervical Cancer in the HPV Era: State of the Art
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Cervical Cancer Screening Tests
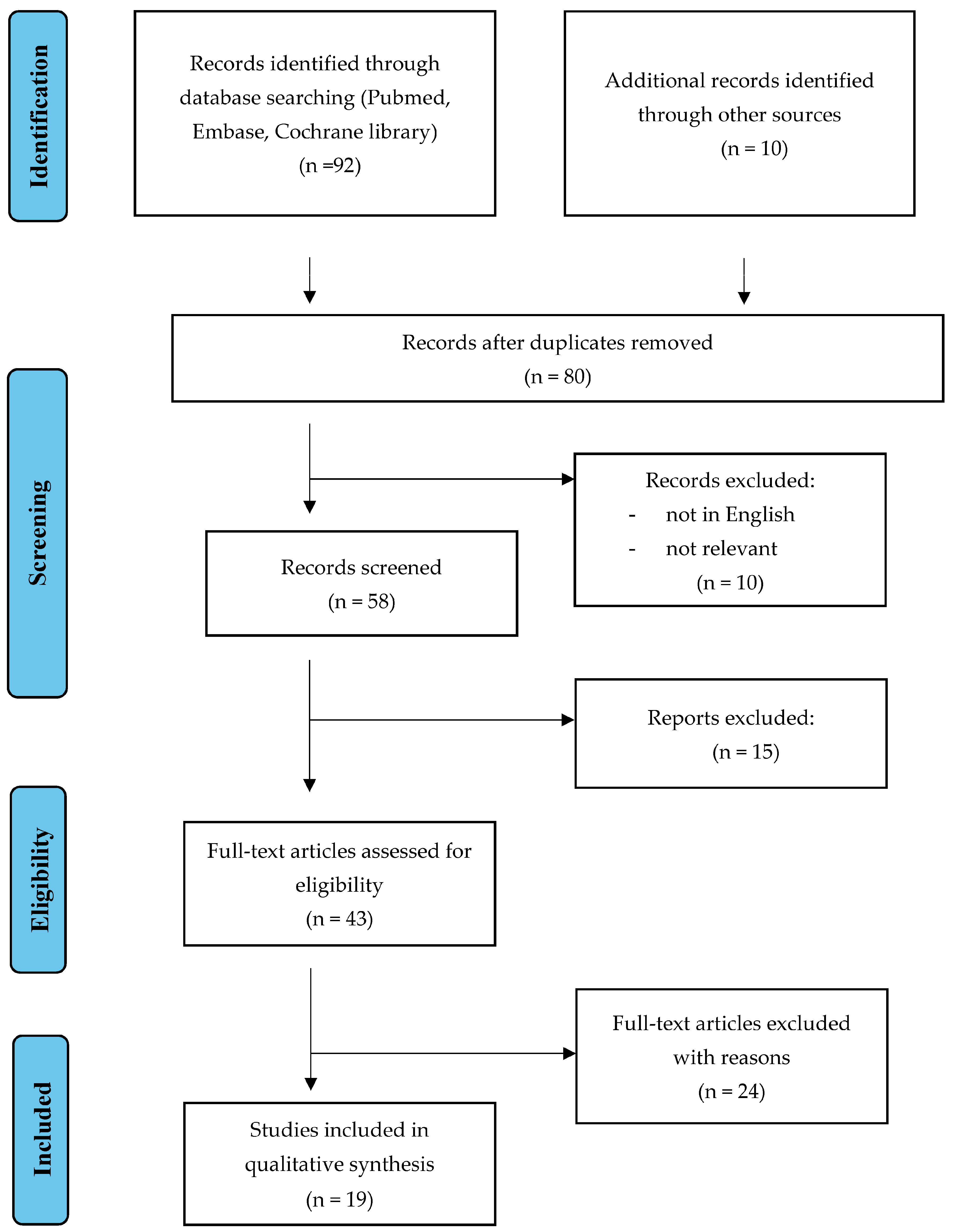
2. Materials and Methods
3. Results
3.1. Excision Procedure
3.2. Trachelectomy and SLN Mapping
3.3. Laparotomy Hysterectomy versus Laparoscopy Management
3.4. Laparotomy Hysterectomy versus Robotic Management
3.5. Uterine Manipulator for Early-Stage Cervical Cancer
3.6. Fertility Sparing-Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Centre for Disease Control and Prevention. Sexually Transmitted Diseases (STD)—Human Papillomavirus Infection. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf (accessed on 1 May 2023).
- Castle, P.E.; Rodriguez, A.C.; Burk, R.D.; Herrero, R.; Wacholder, S.; Alfaro, M.; Morales, J.; Guillen, D.; Sherman, M.; Solomon, D.; et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: Population based cohort study. BMJ 2009, 339, b2569. [Google Scholar] [CrossRef]
- Voidăzan, S.; Morariu, S.H.; Tarcea, M.; Moldovan, H.; Curticăpian, I.; Dobreanu, M. Human Papillomavirus (HPV) Infection and HPV Vaccination: Assessing the Level of Knowledge among Students of the University of Medicine and Pharmacy of Tîrgu Mureş, Romania. Acta Dermatovenerol. Croat. 2016, 24, 193–202. [Google Scholar] [PubMed]
- Afonso, N.M.; Kavanagh, M.J.; Swanberg, S.M.; Schulte, J.M.; Wunderlich, T.; Lucia, V.C. Will they lead by example? Assessment of vaccination rates and attitudes to human papilloma virus in millennial medical students. BMC Public Health 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100B: A Review of Human Carcinogens: Biological Agents. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: Collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet 2007, 370, 1609–1621. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer; Appleby, P.; Beral, V.; Berrington de González, A.; Colin, D.; Franceschi, S.; Goodill, A.; Green, J.; Peto, J.; Plummer, M.; et al. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int. J. Cancer 2006, 118, 1481–1495. [Google Scholar]
- Dahlström, L.A.; Andersson, K.; Luostarinen, T.; Thoresen, S.; Ögmundsdottír, H.; Tryggvadottír, L.; Wiklund, F.; Skare, G.B.; Eklund, C.; Sjölin, K.; et al. Prospective Seroepidemiologic Study of Human Papillomavirus and Other Risk Factors in Cervical Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2541–2550. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: Collaborative reanalysis of individual data on 8097 women with squamous cell carcinoma and 1374 women with adenocarcinoma from 12 epidemiological studies. Int. J. Cancer 2007, 120, 885–891. [Google Scholar] [CrossRef]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Woodman, C.B.J.; Collins, S.I.; Young, L.S. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Arbyn, M.; Bergeron, C.; Bosch, F.X.; Dillner, J.; Jit, M.; Kim, J.; Poljak, M.; Nieminen, P.; Sasieni, P.; et al. Cervical screening: ESGO-EFC position paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br. J. Cancer 2020, 123, 510–517. [Google Scholar] [CrossRef]
- Peirson, L.; Fitzpatrick-Lewis, D.; Ciliska, D.; Warren, R. Screening for cervical cancer: A systematic review and meta-analysis. Syst. Rev. 2013, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, J.; Castle, P.E.; Temin, S.; Denny, L.; Gupta, V.; Kim, J.J.; Luciani, S.; Murokora, D.; Ngoma, T.; Qiao, Y.; et al. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J. Glob. Oncol. 2016, 3, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, G.F.; Kulasingam, S.; Denberg, T.D.; Qaseem, A. Clinical Guidelines Committee of the American College of Physicians Cervical Cancer Screening in Average-Risk Women: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann. Intern. Med. 2015, 162, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, T.; Hosono, S.; Sasaki, S.; Hoshi, K.; Hamashima, Y.; Katayama, T.; Hamashima, C. Comparative accuracy of cervical cancer screening strategies in healthy asymptomatic women: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 94. [Google Scholar] [CrossRef]
- Huh, W.K.; Ault, K.A.; Chelmow, D.; Davey, D.D.; Goulart, R.A.; Garcia, F.A.; Kinney, W.K.; Massad, L.S.; Mayeaux, E.J.; Saslow, D.; et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol. Oncol. 2015, 125, 330–337. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- NICE. Guidance on the Use of Liquid-Based Cytology for Cervical Screening. 2003. Available online: http://www.nice.org.uk (accessed on 1 May 2023).
- Arbyn, M.; Anttila, A.; Jordan, J.; Ronco, G.; Schenck, U.; Segnan, N.; Wiener, H.; Herbert, A.; von Karsa, L. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document. Ann. Oncol. 2010, 21, 448–458. [Google Scholar] [CrossRef]
- Tax, C.; Rovers, M.M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.A.O.; Aaltonen, R.; Cárdenas, J.; Hernándes, J.C.; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ 2018, 360, k499. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Directorate-General for Health and Food Safety; Karsa, L.; Dillner, J.; Suonio, E. European Guidelines for Quality Assurance in Cervical Cancer Screening, 2nd ed.; Karsa, L., Dillner, J., Suonio, E., Törnberg, S., Anttila, A., Ronco, G., Franceschi, S., De Vuyst, H., Dillner, L., Patnick, J., et al., Eds.; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.E.; Kesic, V.; Cruickshank, M.E.; Gultekin, M.; Carcopino, X.; Castro Sanchez, M.; Grigore, M.; Jakobsson, M.; Kuppers, V.; Pedro, A.; et al. European consensus statement on essential colposcopy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Burness, J.V.; Schroeder, J.M.; Warren, J.B. Cervical Colposcopy: Indications and Risk Assessment. Am. Fam. Physician 2020, 102, 39–48. [Google Scholar] [PubMed]
- Feng, Y.; Zhang, Z.; Lou, T.; Wang, S.; Bai, H.; Zhang, Z. The security of radical trachelectomy in the treatment of IA–IIA cervical carcinoma requires further evaluation: Updated meta-analysis and trial sequential analysis. Arch. Gynecol. Obstet. 2019, 299, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Ishioka, S.; Mariya, T.; Fujibe, Y.; Kim, M.; Someya, M.; Saito, T. Pregnancies after vaginal radical trachelectomy (RT) in patients with early invasive uterine cervical cancer: Results from a single institute. BMC Pregnancy Childbirth 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.R.; Burrell, M.O.; Nezhat, F.R.; Benigno, B.B.; Welander, C.E. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am. J. Obstet. Gynecol. 1992, 166, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; dos Reis, R.; Sun, C.C.; Milam, M.R.; Bevers, M.W.; Brown, J.; Slomovitz, B.M.; Ramirez, P.T. Comparison of Total Laparoscopic and Abdominal Radical Hysterectomy for Patients With Early-Stage Cervical Cancer. Obstet. Gynecol. 2007, 110, 96–102. [Google Scholar] [CrossRef]
- Hauspy, J.; Beiner, M.; Harley, I.; Ehrlich, L.; Rasty, G.; Covens, A. Sentinel lymph nodes in early stage cervical cancer. Gynecol. Oncol. 2007, 105, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2006, 95, S43–S103. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Falconer, H.; Palsdottir, K.; Stalberg, K.; Dahm-Kähler, P.; Ottander, U.; Lundin, E.S.; Wijk, L.; Kimmig, R.; Jensen, P.T.; Zahl Eriksson, A.G.; et al. Robot-assisted approach to cervical cancer (RACC): An international multi-center, open-label randomized controlled trial. Int. J. Gynecol. Cancer 2019, 29, 1072–1076. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.-P.; Wen, N.; Qiao, X.; Meng, Y.-G. Comparative analysis of robotic vs laparoscopic radical hysterectomy for cervical cancer. World J. Clin. Cases 2019, 7, 3185–3193. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Ming, X.; Jing, H.; Li, Z. Surgical Approach and Use of Uterine Manipulator Are Not Associated with LVSI in Surgery for Early-stage Cervical Cancer. J. Minim. Invasive Gynecol. 2021, 28, 1573–1578. [Google Scholar] [CrossRef]
- Kampers, J.; Gerhardt, E.; Sibbertsen, P.; Flock, T.; Klapdor, R.; Hertel, H.; Jentschke, M.; Hillemanns, P. Protective operative techniques in radical hysterectomy in early cervical carcinoma and their influence on disease-free and overall survival: A systematic review and meta-analysis of risk groups. Arch. Gynecol. Obstet. 2021, 304, 577–587. [Google Scholar] [CrossRef]
- Fusegi, A.; Kanao, H.; Tsumura, S.; Murakami, A.; Abe, A.; Aoki, Y.; Nomura, H. Minimally invasive radical hysterectomy and the importance of avoiding cancer cell spillage for early-stage cervical cancer: A narrative review. J. Gynecol. Oncol. 2023, 34, e5. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Obermair, A.; Coleman, R.L.; Pareja, R.; Lopez, A.; Ribero, R.; Isla, D.; Rendon, G.; Bernardini, M.Q.; Buda, A.; et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): A secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 851–860. [Google Scholar] [CrossRef]
- Copeland, L.J.; Silva, E.G.; Gershenson, D.M.; Morris, M.; Young, D.C.; Wharton, J. Superficially invasive squamous cell carcinoma of the cervix. Gynecol. Oncol. 1992, 45, 307–312. [Google Scholar] [CrossRef]
- Kim, S.I.; Cho, J.H.; Seol, A.; Kim, Y.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1–IIA2 cervical cancer. Gynecol. Oncol. 2019, 153, 3–12. [Google Scholar] [CrossRef]
- Paik, E.S.; Lim, M.C.; Kim, M.-H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.-M.; Lee, C.; Choi, C.H. Prognostic Model for Survival and Recurrence in Patients with Early-Stage Cervical Cancer: A Korean Gynecologic Oncology Group Study (KGOG 1028). Cancer Res. Treat. 2020, 52, 320–333. [Google Scholar] [CrossRef]
- De Rosa, N.; Lavitola, G.; Della Corte, L.; Bifulco, G. Diagnostic Accuracy of Endocervicoscopy in Identifying and Grading Cervical Intraepithelial Neoplasia Lesion. Gynecol. Obstet. Investig. 2020, 85, 196–205. [Google Scholar] [CrossRef]
- Odetto, D.; Puga, M.C.; Saadi, J.; Noll, F.; Perrotta, M. Minimally invasive radical hysterectomy: An analysis of oncologic outcomes from Hospital Italiano (Argentina). Int. J. Gynecol. Cancer 2019, 29, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Baxter, N.N.; Gien, L.T.; Moineddin, R.; Liu, N.; Dossa, F.; Willows, K.; Ferguson, S.E. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am. J. Obstet. Gynecol. 2019, 221, 619.e1–619.e24. [Google Scholar] [CrossRef]
- Casarin, J.; Bogani, G.; Papadia, A.; Ditto, A.; Pinelli, C.; Garzon, S.; Donadello, N.; Laganà, A.S.; Cromi, A.; Mueller, M.; et al. Preoperative Conization and Risk of Recurrence in Patients Undergoing Laparoscopic Radical Hysterectomy for Early Stage Cervical Cancer: A Multicenter Study. J. Minim. Invasive Gynecol. 2020, 28, 117–123. [Google Scholar] [CrossRef]
- Bizzarri, N.; Anchora, L.P.; Kucukmetin, A.; Ratnavelu, N.; Korompelis, P.; Carbone, V.; Fedele, C.; Bruno, M.; Vizzielli, G.; Gallotta, V.; et al. Protective Role of Conization Before Radical Hysterectomy in Early-Stage Cervical Cancer: A Propensity-Score Matching Study. Ann. Surg. Oncol. 2021, 28, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- D’asta, M.; Gulino, F.A.; Cannone, F.; Ettore, C.; Bonanno, G.; Ettore, G. Early Cervical Cancer and Recurrence after Minimally Invasive Surgery without Uterine Manipulator. Surgeries 2022, 3, 277–283. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Y.; Xia, H.; Zhu, X. Does the Use of a Uterine Manipulator or Intracorporeal Colpotomy Confer an Inferior Prognosis in Minimally Invasive Surgery–Treated Early-stage Cervical Cancer? J. Minim. Invasive Gynecol. 2023, 30, 156–163. [Google Scholar] [CrossRef]
- Pedone Anchora, L.; Turco, L.C.; Bizzarri, N.; Capozzi, V.A.; Lombisani, A.; Chiantera, V.; De Felice, F.; Gallotta, V.; Cosentino, F.; Fagotti, A.; et al. How to Select Early-Stage Cervical Cancer Patients Still Suitable for Laparoscopic Radical Hysterectomy: A Propensity-Matched Study. Ann. Surg. Oncol. 2020, 27, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Charo, L.M.; Vaida, F.; Eskander, R.N.; Binder, P.; Saenz, C.; McHale, M.; Plaxe, S. Rapid dissemination of practice-changing information: A longitudinal analysis of real-world rates of minimally invasive radical hysterectomy before and after presentation of the LACC trial. Gynecol. Oncol. 2020, 157, 494–499. [Google Scholar] [CrossRef]
- Cubal, A.F.R.; Carvalho, J.I.F.; Costa, M.F.M.; Branco, A.P.T. Fertility-Sparing Surgery for Early-Stage Cervical Cancer. Int. J. Surg. Oncol. 2012, 2012, 936534. [Google Scholar] [CrossRef]
- He, Z.; Bian, C.; Xie, C. Fertility-sparing surgery in early-stage cervical cancer: Laparoscopic versus abdominal radical trachelectomy. BMC Women’s Health 2022, 22, 241. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph Node Metastasis in Patients with Clinical Early-Stage Cervical Cancer: Detection with Integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef]
- Pecorino, B.; D’agate, M.G.; Scibilia, G.; Scollo, P.; Giannini, A.; Di Donna, M.C.; Chiantera, V.; Laganà, A.S. Evaluation of Surgical Outcomes of Abdominal Radical Hysterectomy and Total Laparoscopic Radical Hysterectomy for Cervical Cancer: A Retrospective Analysis of Data Collected before the LACC Trial. Int. J. Environ. Res. Public Health 2022, 19, 13176. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, Y.M.; Godoy, L.R.; Longatto-Filho, A.; dos Reis, R. Management of Early-Stage Cervical Cancer: A Literature Review. Cancers 2022, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Buda, A.; Bogani, G.; Fanfani, F.; Papadia, A.; Ceccaroni, M.; Malzoni, M.; Pellegrino, A.; Ferrari, F.; Greggi, S.; et al. Predictors of recurrence following laparoscopic radical hysterectomy for early-stage cervical cancer: A multi-institutional study. Gynecol. Oncol. 2020, 159, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef]
- Lukas, R.; Helena, R.; Jiri, H.M.; Martin, H.; Petr, S. Current status of sentinel lymph node mapping in the management of cervical cancer. Expert Rev. Anticancer. Ther. 2013, 13, 861–870. [Google Scholar] [CrossRef]
- Atri, M.; Zhang, Z.; Dehdashti, F.; Lee, S.I.; Ali, S.; Marques, H.; Koh, W.-J.; Moore, K.; Landrum, L.; Kim, J.W. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol. Oncol. 2016, 142, 413–419. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Levenback, C.; Coleman, R.L.; Burke, T.W.; Lin, W.M.; Erdman, W.; Deavers, M.; Delpassand, E.S. Lymphatic Mapping and Sentinel Node Identification in Patients With Cervix Cancer Undergoing Radical Hysterectomy and Pelvic Lymphadenectomy. J. Clin. Oncol. 2002, 20, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bats, A.-S.; Buénerd, A.; Querleu, D.; Leblanc, E.; Daraï, E.; Morice, P.; Marret, H.; Gillaizeau, F.; Mathevet, P.; Lécuru, F. Diagnostic value of intraoperative examination of sentinel lymph node in early cervical cancer: A prospective, multicenter study. Gynecol. Oncol. 2011, 123, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Slama, J.; Dundr, P.; Dusek, L.; Cibula, D. High false negative rate of frozen section examination of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2013, 129, 384–388. [Google Scholar] [CrossRef]
- Plante, M.; Gregoire, J.; Renaud, M.-C.; Roy, M. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011, 121, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.P.; Sonoda, Y.; Leitao, M.M.; Zivanovic, O.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol. Oncol. 2008, 111, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Boss, E.; van Golde, R.; Beerendonk, C.; Massuger, L. Pregnancy after radical trachelectomy: A real option? Gynecol. Oncol. 2005, 99, S152–S156. [Google Scholar] [CrossRef]
| Procedure Type | Description |
|---|---|
| Punch biopsy | Surgical procedure consisting in the removal of a round-shaped tissue sample for pathological analysis. |
| Endocervical curettage | Surgical procedure consisting in the collection of tissue from the cervical canal to find a glandular lesion or an endocervical squamouslesion which cannot be found with abiopsyincolposcopy. |
| Loop electrosurgical excision procedure (LEEP) | Diagnostic and therapeutic technique employing energy for removing atypical cells from the cervix for subsequent histological examination. |
| Cone biopsy | Diagnostic and therapeutic technique in which a cone-shaped piece of tissue from the cervix and cervical canal is removed. The aim of this procedure is the removal of precancerous lesion or early-stage cancer. A synonym for cone biopsy is cervical conization. |
| Procedure Type | Description |
|---|---|
| Total hysterectomy (TH) | Removal of the uterus and the cervix. Further subclassification according to access techniques in:
|
| Radical hysterectomy (RH) | Removal of the uterus, cervix, part of the vagina, and a wide area of ligaments and tissues around them; also ovaries, fallopian tubes, nearby lymph nodes. |
| Modified radical hysterectomy (MRH) | Removal of the uterus, cervix, upper part of the vagina, and ligaments and tissues that closely surround these organs; also ovaries, fallopian tubes, nearby lymph nodes. |
| Radical trachelectomy (RT) | Removal of the cervix, nearby tissue, the upper part of the vagina with/without removal of regional lymph nodes. |
| Cervical Cancer Stage | Treatment Description |
|---|---|
| Treatment of stage IA cervical cancer | STAGE IA1
|
| Treatment of stages IB and IIA cervical cancer |
** chemotherapy drugs: cisplatin or carboplatin and/or radiation therapy. |
| Treatment of stages IIB, III, and IVA cervical cancer |
|
| Treatment of stage IVB cervical cancer |
|
| Treatment of recurrent cervical cancer |
|
| Study/Year | Country | Type of Study | Stage/Types of Tumors | Sample Size, n° | Age (Years-Mean ± SD or Median (Range)) | Surgical Treatment | Primary Outcomes | Results |
|---|---|---|---|---|---|---|---|---|
| Ramirez et al., 2018 [41] | US | Prospective (randomized trial) “LAAC TRIAL” | Stage IA1, IA2, or IB1 CC and a histologic subtype of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma | 319 (MIS) versus 312 (AH) | 46.0 ± 10.6 46.1 ± 11.0 | MIS versus AH | The rate of DFS and OS | MIS reported lower rates of DFS and OS |
| Melamed et al., 2018 [47] | US | Retrospective (cohort study) | Stage IA2 or IB1CC and a histologic subtype of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma | 2461 patients | NA | MIS versus AH | The rate of OS | MIS reported a lower rates of OS |
| Falconer et al., 2019 [42] | Sweden | Prospective multi- institutional international open-label randomized clinical trial “RAAC TRIAL” | Stages IB1, IB2, IIA CC and a histologic subtype of squamous, adenocarcinoma, or adenosquamous | NA (interim analysis): 3 years after the first patient is randomized or when 300 patients have been included in the study | NA | TLRH versus AH | Recurrence-free survival at 5 years | The clinical non-inferiority margin is defined with a cut-off by >7.5% |
| Casarin et al., 2020 [68] | Italy | Retrospective multi-institutional study | Stage IA1, IA2 and IB1 CC, and a histologic subtype of squamous, adenocarcinoma, or adenosquamous | 428 patients | 45 (recurence NO) - 48 (recurence YES) | TLRH | To assess predictors of recurrence following TLRH | Independent predictor of recurrence after TLRH (high-volume disease) |
| Chiva et al., 2020 [69] | Europe (multicenter) | European multicenter retrospective observational cohort study “SUCCOR study” | Stage IB1 CC and a histologic subtype of squamous, adenocarcinoma, or adenosquamous | 693 patients | 48.3 (23–83) | MIS versus AH | To compare DFS, OS, and the role of uterine manipulator | MIS in CC increased the risk of relapse and death compared with AH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, M.; Della Corte, L.; Ronsini, C.; Guerra, S.; Giampaolino, P.; Bifulco, G. Surgical Treatment for Early Cervical Cancer in the HPV Era: State of the Art. Healthcare 2023, 11, 2942. https://doi.org/10.3390/healthcare11222942
Palumbo M, Della Corte L, Ronsini C, Guerra S, Giampaolino P, Bifulco G. Surgical Treatment for Early Cervical Cancer in the HPV Era: State of the Art. Healthcare. 2023; 11(22):2942. https://doi.org/10.3390/healthcare11222942
Chicago/Turabian StylePalumbo, Mario, Luigi Della Corte, Carlo Ronsini, Serena Guerra, Pierluigi Giampaolino, and Giuseppe Bifulco. 2023. "Surgical Treatment for Early Cervical Cancer in the HPV Era: State of the Art" Healthcare 11, no. 22: 2942. https://doi.org/10.3390/healthcare11222942
APA StylePalumbo, M., Della Corte, L., Ronsini, C., Guerra, S., Giampaolino, P., & Bifulco, G. (2023). Surgical Treatment for Early Cervical Cancer in the HPV Era: State of the Art. Healthcare, 11(22), 2942. https://doi.org/10.3390/healthcare11222942







