The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review
Abstract
1. Background
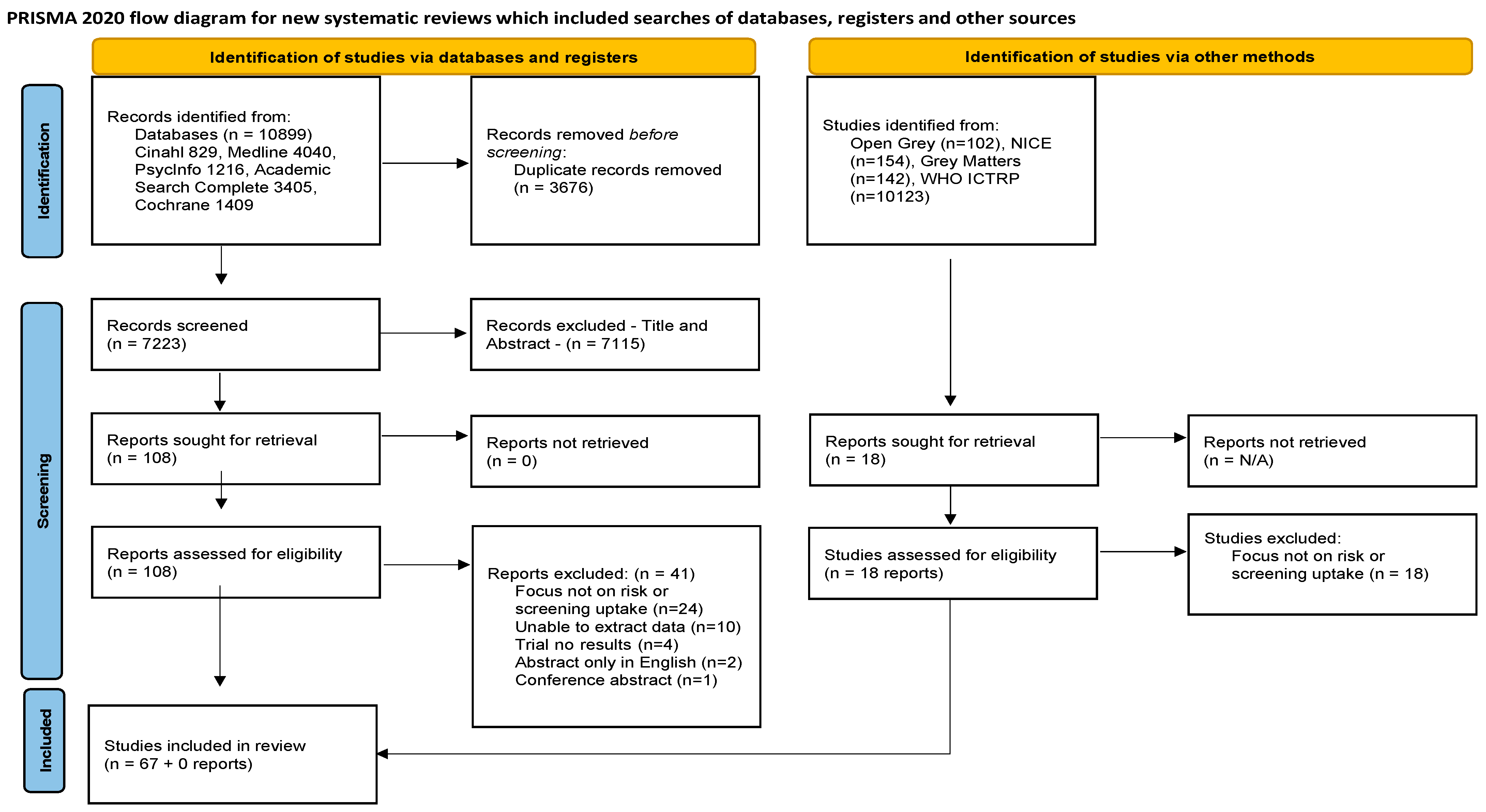
2. Methodology
2.1. Identifying the Research Question
- What risk factors for prostate cancer are evident within the literature?
- What screening and diagnostic tools for prostate cancer are evident within the literature?
- What is the screening uptake for prostate cancer evident within the literature?
2.2. Identifying Relevant Studies
Search Strategy
2.3. Selection of Studies
2.4. Charting the Data
2.5. Collating, Summarising and Reporting Results
2.6. Patient and Public Involvement
3. Results
- (A)
- What risk factors for prostate cancer are evident within the literature?
- (B)
- What screening and diagnostic tools for prostate cancer are evident within the literature?
- (C)
- What is the screening uptake for prostate cancer evident within the literature?
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Irish Cancer Society. Cancer Statistics: Irish Cancer Society 2020. Available online: https://www.cancer.ie/cancer-information-and-support/cancer-information/about-cancer/cancer-statistics (accessed on 1 April 2022).
- Chehal, A. Duodenal Metastasis from Colorectal Cancer: A Case Report. Saudi J. Pathol. Microbiol. 2023, 8, 210–215. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Pettersson, A.; Graff, R.E.; Giunchi, F.; Ahearn, T.U.; Gonzalez-Feliciano, A.G.; Markt, S.C.; Wilson, K.M.; Stopsack, K.H.; et al. A prospective study of the association between physical activity and risk of prostate cancer defined by clinical features and TMPRSS2: ERG. Eur. Urol. 2019, 76, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, E.O.; Liu, X.; Lok, V.; Ngai, C.H.; Zhang, L.; Xu, W.; Zheng, Z.J.; Chiu, P.K.; Vasdev, N.; et al. Global Trends of Prostate Cancer by Age, and Their Associations with Gross Domestic Product (GDP), Human Development Index (HDI), Smoking, and Alcohol Drinking. Clin. Genitourin. Cancer 2023, 21, e261–e270.e50. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. J. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Giona, S. The Epidemiology for Prostate Cancer; Exon Publications: Brisbane, Australia, 2021; pp. 1–15. [Google Scholar] [CrossRef]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef]
- Gulati, R.; Psutka, S.P.; Etzioni, R. Personalized risks of over diagnosis for screen detected prostate cancer incorporating patient comorbidities: Estimation and communication. J. Urol. 2019, 202, 936–943. [Google Scholar] [CrossRef]
- Khazaei, Z.; Sohrabivafa, M.; Momenabadi, V.; Moayed, L.; Goodarzi, E. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide prostate cancers and their relationship with the human development index. Adv. Hum. Biol. 2019, 9, 245. [Google Scholar] [CrossRef]
- Irish Cancer Society. Understanding Metastatic Prostate Cancer: Caring for People with Cancer. Ireland. 2021. Available online: https://www.cancer.ie/cancer-information-and-support/cancer-types/prostate-cancer (accessed on 31 March 2022).
- National Health Service. Overview: Prostate Cancer. 2021. Available online: https://www.nhs.uk/conditions/prostate-cancer/ (accessed on 30 March 2022).
- Baden, M.; Lu, L.; Drummond, F.J.; Gavin, A.; Sharp, L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support. Care Cancer 2020, 28, 4813–4824. [Google Scholar] [CrossRef]
- Sakellakis, M.; Flores, L.J.; Ramachandran, S. Patterns of indolence in prostate cancer. Exp. Ther. Med. 2022, 23, 351. [Google Scholar] [CrossRef]
- Chan, J.S.; Lee, Y.H.; Hui, J.M.; Liu, K.; Dee, E.C.; Ng, K.; Liu, T.; Tse, G.; Ng, C.F. Long-term prognostic impact of cardiovascular comorbidities in patients with prostate cancer receiving androgen deprivation therapy: A population-based competing risk analysis. Int. J. Cancer 2023, 153, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 SignalingHFD-induced inflammation and prostate cancer growth. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, inflammation, and prostate cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, G.K.; Praharaj, P.P.; Kittaka, H.; Mridha, A.R.; Black, O.M.; Singh, R.; Mercer, R.; van Bokhoven, A.; Torkko, K.C.; Agarwal, C.; et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med. 2019, 8, 1110–1123. [Google Scholar] [CrossRef]
- Grozescu, T.; Popa, F. Prostate cancer between prognosis and adequate/proper therapy. J. Med. Life 2017, 10, 5. [Google Scholar]
- Heidegger, I. PSA screening—A matter of debate? Memo-Mag. Eur. Med. Oncol. 2019, 12, 244–248. [Google Scholar] [CrossRef]
- Gandaglia, G.; Albers, P.; Abrahamsson, P.A.; Briganti, A.; Catto, J.W.F.; Chapple, C.R.; Montorsi, F.; Mottet, N.; Roobol, M.J.; Sønksen, J.; et al. Structured population-based prostate-specific antigen screening for prostate cancer: The European Association of Urology position in 2019. Eur. Urol. 2019, 76, 142–150. [Google Scholar] [CrossRef]
- Wade, C.A.; Kyprianou, N. Profiling prostate cancer therapeutic resistance. Int. J. Mol. Sci. 2018, 19, 904. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European Association of Urology position and recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef]
- Carberry, C.; McCombe, G.; Tobin, H.; Stokes, D.; Last, J.; Bury, G.; Cullen, W. Curriculum initiatives to enhance research skills acquisition by medical students: A scoping review. BMC Med. Educ. 2021, 21, 312. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Al Shareef, Z.; Al-Shahrabi, R.; Saheb Sharif-Askari, F.; Alshamsi, Y.; Zarooni, A.A.; AlKhayyal, N.; Soliman, S.S.; Bendardaf, R.; Halwani, R. Risks of prostate cancer and mortality in the city of Sharjah, United Arab Emirates. Front. Oncol. 2023, 13, 1180902. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Steginga, S.K.; Aitken, J.F.; Pinnock, C.B. Communicating prostate cancer risk: What should we be telling our patients? Med. J. Aust. 2005, 182, 472–475. [Google Scholar] [CrossRef]
- Beaulac, J.A.; Fry, R.N.; Onysko, J. Lifetime and recent prostate specific antigen (PSA) Screening of men for prostate cancer in Canada. Can. J. Public Health 2006, 97, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on prostate cancer epidemiology and risk factors: A systematic review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Brawley, O.W.; Ankerst, D.P.; Thompson, I.M. Screening for prostate cancer. CA Cancer J. Clin. 2009, 59, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, D.A. Prostate cancer in older men. Urol. Nurs. 2004, 24, 258–269. [Google Scholar]
- Castillejos-Molina, R.A.; Gabilondo-Navarro, F.B. Prostate cancer. Salud Publica Mex. 2016, 58, 279–284. [Google Scholar] [CrossRef]
- Chang, H.J.; Pong, Y.H.; Chiang, C.Y.; Huang, P.C.; Wang, M.H.; Chan, Y.J.; Lan, T.Y. A matched case-control study in Taiwan to evaluate potential risk factors for prostate cancer. Sci. Rep. 2023, 13, 4382. [Google Scholar] [CrossRef]
- Chen, R.; Ren, S.; Yiu, M.K.; Fai, N.C.; Cheng, W.S.; Ian, L.H.; Naito, S.; Matsuda, T.; Kehinde, E.; Kural, A.; et al. Prostate cancer in Asia: A collaborative report. Asian J. Urol. 2014, 1, 15–29. [Google Scholar] [CrossRef]
- Childre, F.; Vetrosky, D.; White, G.L., Jr. Prostate cancer: Clinical perspectives. AAOHN J. 1998, 46, 434–442. [Google Scholar] [CrossRef]
- Chung, B.H.; Horie, S.; Chiong, E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E. Diet, nutrition, and prostate cancer. Annu. Rev. Nutr. 1998, 18, 413. [Google Scholar] [CrossRef]
- De Silva, F.; Alcorn, J. A tale of two cancers: A current concise overview of breast and prostate cancer. Cancers 2022, 14, 2954. [Google Scholar] [CrossRef] [PubMed]
- Drudge-Coates, L.; Turner, B. Prostate cancer overview. Part 1: Non-metastatic disease. Br. J. Nurs. 2012, 21 (Suppl. S9), S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.W.; Kazer, M.W. Prostate cancer overview. Semin. Oncol. Nurs. 2011, 27, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Firzara, A.M.; Ng, C.J. Knowledge and practice of prostate cancer screening among general practitioners in Malaysia: A cross-sectional study. BMJ Open 2016, 6, e011467. [Google Scholar] [CrossRef]
- Gaines, K.K. Controversies in prostate cancer screening and treatment. Urol. Nurs. 2010, 30, 165–166. [Google Scholar] [CrossRef]
- Gontijo Gomes, C.R.; Resende Izidoro, L.C.; Ferreira da Mata, L.R. Risk factors for prostate cancer, and motivational and hindering aspects in conducting preventive practices. Investig. Educ. Enferm. 2015, 33, 415–423. [Google Scholar] [CrossRef]
- Gray, M. A prostate cancer primer. Urol. Nurs. 2002, 22, 151–167. [Google Scholar]
- Harvard Health Publication. Prostate cancer: What’s your risk? Harv. Men’s Health Watch 2015, 19, 6–7. [Google Scholar]
- Hoffman, S.S.; Smith, A.W.; Kent, E.E.; Doria-Rose, V.P.; Kobrin, S.C.; Mollica, M.A. Examination of prostate-specific antigen (PSA) screening in military and civilian men: Analysis of the 2018 behavioral risk factor surveillance system. Cancer Causes Control 2022, 33, 393–402. [Google Scholar] [CrossRef]
- Johnson, H. Prostate cancer: Can I have a PSA test please nurse? Pract. Nurs. 2016, 27, 22–26. [Google Scholar] [CrossRef]
- Maladze, N.; Maphula, A.; Maluleke, M.; Makhado, L. Knowledge and Attitudes towards Prostate Cancer and Screening among Males in Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 5220. [Google Scholar] [CrossRef]
- Morrison, B.F.; Aiken, W.D.; Mayhew, R.; Gordon, Y.; Odedina, F.T. Prostate cancer knowledge, prevention, and screening behaviours in Jamaican men. J. Canc. Educ. 2017, 32, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The epidemiology of prostate cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, M.; Müller, A.; Carlsson, S.; Eberli, D.; Huber, A.; Grobholz, R.; Manka, L.; Mortezavi, A.; Sulser, T.; Recker, F.; et al. A positive family history as risk factor for prostate cancer in a population-based study with organized PSA-screening: Results of the Swiss ERSPC (Aarau). BJU Int. 2016, 117, 576. [Google Scholar] [CrossRef]
- Sasagawa, I.; Nakada, T. Epidemiology of prostatic cancer in East Asia. Arch. Androl. 2001, 47, 195–201. [Google Scholar] [CrossRef]
- Schreiber-Agus, N. Prostate cancer, basic characteristics and experimental models. In Encyclopedic Reference of Cancer; Springer: Berlin/Heidelberg, Germany, 2001; pp. 716–720. [Google Scholar]
- Terris, M.K.; Ruff, P.A.; Marotte, J.B.; Ruff, P.A.; Marotte, J.B.; Terris, M.K. Primary care providers’ attitudes toward prostate cancer risk factors at a Veterans Affairs health care facility. Mil. Med. 2005, 170, 154–157. [Google Scholar]
- Turner, B.; Drudge-Coates, L. Prostate cancer: Risk factors, diagnosis and management. Cancer Nurs. Pract. 2010, 9, 29–36. [Google Scholar] [CrossRef]
- Vane, S. Prostate Cancer Screening: A Review of Current Recommendations. Urol. Nurs. 2019, 39, 133–138. [Google Scholar] [CrossRef]
- Watson, E.; Austoker, J. Testing for prostate cancer. Pract. Nurs. 2008, 35, 14–19. [Google Scholar]
- Aiken, W.D.; Eldemire-Shearer, D. Prostate cancer in Jamaica and the wider Caribbean: It is time to consider screening. West Indian Med. J. 2012, 61, 90. [Google Scholar]
- Åkerstedt, T.; Narusyte, J.; Svedberg, P.; Kecklund, G.; Alexanderson, K. Night work and prostate cancer in men: A Swedish prospective cohort study. BMJ Open 2017, 7, e015751. [Google Scholar] [CrossRef]
- Kabore, F.A.; Zango, B.; Sanou, A.; Yameogo, C.; Kirakoya, B. Prostate cancer outcome in Burkina Faso. Infect. Agents Cancer 2011, 6, S6. [Google Scholar] [CrossRef] [PubMed]
- Syrigos, K.N.; Karapanagiotou, E.; Harrington, K.J. Prostate cancer in the elderly. Anticancer Res. 2005, 25, 4527–4533. [Google Scholar] [PubMed]
- Alkhatib, K.; Labban, M.; Briggs, L.; Nguyen, D.D.; Herzog, P.; Cole, A.P.; Haag, A.; Trinh, Q.D. Does veteran status mitigate racial disparities in prostate cancer screening: Analysis of prostate specific antigen screening patterns in the 2018 Behavioral Risk Factor Surveillance System data. J. Urol. 2022, 207, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, K.; Proverbs-Singh, T.; Katner, A.; Lifsey, D.; Pollard, S.; Rayford, W. Knowledge, attitudes and beliefs of women about the importance of prostate cancer screening. J. Natl. Med. Assoc. 2005, 97, 1378. [Google Scholar]
- Held-Warmkessel, J. What your patient needs to know about prostate cancer. Nursing 2020, 32, 36–43. [Google Scholar] [CrossRef]
- Trudeau, K.; Rousseau, M.C.; Barul, C.; Csizmadi, I.; Parent, M.É. Dietary patterns are associated with risk of prostate cancer in a population-based case-control study in Montreal, Canada. Nutrients 2020, 12, 1907. [Google Scholar] [CrossRef]
- Adler, C.; Friesen, M.C.; Yeboah, E.D.; Tettey, Y.; Biritwum, R.B.; Adjei, A.A.; Tay, E.; Okyne, V.; Mensah, J.E.; Truelove, A.; et al. Usual adult occupation and risk of prostate cancer in West African men: The Ghana Prostate Study. J. Occup. Environ. Med. 2019, 76, 71–77. [Google Scholar] [CrossRef]
- Jochems, S.H.; Fritz, J.; Häggström, C.; Järvholm, B.; Stattin, P.; Stocks, T. Smoking and risk of prostate cancer and prostate cancer death: A pooled study. Eur. Urol. 2023, 83, 422–431. [Google Scholar] [CrossRef]
- Ito, K. Prostate cancer in Asian men. Nat. Rev. Urol. 2014, 11, 197–212. [Google Scholar] [CrossRef]
- Consumer Protection. Best test and care for prostate cancer. Consum. Rep. Health 2001, 12, 1. [Google Scholar]
- da Rocha Araujo, F.A.; Barroso, U.D., Jr. Prostate cancer screening: Beliefs and practices of the Brazilian physicians with different specialties. J. Eval. Clin. Pract. 2018, 24, 508–513. [Google Scholar] [CrossRef]
- Linn, M.M.; Ball, R.A.; Maradiegue, A. Prostate-specific antigen screening: Friend or foe. Urol. Nurs. 2007, 27, 481–489. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02107818/full (accessed on 28 August 2023). [PubMed]
- Kim, Y.; Alhassan, M. Analyzing Factors Enabling Prostate Cancer Screening Behaviors Among African American Males in the South Region Using the Andersen’s Behavioral Model of Healthcare Services Utilization. J. Prev. 2023, 44, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Riviere, P.; Kalavacherla, S.; Banegas, M.P.; Javier-Desloges, J.; Martinez, M.E.; Garraway, I.P.; Murphy, J.D.; Rose, B.S. Patient perspectives of prostate cancer screening vary by race following 2018 guideline changes. Cancer 2023, 129, 82–88. [Google Scholar] [CrossRef]
- Akinremi, T.O.; Adeniyi, A.; Olutunde, A.; Oduniyi, A.; Ogo, C.N. Need for and relevance of prostate cancer screening in Nigeria. Ecancer 2014, 8, 457. [Google Scholar] [CrossRef]
- Kagotho, N.; Tan, J. Predictors of prostate cancer screening among older immigrant men. J. Stud. Natl. Med. Assoc. 2008, 100, 1168–1174. [Google Scholar] [CrossRef]
- Ogunsanya, M.E.; Brown, C.M.; Odedina, F.T.; Barner, J.C.; Adedipe, T.B.; Corbell, B. Knowledge of prostate cancer and screening among young multiethnic black men. Am. J. Men’s Health 2017, 11, 1008–1018. [Google Scholar] [CrossRef]
- Austin, O.J. Prostate-specific antigen prostate cancer screening: Answers to the critical questions. Ann. Long-Term Care 2012, 20, 16–21. [Google Scholar]
- Bowen, D.J.; Hannon, P.A.; Harris, J.R.; Martin, D.P. Prostate cancer screening and informed decision-making: Provider and patient perspectives. Prostate Cancer Prostatic Dis. 2011, 14, 155–161. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Vickers, A.J. Screening for prostate cancer. Med. Care. 2020, 104, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B. Assessing risk: Does this patient have prostate cancer? J. Natl. Cancer Inst. 2006, 98, 506–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caso, J.R.; Mouraviev, V.; Tsivian, M.; Polascik, T.J.; Moul, J.W. Prostate cancer: An evolving paradigm. J. Endourol. 2010, 24, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Crittendon, D.R.; LaNoue, M.; George, B. Does perceived racism affect prostate cancer screening rates and patient-provider shared discussions among black and white men. J. Health Care Poor Underserved 2022, 33, 5–19. [Google Scholar] [CrossRef]
- de Bekker-Grob, E.W.; Rose, J.M.; Donkers, B.; Essink-Bot, M.L.; Bangma, C.H.; Steyerberg, E.W. Men’s preferences for prostate cancer screening: A discrete choice experiment. Br. J. Cancer. 2013, 108, 533–541. [Google Scholar] [CrossRef]
- Kabore, F.A.; Kambou, T.; Zango, B.; Ouédraogo, A. Knowledge and awareness of prostate cancer among the general public in Burkina Faso. J. Cancer Educ. 2014, 29, 69–73. [Google Scholar] [CrossRef]
- Kalavacherla, S.; Riviere, P.; Javier-DesLoges, J.; Banegas, M.P.; McKay, R.R.; Murphy, J.D.; Rose, B.S. Low-Value Prostate-Specific Antigen Screening in Older Males. JAMA Netw. Open 2023, 6, e237504. [Google Scholar] [CrossRef]
- Maclean. Probing the prostate: What everyone should know about the most commonly diagnosed cancer in Canadian men. Maclean’s 2020, 133, 100. [Google Scholar]
- Taneja, S.S. Imaging in the diagnosis and management of prostate cancer. Rev. Urol. 2004, 6, 101–113. [Google Scholar]
- Kaninjing, E.; Lopez, I.; Nguyen, J.; Odedina, F.; Young, M.E. Prostate cancer screening perception, beliefs, and practices among men in Bamenda, Cameroon. Am. J. Mens Health 2018, 12, 1463–1472. [Google Scholar] [CrossRef]
- Kilpeläinen, T.P.; Talala, K.; Raitanen, J.; Taari, K.; Kujala, P.; Tammela, T.L.; Auvinen, A. Prostate cancer and socioeconomic status in the finnish randomized study of screening for prostate cancer. Am. J. Epidemiol. 2016, 184, 720–731. [Google Scholar] [CrossRef]
- Koitsalu, M.; Eklund, M.; Adolfsson, J.; Sprangers, M.A.; Grönberg, H.; Brandberg, Y. Predictors of participation in risk-based prostate cancer screening. PLoS ONE 2018, 13, e0200409. [Google Scholar] [CrossRef] [PubMed]
- Nijs, H.G.; Essink-Bot, M.L.; DeKoning, H.J.; Kirkels, W.J.; Schröder, F.H. Why do men refuse or attend population-based screening for prostate cancer. J Public Health 2000, 22, 312–316. [Google Scholar] [CrossRef]
- Mons, U.; Gredner, T.; Behrens, G.; Stock, C.; Brenner, H. Cancers Due to Smoking and High Alcohol Consumption. Dtsch. Arztebl. Int. 2018, 115, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, M.S.; Lynch, J.; Karunamuni, R.; Alba, P.R.; Lee, K.M.; Agiri, F.Y.; Anglin, T.; Carter, H.; Gaziano, J.M.; Jasuja, G.K.; et al. Polygenic risk of any, metastatic, and fatal prostate cancer in the Million Veteran Program. Natl. Cancer Inst. 2022, 115, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.; Giovannucci, E.; Parmigiani, G.; Mucci, L.A. Family history of breast or prostate cancer and prostate cancer risk genetic link between prostate cancer and breast cancer. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary prostate cancer: Genes related, target therapy and prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef]
- Clouston, S.A.; Kuan, P.; Kotov, R.; Mukherjee, S.; Thompson-Carino, P.; Bromet, E.J.; Luft, B.J. Risk factors for incident prostate cancer in a cohort of world trade center responders. BMC Psychiatry 2019, 19, 389. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Pettersson, A.; Robinson, D.; Garmo, H.; Holmberg, L.; Stattin, P. Age at diagnosis and prostate cancer treatment and prognosis: A population-based cohort study. Ann. Oncol. 2018, 29, 377–385. [Google Scholar] [CrossRef]
- Macke, A.J.; Petrosyan, A. Alcohol and prostate cancer: Time to draw conclusions. Biomolecules 2022, 12, 375. [Google Scholar] [CrossRef]
- Dovey, Z.S.; Nair, S.S.; Chakravarty, D.; Tewari, A.K. Racial disparity in prostate cancer in the African American population with actionable ideas and novel immunotherapies. Cancer Rep. 2021, 4, e1340. [Google Scholar] [CrossRef]
- Rayford, W.; Beksac, A.T.; Alger, J.; Alshalalfa, M.; Ahmed, M.; Khan, I.; Falagario, U.G.; Liu, Y.; Davicioni, E.; Spratt, D.E.; et al. Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences. Commun. Biol. 2021, 4, 670. [Google Scholar] [CrossRef] [PubMed]
- Zeigler-Johnson, C.; McDonald, A.C.; Pinheiro, P.; Lynch, S.; Taioli, E.; Joshi, S.; Alpert, N.; Baudin, J.; Joachim, C.; Deloumeaux, J.; et al. Trends in prostate cancer incidence among Black men in the Caribbean and the United States. Prostate 2023, 83, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, Y.S.; Ohanian, A.; Huang, F.W. Prostate cancer in the Caribbean: A baseline assessment of current practices and potential needs. Cancer Control 2022, 29, 10732748221082372. [Google Scholar] [CrossRef]
- Chowdhury-Paulino, I.M.; Ericsson, C.; Vince, R., Jr.; Spratt, D.E.; George, D.J.; Mucci, L.A. Racial disparities in prostate cancer among black men: Epidemiology and outcomes. Prostate Cancer Prostatic Dis. 2022, 25, 397–402. [Google Scholar] [CrossRef]
- Hinata, N.; Fujisawa, M. Racial differences in prostate cancer characteristics and cancer-specific mortality: An overview. World J. Men’s Health 2022, 40, 217. [Google Scholar] [CrossRef]
- Zhu, Y.; Mo, M.; Wei, Y.; Wu, J.; Pan, J.; Freedland, S.J.; Zheng, Y.; Ye, D. Epidemiology and genomics of prostate cancer in Asian men. Nat. Rev. Urol. 2021, 18, 282–301. [Google Scholar] [CrossRef]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen receptor signaling pathway in prostate cancer: From genetics to clinical applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 2018, 173, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Tests to Diagnose and Stage Prostate Cancer. 2022. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/how-diagnosed.html (accessed on 18 August 2022).
- Montenegro, J.; Freitas-Silva, O.; Teodoro, A.J. Molecular Mechanisms of Coffee on Prostate Cancer Prevention. BioMed Res. Int. 2022, 2022, 3254420. [Google Scholar] [CrossRef]
- Barul, C.; Parent, M.E. Occupational exposure to polycyclic aromatic hydrocarbons and risk of prostate cancer. Environ. Health 2021, 20, 71. [Google Scholar] [CrossRef]
- Troeschel, A.N.; Hartman, T.J.; Jacobs, E.J.; Stevens, V.L.; Gansler, T.; Flanders, W.D.; McCullough, L.E.; Wang, Y. Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. J. Clin. Oncol. 2020, 38, 2018. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early detection of prostate cancer: AUA/SUO guideline part I: Prostate cancer screening. J. Urol. 2023, 210, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Srinivasan, S.; Clements, J.; Batra, J. Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019, 38, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.C. A discussion on controversies and ethical dilemmas in prostate cancer screening. J. Med. Ethics 2021, 47, 152–158. [Google Scholar] [CrossRef]
- Hamdy, F.C. Prostate-Specific Antigen testing for prostate cancer screening—Is the message getting through? JAMA Oncol. 2022, 8, 47–49. [Google Scholar] [CrossRef]
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: Past, present and future. Nat. Rev. Urol. 2022, 19, 562–572. [Google Scholar] [CrossRef]
- Nielsen, S.B.; Spalletta, O.; Toft Kristensen, M.A.; Brodersen, J. Psychosocial consequences of potential overdiagnosis in prostate cancer a qualitative interview study. Scand. J. Prim. Health Care 2020, 38, 439–446. [Google Scholar] [CrossRef]
- Soronen, V.; Talala, K.; Raitanen, J.; Taari, K.; Tammela, T.; Auvinen, A. Digital rectal examination in prostate cancer screening at PSA level 3.0–3.9 ng/mL: Long-term results from a randomized trial. Scand. J. Urol. 2021, 55, 348–353. [Google Scholar] [CrossRef]
- Pepe, P.; Garufi, A.; Priolo, G.D.; Galia, A.; Fraggetta, F.; Pennisi, M. Is it time to perform only MRI targeted biopsy? Our experience in 1032 men submitted to prostate biopsy. J. Urol. 2018, 200, 774–778. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, G.; Pepe, L.; Garufi, A.; Priolo, G.D.; Pennisi, M. Cost-effectiveness of multiparametric MRI in 800 men submitted to repeat prostate biopsy: Results of a public health model. Anticancer. Res. 2018, 38, 2395–2398. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.; Hayne, D. Transrectal ultrasound biopsy of the prostate: Does it still have a role in prostate cancer diagnosis. Transl. Androl. Urol. 2020, 9, 3018–3024. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Carroll, P.R.; Eggener, S.; Fulgham, P.F.; Margolis, D.J.; Pinto, P.A.; Rosenkrantz, A.B.; Rubenstein, J.N.; Rukstalis, D.B.; Taneja, S.S.; et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J. Uurol. 2020, 203, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhu, D.; Byanju, S.; Chen, J.; Zhang, H.; Wang, Y.; Liao, M. Magnetic resonance spectroscopy imaging in diagnosis of suspicious prostate cancer: A meta-analysis. Medicine 2019, 98, e14891. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Ahmad, F.; Beaton, D.; Bierman, A.S. Cancer screening behaviours among S outh A sian immigrants in the UK, US and Canada: A scoping study. Health Soc. Care Community 2016, 24, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Mbugua, R.G.; Oluchina, S.; Karanja, S. Prostate cancer awareness and screening among men in a rural community in Kenya: A cross-sectional study. Afr. J. Urol. 2021, 27, 7. [Google Scholar] [CrossRef]
- Aragona, F.; Pepe, P.; Motta, M.; Saita, A.; Raciti, G.; La Rosa, P.; Nicolosi, D.; Dammino, A.; Minaldi, G.; Rizza, G.; et al. Incidence of prostate cancer in Sicily: Results of a multicenter case-findings protocol. Eur. Urol. 2005, 47, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wachira, B.W.; Meng’anyi, L.W.; Mbugua, G.R. Knowledge, perception and uptake of prostate cancer screening: A cross sectional study at a level III hospital in Kenya. Public Health Res. 2018, 8, 81–87. [Google Scholar] [CrossRef]
- Cincidda, C.; Pizzoli, S.F.M.; Ongaro, G.; Oliveri, S.; Pravettoni, G. Caregiving and Shared Decision Making in Breast and Prostate Cancer Patients: A Systematic Review. Curr. Oncol. 2023, 30, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Golijanin, B.; Bhatt, V.; Homer, A.; Pareek, G.; Hyams, E. Shared Decision-Making for Prostate Cancer Screening: Is It a Marker of Quality Preventative Healthcare. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4489526 (accessed on 28 August 2023).
- Woods-Burnham, L.; Stiel, L.; Wilson, C.; Montgomery, S.; Durán, A.M.; Ruckle, H.R.; Thompson, R.A.; De León, M.; Casiano, C.A. Physician consultations, prostate cancer knowledge, and PSA screening of African American men in the era of shared decision-making. Am. J. Men’s Health 2018, 12, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Salmon, C.; Parent, M.É.; Quesnel-Vallée, A.; Barnett, T.A. A scoping review of social relationships and prostate cancer screening. Prev. Med. 2022, 154, 106892. [Google Scholar] [CrossRef] [PubMed]
- Ceres, M.; Quinn, G.P.; Loscalzo, M.; Rice, D. Cancer screening considerations and cancer screening uptake for lesbian, gay, bisexual, and transgender persons. Semin. Oncol. Nurs. 2018, 34, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Oladeru, O.T.; Wang, K.; Attwood, K.; Singh, A.K.; Haas-Kogan, D.A.; Neira, P.M. Prostate cancer screening patterns among sexual and gender minority individuals. Eur. Urol. 2021, 79, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, J.; Takizawa, C.; Valentine, W. Literature review of the burden of prostate cancer in Germany, France, the United Kingdom and Canada. BMC Urol. 2019, 19, 19. [Google Scholar] [CrossRef]
- Pollock, A.; Campbell, P.; Struthers, C.; Synnot, A.; Nunn, J.; Hill, S.; Goodare, H.; Morris, J.; Watts, C.; Morley, R. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J. Health Serv. Res. Policy 2019, 24, 245–255. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef] [PubMed]
| P | Population | Males |
| E | Exposure | Prostate cancer |
| O | Outcome | Risk factors and/or screening uptake |
| Search | Search Terms |
|---|---|
| S1 | (MM “Prostate”) OR (MM “Prostatic Neoplasms+”) |
| S2 | (MM “Neoplasms, Second Primary”) |
| S3 | prostate cancer OR prostatic neoplasm * OR prostate carcinoma (Title or Abstract) |
| S4 | (MM “Early Detection of Cancer”) |
| S5 | (MM “Mass Screening+”) |
| S6 | screening OR assessment * OR test * OR diagnosis OR early detection OR detect * (Title or Abstract) |
| S7 | risk factor * OR contributing factor * OR predisposing factor * (Title or Abstract) |
| S8 | S1 OR S2 OR S3 |
| S9 | S4 OR S5 OR S6 |
| S10 | S7 AND S8 AND S9 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Grey literature and peer reviews or primary research articles. | Treatment and management of prostate cancer. |
| Articles addressing risk factors and screening for prostate cancer. | Articles published after 30 June 2023. |
| Articles published before 30 June 2023. | Non-English papers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mumuni, S.; O’Donnell, C.; Doody, O. The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review. Healthcare 2023, 11, 2780. https://doi.org/10.3390/healthcare11202780
Mumuni S, O’Donnell C, Doody O. The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review. Healthcare. 2023; 11(20):2780. https://doi.org/10.3390/healthcare11202780
Chicago/Turabian StyleMumuni, Seidu, Claire O’Donnell, and Owen Doody. 2023. "The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review" Healthcare 11, no. 20: 2780. https://doi.org/10.3390/healthcare11202780
APA StyleMumuni, S., O’Donnell, C., & Doody, O. (2023). The Risk Factors and Screening Uptake for Prostate Cancer: A Scoping Review. Healthcare, 11(20), 2780. https://doi.org/10.3390/healthcare11202780






