Management Strategies in Septic Coagulopathy: A Review of the Current Literature
Abstract
1. Introduction
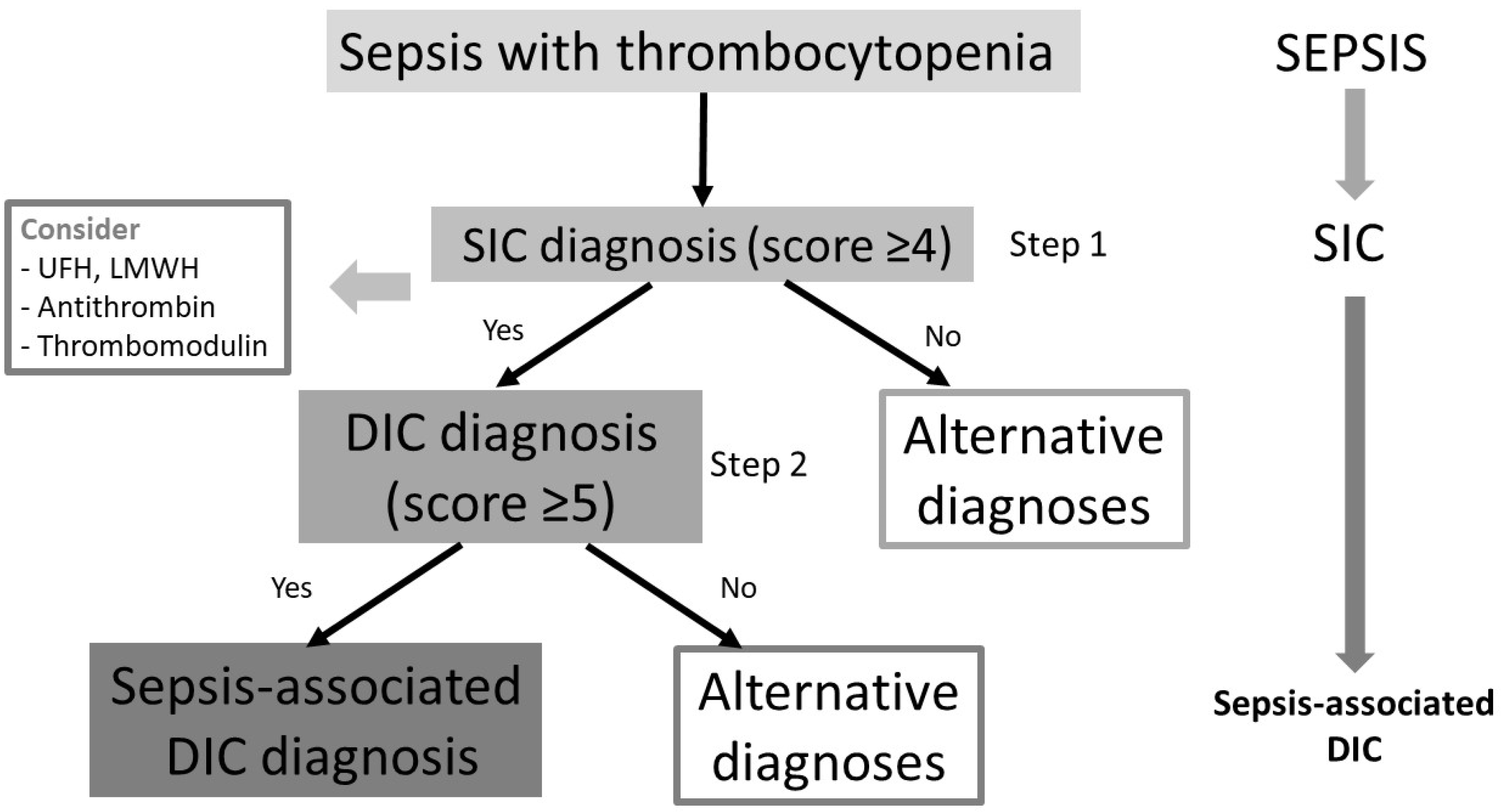
2. Diagnostic Criteria for SIC and Sepsis-Associated DIC
2.1. International Society on Thrombosis and Hemostasis (ISTH)
2.2. Japanese Association for Acute Medicine (JAAM)
2.3. Thromboelastometry and Thromboelastography in SIC and Sepsis-Induced DIC
3. Management Strategies in SIC and Sepsis-Associated DIC
3.1. Causal Treatment of Sepsis
3.2. Unfractionated Heparin (UFH)
3.3. Low-Molecular-Weight Heparin (LMWH)
3.4. Antithrombin (AT)
3.5. Activated Protein C (APC)
3.6. Thrombomodulin (TM)
3.7. Tissue Factor Pathway Inhibitor (TFPI)
3.8. Transfusion of Blood Components
3.9. Therapeutic Plasma Exchange (TPE)
4. Future Directions in Management of SIC and Sepsis-Associated DIC
4.1. Dynamic/Continuous SIC/Sepsis-Associated DIC Prediction Scores
4.2. New Markers for SIC
4.3. Toward Personalized Diagnostics
4.4. Inhibitor of Plasminogen Activator Inhibitor-1 (PAI-1)
5. Authors’ Perspective on Practical Management Approaches in Different Clinical Scenarios
5.1. Patients with SIC
5.2. Patients with Early Sepsis-Associated DIC
5.3. Patients with Late Sepsis-Associated DIC
5.4. Patients with Purpura Fulminans
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Nisio, M.D.; Levy, J.H.; Kitamura, N.; Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open 2017, 7, e017046. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.B.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.C.F.; Manolov, V.; Morgenstern, J.; Fleming, T.; Heitmeier, S.; Uhle, F.; Al-Saeedi, M.; Hackert, T.; Bruckner, T.; Schöchl, H.; et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: Results of an observational pilot study. Ann. Intensive Care 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; Ishikura, H.; et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006, 34, 625–631. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Egi, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Iba, T.; Kakihana, Y.; Kawasaki, T.; Kushimoto, S.; Kuroda, Y.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J. Intensive Care 2021, 9, 53. [Google Scholar] [CrossRef]
- Dhainaut, J.F.; Yan, S.B.; Joyce, D.E.; Pettilä, V.; Basson, B.; Brandt, J.T.; Sundin, D.P.; Levi, M. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J. Thromb. Haemost. 2004, 2, 1924–1933. [Google Scholar] [CrossRef]
- Yamakawa, K.; Yoshimura, J.; Ito, T.; Hayakawa, M.; Hamasaki, T.; Fujimi, S. External Validation of the Two Newly Proposed Criteria for Assessing Coagulopathy in Sepsis. Thromb. Haemost. 2019, 119, 203–212. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [CrossRef]
- Iba, T.; Arakawa, M.; Di Nisio, M.; Gando, S.; Anan, H.; Sato, K.; Ueki, Y.; Levy, J.H.; Thachil, J. Newly Proposed Sepsis-Induced Coagulopathy Precedes International Society on Thrombosis and Haemostasis Overt-Disseminated Intravascular Coagulation and Predicts High Mortality. J. Intensive Care Med. 2020, 35, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, C.; Jourdi, G.; Adjambri, E.; Walborn, A.; Patel, P.; Fareed, J.; Elalamy, I.; Hoppensteadt, D.; Gerotziafas, G.T. Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clin. Appl. Thromb. Hemost. 2018, 24 (Suppl. 9), 8S–28S. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wang, Z.; Lin, Y.; Liu, B.; Zhang, Z.; Ma, X. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: A retrospective study. Blood Coagul. Fibrinolysis 2018, 29, 551–558. [Google Scholar] [CrossRef]
- Tanaka, C.; Tagami, T.; Kudo, S.; Takehara, A.; Fukuda, R.; Nakayama, F.; Kaneko, J.; Ishiki, Y.; Sato, S.; Kuno, M.; et al. Validation of sepsis-induced coagulopathy score in critically ill patients with septic shock: Post hoc analysis of a nationwide multicenter observational study in Japan. Int. J. Hematol. 2021, 114, 164–171. [Google Scholar] [CrossRef]
- Iba, T.; Arakawa, M.; Levy, J.H.; Yamakawa, K.; Koami, H.; Hifumi, T.; Sato, K. Sepsis-Induced Coagulopathy and Japanese Association for Acute Medicine DIC in Coagulopathic Patients with Decreased Antithrombin and Treated by Antithrombin. Clin. Appl. Thromb. Hemost. 2018, 24, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.C.; Meijers, J.C.; Vroom, M.B.; Juffermans, N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit. Care 2014, 18, R30. [Google Scholar] [CrossRef]
- Boscolo, A.; Spiezia, L.; De Cassai, A.; Pasin, L.; Pesenti, E.; Zatta, M.; Zampirollo, S.; Andreatta, G.; Sella, N.; Pettenuzzo, T.; et al. Are thromboelastometric and thromboelastographic parameters associated with mortality in septic patients? A systematic review and meta-analysis. J. Crit. Care 2021, 61, 5–13. [Google Scholar] [CrossRef]
- Luo, C.; Hu, H.; Gong, J.; Zhou, Y.; Chen, Z.; Cai, S. The Value of Thromboelastography in the Diagnosis of Sepsis-Induced Coagulopathy. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620951847. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.L.; Zheng, Z.; Liu, Y.N.; Ma, X.C. Unfractionated heparin ameliorates lipopolysaccharide-induced lung inflammation by downregulating nuclear factor-κB signaling pathway. Inflammation 2013, 36, 1201–1208. [Google Scholar] [CrossRef]
- Ding, R.Y.; Zhao, D.M.; Guo, R.X.; Zhang, Z.D.; Ma, X.C. Treatment with unfractionated heparin attenuates coagulation and inflammation in endotoxemic mice. Thromb. Res. 2011, 128, e160–e165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.N.; Wang, L.; Li, Z.L.; Ma, X.C. Unfractionated heparin attenuates LPS-induced IL-8 secretion via PI3K/Akt/NF-κB signaling pathway in human endothelial cells. Immunobiology 2015, 220, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Alhamdi, Y.; Abrams, S.T.; Lane, S.; Wang, G.; Toh, C.H. Histone-associated thrombocytopenia in patients who are critically Ill. JAMA 2016, 315, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Zhang, H.; An, X.; Ma, X.C. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015, 59, 160–169. [Google Scholar]
- Huang, X.; Han, S.; Liu, X.; Wang, T.; Xu, H.; Xia, B.; Kong, G.; Li, J.; Zhu, W.; Hu, H.; et al. Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPS-induced sepsis. Exp. Ther. Med. 2020, 19, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wang, X.Z.; Liu, X.X.; Hao, D.; Jaladat, Y.; Lu, F.; Sun, T.; Lv, C.J. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Exp. Ther. Med. 2014, 7, 604–608. [Google Scholar] [CrossRef]
- Peng, J.C.; Nie, F.; Li, Y.J.; Xu, Q.Y.; Xing, S.P.; Li, W.; Gao, Y. Favorable Outcomes of Anticoagulation With Unfractioned Heparin in Sepsis-Induced Coagulopathy: A Retrospective Analysis of MIMIC-III Database. Front. Med. (Lausanne) 2022, 3, 773339. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Huang, J.J.; Luan, Y.Y.; Yang, Z.J.; Zhou, Z.P.; Zhang, J.J.; Yao, Y.M.; Wu, M. Early prophylactic anticoagulation with heparin alleviates mortality in critically ill patients with sepsis: A retrospective analysis from the MIMIC-IV database. Burns Trauma 2022, 10, tkac029. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Shimazaki, R.; Antithrombin Gamma Study Group. An open-label, randomized, phase 3 study of the efficacy and safety of antithrombin gamma in patients with sepsis-induced disseminated intravascular coagulation syndrome. J. Intensive Care 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Nishida, O.; Ogura, H.; Egi, M.; Fujishima, S.; Hayashi, Y.; Iba, T.; Imaizumi, H.; Inoue, S.; Kakihana, Y.; Kotani, J.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 (J-SSCG 2016). Acute Med. Surg. 2018, 5, 3–89. [Google Scholar] [CrossRef] [PubMed]
- Kienast, J.; Juers, M.; Wiedermann, C.J.; Hoffmann, J.N.; Ostermann, H.; Strauss, R.; Keinecke, H.O.; Warren, B.L.; Opal, S.M.; KyberSept investigators. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 2006, 4, 90–97. [Google Scholar] [CrossRef]
- Fourrier, F.; Chopin, C.; Huart, J.J.; Runge, I.; Caron, C.; Goudemand, J. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 1993, 104, 882–888. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kohno, Y.; Koishi, K. Effects of antithrombin and gabexate mesilate on disseminated intravascular coagulation: A preliminary study. Am. J. Emerg. Med. 2012, 30, 1219–1223. [Google Scholar] [CrossRef]
- Gando, S.; Saitoh, D.; Ishikura, H.; Ueyama, M.; Otomo, Y.; Oda, S.; Kushimoto, S.; Tanjoh, K.; Mayumi, T.; Ikeda, T.; et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit. Care 2013, 17, R297. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: Meta-analyses revisited. J. Thromb. Haemost. 2018, 16, 455–457. [Google Scholar] [CrossRef]
- Tagami, T. Antithrombin concentrate use in sepsis-associated disseminated intravascular coagulation: Re-evaluation of a ‘pendulum effect’ drug using a nationwide database. J. Thromb. Haemost. 2018, 16, 458–461. [Google Scholar] [CrossRef]
- Hayakawa, M.; Kudo, D.; Saito, S.; Uchino, S.; Yamakawa, K.; Iizuka, Y.; Sanui, M.; Takimoto, K.; Mayumi, T.; Ono, K.; et al. Antithrombin Supplementation and Mortality in Sepsis-Induced Disseminated Intravascular Coagulation: A Multicenter Retrospective Observational Study. Shock 2016, 46, 623–631. [Google Scholar] [CrossRef]
- Yatabe, T.; Inoue, S.; Sakamoto, S.; Sumi, Y.; Nishida, O.; Hayashida, K.; Hara, Y.; Fukuda, T.; Matsushima, A.; Matsuda, A.; et al. The anticoagulant treatment for sepsis induced disseminated intravascular coagulation; network meta-analysis. Thromb. Res. 2018, 171, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takeba, J.; Matsumoto, H.; Ohshita, M.; Annen, S.; Moriyama, N.; Nakabayashi, Y.; Aibiki, M. Anticoagulation Therapy Using rh-Thrombomodulin and/or Antithrombin III Agent is Associated With Reduction in in-Hospital Mortality in Septic Disseminated Intravascular Coagulation: A Nationwide Registry Study. Shock 2019, 51, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Anticoagulant therapy for septic coagulopathy and disseminated intravascular coagulation: Where do KyberSept and SCARLET leave us? Acute Med. Surg. 2020, 7, e477. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Yamakawa, K.; Kudo, D.; Ono, K. Optimal Antithrombin Activity Threshold for Initiating Antithrombin Supplementation in Patients With Sepsis-Induced Disseminated Intravascular Coagulation: A Multicenter Retrospective Observational Study. Clin. Appl. Thromb. Hemost. 2018, 24, 874–883. [Google Scholar] [CrossRef]
- Akahoshi, T.; Kaku, N.; Shono, Y.; Yamamoto, Y.; Takahashi, K.; Iyonaga, T.; Momii, K.; Nishihara, M.; Maki, J.; Tokuda, K.; et al. Impact of Antithrombin Activity Levels Following Recombinant Antithrombin Gamma Therapy in Patients with Sepsis-Induced Disseminated Intravascular Coagulation. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221135790. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2012, 3, CD004388. [Google Scholar] [CrossRef]
- Opal, S.M.; Dellinger, R.P.; Vincent, J.L.; Masur, H.; Angus, D.C. The next generation of sepsis clinical trial designs: What is next after the demise of recombinant human activated protein C? Crit. Care Med. 2014, 42, 1714–1721. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 89–95. [Google Scholar] [CrossRef]
- François, B.; Fiancette, M.; Helms, J.; Mercier, E.; Lascarrou, J.B.; Kayanoki, T.; Tanaka, K.; Fineberg, D.; Vincent, J.L.; Wittebole, X. Efficacy and safety of human soluble thrombomodulin (ART-123) for treatment of patients in France with sepsis-associated coagulopathy: Post hoc analysis of SCARLET. Ann. Intensive Care 2021, 11, 53. [Google Scholar] [CrossRef]
- Levi, M.; Vincent, J.L.; Tanaka, K.; Radford, A.H.; Kayanoki, T.; Fineberg, D.A.; Hoppensteadt, D.; Fareed, J. Effect of a Recombinant Human Soluble Thrombomodulin on Baseline Coagulation Biomarker Levels and Mortality Outcome in Patients With Sepsis-Associated Coagulopathy. Crit. Care Med. 2020, 48, 1140–1147. [Google Scholar] [CrossRef]
- Kudo, D.; Goto, T.; Uchimido, R.; Hayakawa, M.; Yamakawa, K.; Abe, T.; Shiraishi, A.; Kushimoto, S. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: An analysis of three multicentre observational studies. Crit. Care 2021, 25, 114. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Yamakawa, K.; Hayakawa, M.; Kudo, D.; Fujimi, S. Concomitant Versus Individual Administration of Antithrombin and Thrombomodulin for Sepsis-Induced Disseminated Intravascular Coagulation: A Nationwide Japanese Registry Study. Clin. Appl. Thromb. Hemost. 2018, 24, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Murao, A.; Kato, T.; Yamane, T.; Honda, G.; Eguchi, Y. Benefit Profile of Thrombomodulin Alfa Combined with Antithrombin Concentrate in Patients with Sepsis-Induced Disseminated Intravascular Coagulation. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221077096. [Google Scholar] [CrossRef] [PubMed]
- Broze, G.J., Jr. Tissue factor pathway inhibitor. Thromb. Haemost. 1995, 74, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Reinhart, K.; Svoboda, P.; Seibert, A.; Olthoff, D.; Dal Nogare, A.; Postier, R.; Hempelmann, G.; Butler, T.; Martin, E.; et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: A multicenter, randomized, placebocontrolled, single-blind, dose escalation study. Crit. Care Med. 2001, 29, 2081–2089. [Google Scholar] [CrossRef]
- Asakura, H.; Ontachi, Y.; Mizutani, T.; Kato, M.; Saito, M.; Morishita, E.; Yamazaki, M.; Suga, Y.; Takami, A.; Miyamoto, K.; et al. Elevated levels of free tissue factor pathway inhibitor antigen in cases of disseminated intravascular coagulation caused by various underlying diseases. Blood Coagul. Fibrinolysis 2001, 12, 1–8. [Google Scholar] [CrossRef]
- Abraham, E.; Reinhart, K.; Opal, S.; Demeyer, I.; Doig, C.; Rodriguez, A.L.; Beale, R.; Svoboda, P.; Laterre, P.F.; Simon, S.; et al. Efficacy and safety of tifacogin (recombinant tissue facto pathway inhibitor) in severe sepsis: A randomized controlled trial. JAMA 2003, 290, 238–247. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef]
- Ito, T.; Kakuuchi, M.; Maruyama, I. Endotheliopathy in septic conditions: Mechanistic insight into intravascular coagulation. Crit. Care 2021, 25, 95. [Google Scholar] [CrossRef]
- Walborn, A.; Rondina, M.; Mosier, M.; Fareed, J.; Hoppensteadt, D. Endothelial Dysfunction Is Associated with Mortality and Severity of Coagulopathy in Patients with Sepsis and Disseminated Intravascular Coagulation. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619852163. [Google Scholar] [CrossRef]
- El-Nawawy, A.A.; Elshinawy, M.I.; Khater, D.M.; Moustafa, A.A.; Hassanein, N.M.; Wali, Y.A.; Nazir, H.F. Outcome of Early Hemostatic Intervention in Children With Sepsis and Nonovert Disseminated Intravascular Coagulation Admitted to PICU: A Randomized Controlled Trial. Pediatr Crit. Care Med. 2021, 22, e168–e177. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Chen, M.; Fang, D.; Liu, D.; Guo, R.; Yang, S. Therapeutic Plasma Exchange Protects Patients with Sepsis-Associated Disseminated Intravascular Coagulation by Improving Endothelial Function. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211053313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; Liu, L.P.; Luo, J.C.; Luo, Y.W.; Wang, H.; Zhang, Y.J.; Gui, R.; Tu, G.W.; Luo, Z.A. Machine-Learning Approach for Dynamic Prediction of Sepsis-Induced Coagulopathy in Critically Ill Patients With Sepsis. Front. Med. (Lausanne) 2021, 7, 637434. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, J.; Ren, H.; Teng, T.; Li, B.; Wang, Y.; Xiang, L. Clinical Efficacy of Soluble Thrombomodulin, Tissue Plasminogen Activator Inhibitor complex, Thrombin-Antithrombin complex,α2-Plasmininhibitor-Plasmin complex in Pediatric Sepsis. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221102929. [Google Scholar] [CrossRef]
- Ishikura, H.; Irie, Y.; Kawamura, M.; Hoshino, K.; Nakamura, Y.; Mizunuma, M.; Maruyama, J.; Nakashio, M.; Suzuki-Inoue, K.; Kitamura, T. Early recognition of sepsis-induced coagulopathy using the C2PAC index: A ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets 2022, 33, 935–944. [Google Scholar] [CrossRef]
- Ma, Y.; Vilanova, D.; Atalar, K.; Delfour, O.; Edgeworth, J.; Ostermann, M.; Hernandez-Fuentes, M.; Razafimahatratra, S.; Michot, B.; Persing, D.H.; et al. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS ONE 2013, 8, e75918. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, C.; Zhang, C.; Yang, H.; Gao, R.; Tong, Z. Plasma Hsa-miR-92a-3p in correlation with lipocalin-2 is associated with sepsis-induced coagulopathy. BMC Infect. Dis. 2020, 20, 155. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Wang, Y.; Proulle, V.; Andreasen, P.A.; Hong, W.; Chen, Z.; Huang, M.; Xu, P. Embelin ameliorated sepsis-induced disseminated intravascular coagulation intensities by simultaneously suppressing inflammation and thrombosis. Biomed. Pharmacother. 2020, 130, 110528. [Google Scholar] [CrossRef]
- Darmstadt, G.L. Acute infectious purpura fulminans: Pathogenesis and medical management. Pediatr. Dermatol. 1998, 15, 169–183. [Google Scholar] [CrossRef]
- Chalmers, E.; Cooper, P.; Forman, K.; Grimley, C.; Khair, K.; Minford, A.; Morgan, M.; Mumford, A.D. Purpura fulminans: Recognition, diagnosis and management. Arch. Dis. Child. 2011, 96, 1066–1071. [Google Scholar] [CrossRef]
- Colling, M.E.; Bendapudi, P.K. Purpura Fulminans: Mechanism and Management of Dysregulated Hemostasis. Transfus. Med. Rev. 2018, 32, 69–76. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 0 Points | 1 Point | 2 Points |
|---|---|---|---|
| PLTs 1 (×109/L) | ≥150 | 100–149 | <100 |
| INR 2 | ≤1.2 | 1.3–1.4 | >1.4 |
| SOFA 3 score (points) | - | 1 | ≥2 |
| Parameter | 0 Points | 1 Point | 2 Points | 3 Points |
|---|---|---|---|---|
| Fibrin-related marker | No increase | - | Moderate increase | Severe increase |
| PLTs 1 (×109/L) | ≥100 | 50–100 | <50 | - |
| Prolonged PT 2 (s) | <3 | 3–5 | ≥6 | - |
| Fibrinogen (g/L) | ≥1 | <1 | - | - |
| Parameter | 0 Points | 1 Point | 2 Points | 3 Points |
|---|---|---|---|---|
| FDPs 1 (µg/mL) | ≤10 | 10–25 | - | ≥25 |
| PLTs 2 (×109/L) | ≥120 | 80–120 or >30%↓/24 h | - | <80 or >50%↓/24 h |
| Fibrinogen (g/L) | >1.5 | 1.0–1.5 | <1.0 | - |
| INR 3 | <1.2 | ≥1.2 | - | - |
| SIRS 4 criteria (number) | 0–2 | ≥3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czempik, P.F.; Wiórek, A. Management Strategies in Septic Coagulopathy: A Review of the Current Literature. Healthcare 2023, 11, 227. https://doi.org/10.3390/healthcare11020227
Czempik PF, Wiórek A. Management Strategies in Septic Coagulopathy: A Review of the Current Literature. Healthcare. 2023; 11(2):227. https://doi.org/10.3390/healthcare11020227
Chicago/Turabian StyleCzempik, Piotr F., and Agnieszka Wiórek. 2023. "Management Strategies in Septic Coagulopathy: A Review of the Current Literature" Healthcare 11, no. 2: 227. https://doi.org/10.3390/healthcare11020227
APA StyleCzempik, P. F., & Wiórek, A. (2023). Management Strategies in Septic Coagulopathy: A Review of the Current Literature. Healthcare, 11(2), 227. https://doi.org/10.3390/healthcare11020227







