Short-Term (4 Day) Effects of Oral Rinsing with Miswak and Green Tea on Gingival Crevicular Fluid Flow and IL-1β Levels: A Pilot Study
Abstract
1. Introduction
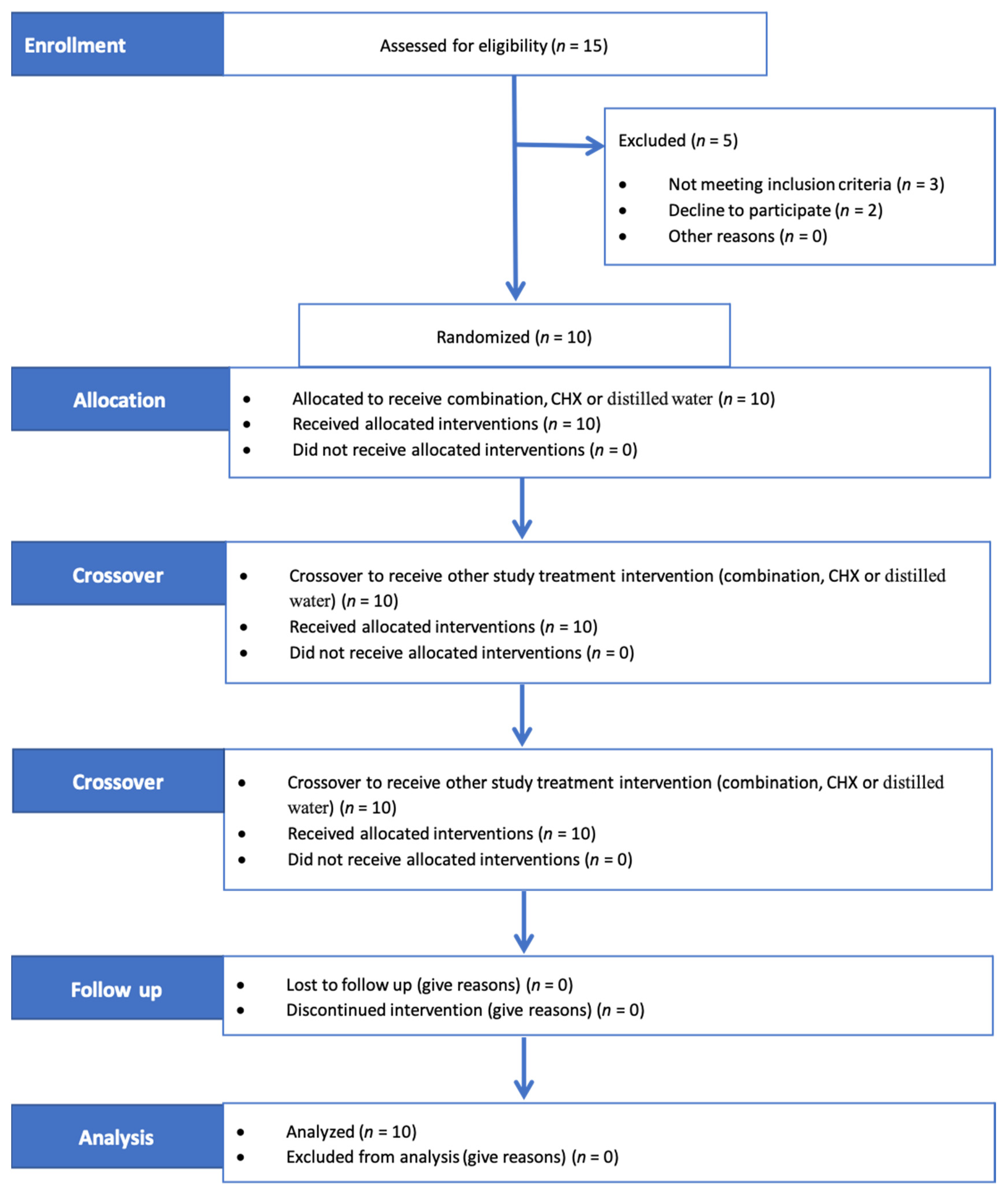
2. Materials and Methods
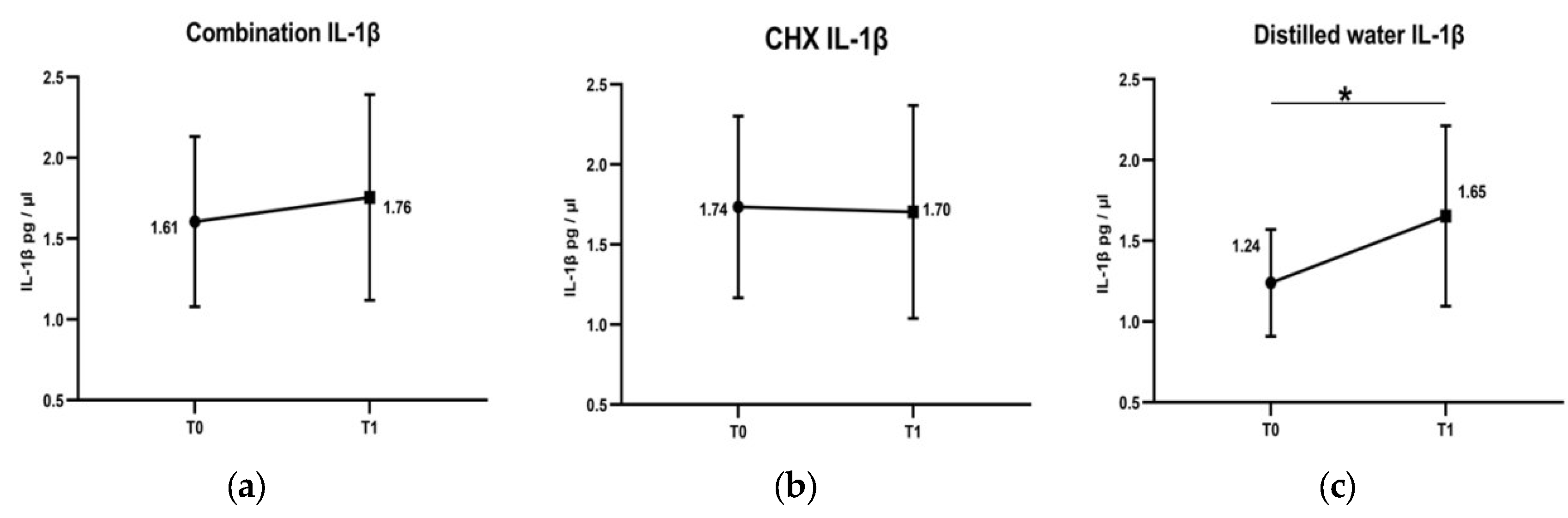
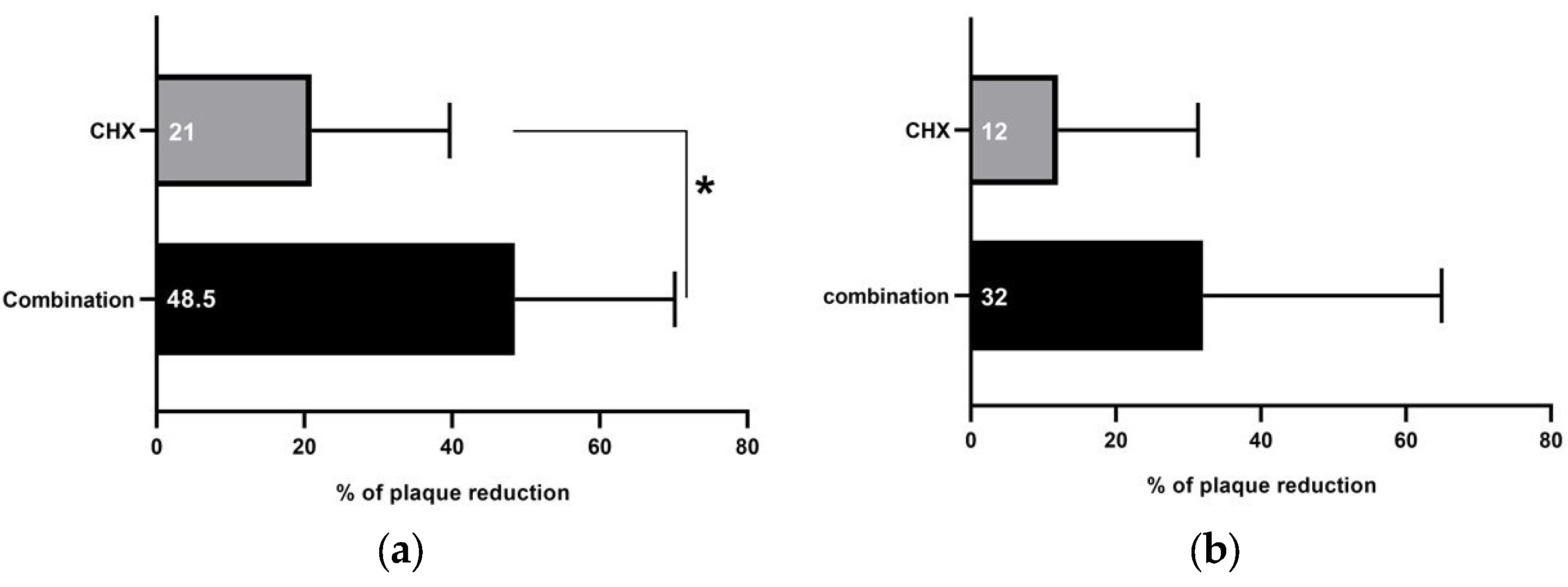
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armitage, G.C. Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontology 2000 2004, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Wassall, R.R.; Preshaw, P.M. Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000 2016, 70, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chhina, S.; Arora, S.A. A systematic review of biomarkers of gingival crevicular fluid: Their predictive role in diagnosis of periodontal disease status. J. Oral Biol. Craniofacial Res. 2018, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Engebretson, S.P.; Grbic, J.T.; Singer, R.; Lamster, I.B. GCF IL-1β profiles in periodontal disease. J. Clin. Periodontol. 2002, 29, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Trombelli, L.; Scapoli, C.; Carrieri, A.; Giovannini, G.; Calura, G.; Farina, R. Interleukin-1β levels in gingival crevicular fluid and serum under naturally occurring and experimentally induced gingivitis. J. Clin. Periodontol. 2010, 37, 697–704. [Google Scholar] [CrossRef]
- Takashiba, S.; Naruishi, K.; Murayama, Y. Perspective of cytokine regulation for periodontal treatment: Fibroblast biology. J. Periodontol. 2003, 74, 103–110. [Google Scholar] [CrossRef]
- Ebersole, J.; Singer, R.; Steffensen, B.; Filloon, T.; Kornman, K. Inflammatory mediators and immunoglobulins in GCF from healthy, gingivitis and periodontitis sites. J. Periodontal Res. 1993, 28, 543–546. [Google Scholar] [CrossRef]
- Zhang, J.; Kashket, S.; Lingström, P. Evidence for the early onset of gingival inflammation following short-term plaque accumulation. J. Clin. Periodontol. 2002, 29, 1082–1085. [Google Scholar] [CrossRef]
- Löe, H.; Theilade, E.; Jensen, S.B. Experimental gingivitis in man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef]
- Van der Weijden, G.; Hioe, K. A systematic review of the effectiveness of self-performed mechanical plaque removal in adults with gingivitis using a manual toothbrush. J. Clin. Periodontol. 2005, 32, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, H.R.; Himratul-Aznita, W.H.; Baharuddin, N.A. Anti-plaque effect of a synergistic combination of green tea and Salvadora persica L. against primary colonizers of dental plaque. Arch. Oral Biol. 2016, 70, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, H.R.; Himratul-Aznita, W.H.; Baharuddin, N.A. Evaluation of Salvadora persica L. and green tea anti-plaque effect: A randomized controlled crossover clinical trial. BMC Complement. Altern. Med. 2016, 16, 493. [Google Scholar] [CrossRef]
- Salah, R.; Abdulbaqi, H.R.; Mohammed, A.N.; Abdulkareem, A.A. Four-day randomized controlled crossover trial evaluating the antiplaque effect of a combination of green tea and Salvadora persica L. mouthwash. J. Herb. Med. 2020, 23, 100357. [Google Scholar] [CrossRef]
- Ahmad, H.; Rajagopal, K. Biological activities of Salvadora persica L. Meswak). Med. Aromat Plants 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Von Staszewski, M.; Pilosof, A.M.; Jagus, R.J. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011, 125, 186–192. [Google Scholar] [CrossRef]
- Ramberg, P.; Furuichi, Y.; Volpe, A.; Gaffar, A.; Lindhe, J. The effects of antimicrobial mouthrinses on de novo plaque formation at sites with healthy and inflamed gingivae. J. Clin. Periodontol. 1996, 23, 7–11. [Google Scholar] [CrossRef]
- Hefti, A.F.; Preshaw, P.M. Examiner alignment and assessment in clinical periodontal research. Periodontology 2000 2012, 59, 41–60. [Google Scholar] [CrossRef]
- Goodson, J.M. Gingival crevice fluid flow. Periodontology 2000 2003, 31, 43–54. [Google Scholar] [CrossRef]
- Eltas, A.; Orbak, R. Effect of 1064-nm Nd: YAG laser therapy on GCF IL-1β and MMP-8 levels in patients with chronic periodontitis. Lasers Med. Sci. 2012, 27, 543–550. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef]
- Brill, N. Gingival conditions related to flow of tissue fluid into gingival pockets. Acta Odontol. Scand. 1960, 18, 421–446. [Google Scholar] [CrossRef]
- Loe, H. Absence and presence of fluid normal and inflamed gingivae. Periodontics 1965, 3, 171–177. [Google Scholar]
- Özkavaf, A.; Aras, H.; Huri, C.B.; Mottaghian-Dini, F.; Tözüm, T.F.; Etikan, I.; Caglayan, F. Relationship between the quantity of gingival crevicular fluid and clinical periodontal status. J. Oral Sci. 2000, 42, 231–238. [Google Scholar] [CrossRef]
- Oliver, R.; Holm-Pedersen, P.; Löe, H. The correlation between clinical scoring, exudate measurements and microscopic evaluation of inflammation in the gingiva. J. Periodontol. Periodontics 1969, 40, 201–209. [Google Scholar] [CrossRef]
- Gartenmann, S.J.; Steppacher, S.L.; von Weydlich, Y.; Heumann, C.; Attin, T.; Schmidlin, P.R. The Effect of Green Tea on plaque and gingival inflammation: A systematic review. J. Herb. Med. 2020, 21, 100337. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Niazi, F.H.; Noushad, M.; Tanvir, S.B.; Ali, S.; Al-Khalifa, K.S.; Qamar, Z.; Al-Sheikh, R. Antimicrobial efficacy of indocyanine green-mediated photodynamic therapy compared with Salvadora persica gel application in the treatment of moderate and deep pockets in periodontitis. Photodiagnosis Photodyn. Ther. 2020, 29, 101665. [Google Scholar] [CrossRef]
- Mathur, S.; Mathur, T.; Srivastava, R.; Khatri, R. Chlorhexidine: The gold standard in chemical plaque control. Natl. J. Physiol. Pharm. Pharmacol. 2011, 1, 45. [Google Scholar]
- Sparrow, T.V.; Dodington, D.W.; Yumol, J.L.; Fritz, P.C.; Ward, W.E. Higher intakes of flavonoids are associated with lower salivary IL-1β and maintenance of periodontal health 3–4 years after scaling and root planing. J. Clin. Periodontol. 2020, 47, 461–469. [Google Scholar] [CrossRef]
- de Almeida, J.M.; Marques, B.M.; Novaes, V.C.N.; de Oliveira, F.L.P.; Matheus, H.R.; Fiorin, L.G.; Ervolino, E. Influence of adjuvant therapy with green tea extract in the treatment of experimental periodontitis. Arch. Oral Biol. 2019, 102, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Payan, B.; Zambrano, A.; Du, Y.; Bondesson, M.; Mohan, C. Epigallocatechin-3-gallate suppresses neutrophil migration speed in a transgenic zebrafish model accompanied by reduced inflammatory mediators. J. Inflamm. Res. 2019, 12, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, D.; Alqerban, A.; Samran, A. Treatment efficacy of photoactivated disinfection versus Salvadora persica gel in experimental gingivitis. Photodiagnosis Photodyn. Ther. 2020, 29, 101641. [Google Scholar] [CrossRef]
- Azaripour, A.; Mahmoodi, B.; Habibi, E.; Willershausen, I.; Schmidtmann, I.; Willershausen, B. Effectiveness of a miswak extract-containing toothpaste on gingival inflammation: A randomized clinical trial. Int. J. Dent. Hyg. 2017, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Abeer, Y.I.; Souad, E.E.-G.; Hemaia, M.M.; Amany, A.S. Anti-Inflammatory Activity of Salvadora persica L. against Carrageenan Induced Paw Oedema in Rat Relevant to Inflammatory Cytokines. Not. Sci. Biol. 2011, 3, 22–28. [Google Scholar]
- Wang, D.; Meng, J.; Xu, K.; Xiao, R.; Xu, M.; Liu, Y.; Zhao, Y.; Yao, P.; Yan, H.; Liu, L. Evaluation of oral subchronic toxicity of Pu-erh green tea (Camellia sinensis var. assamica) extract in Sprague Dawley rats. J. Ethnopharmacol. 2012, 142, 836–844. [Google Scholar]
- Ibrahim, A.Y.; El-Gengaihi, S.E. Safety profile of meswak root extract on liver, kidney, sexual hormones and hematological parameters of rats. Not. Sci. Biol. 2012, 4, 18–23. [Google Scholar] [CrossRef]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132, 110728. [Google Scholar] [CrossRef]
| Mean V1 (µL) | Mean V2 (µL) | fi (µL/s) | fi (µL/min) | fi * (µL/h) | Vr ** (µL) | T (min) | ||
|---|---|---|---|---|---|---|---|---|
| Combination | before | 0.009 | 0.007 | 0.00022 | 0.013 | 0.81 | 0.0025 | 4.5 |
| after | 0.008 | 0.006 | 0.00020 | 0.012 | 0.72 | 0.0020 | 5.0 | |
| Chlorhexidine | before | 0.008 | 0.006 | 0.00019 | 0.011 | 0.71 | 0.0028 | 5.2 |
| after | 0.010 | 0.007 | 0.00022 | 0.013 | 0.80 | 0.0033 | 4.5 | |
| Distilled water | before | 0.009 | 0.007 | 0.00020 | 0.012 | 0.72 | 0.0028 | 4.7 |
| after | 0.009 | 0.007 | 0.00022 | 0.013 | 0.79 | 0.0032 | 4.5 | |
| Formula | V2/33 | 60 *fi (µL/s) | 3600 *fi (µL/s) | V1-30 *fi | 1/fi |
| Mean Change ± SD | 95% Confidence Interval | Versus | p Value * | Effect Size ** | Power ** | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Combination | −0.086 ± 0.222 | −0.193 | 0.021 | CHX | <0.05 | 0.74 | 0.81 |
| distilled water | <0.05 | 0.76 | 0.81 | ||||
| Chlorhexidine | 0.088 ± 0.247 | −0.024 | 0.201 | Combination | <0.05 | 0.74 | 0.81 |
| distilled water | >0.05 | 0.06 | 0.80 | ||||
| Distilled water | 0.075 ± 0.201 | −0.046 | 0.197 | Combination | <0.05 | 0.76 | 0.81 |
| CHX | >0.05 | 0.06 | 0.80 | ||||
| Mean Change ± SD | 95% Confidence Interval | p Value * | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Combination | −0.0005 ± 0.0017 | −0.0014 | 0.0003 | >0.05 |
| Chlorhexidine | 0.0004 ± 0.0029 | −0.0010 | 0.0017 | |
| Distilled water | 0.0004 ± 0.0025 | −0.0011 | 0.0018 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, R.; Abdulbaqi, H.R. Short-Term (4 Day) Effects of Oral Rinsing with Miswak and Green Tea on Gingival Crevicular Fluid Flow and IL-1β Levels: A Pilot Study. Healthcare 2023, 11, 226. https://doi.org/10.3390/healthcare11020226
Salah R, Abdulbaqi HR. Short-Term (4 Day) Effects of Oral Rinsing with Miswak and Green Tea on Gingival Crevicular Fluid Flow and IL-1β Levels: A Pilot Study. Healthcare. 2023; 11(2):226. https://doi.org/10.3390/healthcare11020226
Chicago/Turabian StyleSalah, Rasha, and Hayder Raad Abdulbaqi. 2023. "Short-Term (4 Day) Effects of Oral Rinsing with Miswak and Green Tea on Gingival Crevicular Fluid Flow and IL-1β Levels: A Pilot Study" Healthcare 11, no. 2: 226. https://doi.org/10.3390/healthcare11020226
APA StyleSalah, R., & Abdulbaqi, H. R. (2023). Short-Term (4 Day) Effects of Oral Rinsing with Miswak and Green Tea on Gingival Crevicular Fluid Flow and IL-1β Levels: A Pilot Study. Healthcare, 11(2), 226. https://doi.org/10.3390/healthcare11020226






