Environmental Factors Influencing the Dynamics and Evolution of COVID-19: A Systematic Review on the Study of Short-Term Ozone Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Data Collection Process
2.4. Risk of Bias
3. Results
3.1. Study Characteristics
3.2. Study Design and Outcomes
3.3. Study Results
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, H.Y.; Jayabaskaran, C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J. Med. Virol. 2020, 92, 786–790. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Barac, S.; Onofrei, R.R.; Neagoe, P.V.; Popescu, A.I.; Pantea, S.; Rață, A.L. An Observational Study on Patients with Acute Limb Ischemia and SARS-CoV-2 Infection: Early and Late Results in Limb Salvage Rate. J. Clin. Med. 2021, 10, 5083. [Google Scholar] [CrossRef]
- Facciolà, A.; Laganà, P.; Caruso, G. The COVID-19 pandemic and its implications on the environment. Environ. Res. 2021, 201, 111648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Q. The implications of COVID-19 in the ambient environment and psychological conditions. Nano Impact. 2021, 21, 100295. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Cristaldi, A.; Fiore, M.; Grasso, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: A systematic review. Environ. Res. 2020, 191, 110129. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, N.; Asadi, Z. Air pollution impact on the Covid-19 mortality in Iran considering the comorbidity (obesity, diabetes, and hypertension) correlations. Environ. Res. 2022, 204 Pt A, 112020. [Google Scholar] [CrossRef]
- Cortes-Ramirez, J.; Wilches-Vega, J.D.; Paris-Pineda, O.M.; Rod, J.E.; Ayurzana, L.; Sly, P.D. Environmental risk factors associated with respiratory diseases in children with socioeconomic disadvantage. Heliyon 2021, 7, e06820. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Garcia, C.; Bell, M.L.; Thurston, G.D. Long-Term Exposure to Ozone and Cause-Specific Mortality Risk in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1022–1031. [Google Scholar] [CrossRef]
- Woodby, B.; Arnold, M.M.; Valacchi, G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: What is the connection? Ann. N. Y. Acad. Sci. 2021, 1486, 15–38. [Google Scholar] [CrossRef]
- Vo, T.; Paudel, K.; Choudhary, I.; Patial, S.; Saini, Y. Ozone exposure upregulates the expression of host susceptibility protein TMPRSS2 to SARS-CoV-2. Sci. Rep. 2022, 12, 1357, Preprint in: bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Pansini, R.; Fornacca, D. Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries. Atmosphere 2021, 12, 795. [Google Scholar] [CrossRef]
- Ogen, Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020, 726, 138605. [Google Scholar] [CrossRef]
- Domingo, J.L.; Rovira, J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020, 187, 109650. [Google Scholar] [CrossRef]
- Cole, M.A.; Ozgen, C.; Strobl, E. Air Pollution Exposure and COVID-19; IZA Discussion Papers 13367; Institute of Labor Economics (IZA): Bonn, Germany, 2020. [Google Scholar]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Mersha, T.B. Environmental Determinants of Coronavirus Disease 2019 (COVID-19). Curr. Allergy Asthma Rep. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Uta, M.; Neamtu, R.; Bernad, E.; Mocanu, A.G.; Gluhovschi, A.; Popescu, A.; Dahma, G.; Dumitru, C.; Stelea, L.; Citu, C.; et al. The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients 2022, 14, 836. [Google Scholar] [CrossRef]
- Manolescu, D.; Timar, B.; Bratosin, F.; Rosca, O.; Citu, C.; Oancea, C. Predictors for COVID-19 Complete Remission with HRCT Pattern Evolution: A Monocentric, Prospective Study. Diagnostics 2022, 12, 1397. [Google Scholar] [CrossRef]
- Citu, I.M.; Citu, C.; Margan, M.-M.; Craina, M.; Neamtu, R.; Gorun, O.M.; Burlea, B.; Bratosin, F.; Rosca, O.; Grigoras, M.L.; et al. Calcium, Magnesium, and Zinc Supplementation during Pregnancy: The Additive Value of Micronutrients on Maternal Immune Response after SARS-CoV-2 Infection. Nutrients 2022, 14, 1445. [Google Scholar] [CrossRef]
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Foster, E.D.; Deardorff, A. Open Science Framework (OSF). J. Med. Libr. Assoc. 2017, 105, 203. [Google Scholar] [CrossRef]
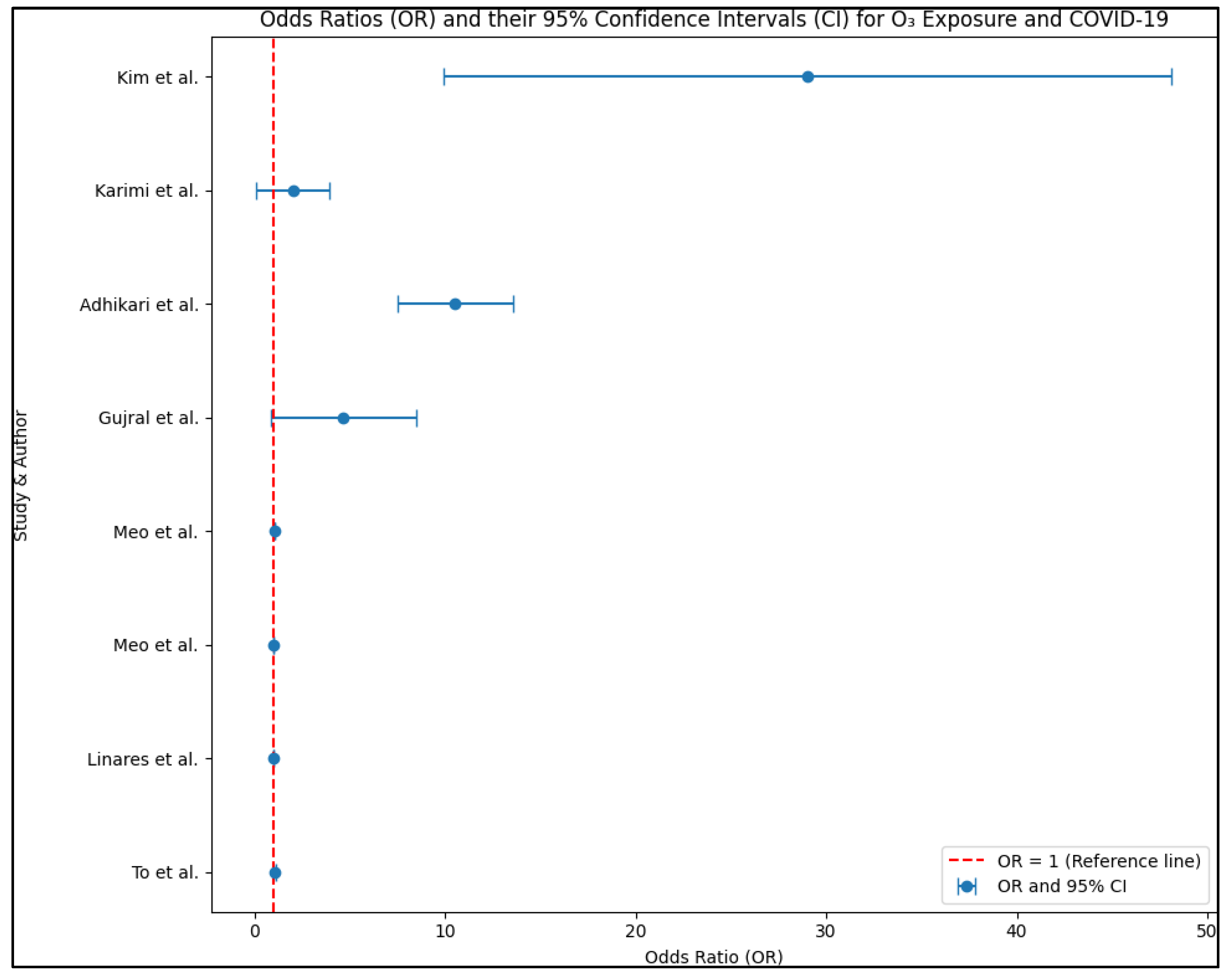
- To, T.; Zhang, K.; Maguire, B.; Terebessy, E.; Fong, I.; Parikh, S.; Zhu, J.; Su, Y. UV, ozone, and COVID-19 transmission in Ontario, Canada using generalised linear models. Environ. Res. 2021, 194, 110645. [Google Scholar] [CrossRef]
- Bilal; Bashir, M.F.; Benghoul, M.; Numan, U.; Shakoor, A.; Komal, B.; Bashir, M.; Tan, D. Environmental pollution and COVID-19 outbreak: Insights from Germany. Air Qual. Atmos. Health 2020, 13, 1385–1394. [Google Scholar] [CrossRef]
- Isphording, I.E.; Pestel, N. Pandemic meets pollution: Poor air quality increases deaths by COVID-19. J. Environ. Econ. Manag. 2021, 108, 102448. [Google Scholar] [CrossRef]
- Dragone, R.; Licciardi, G.; Grasso, G.; Del Gaudio, C.; Chanussot, J. Analysis of the Chemical and Physical Environmental Aspects that Promoted the Spread of SARS-CoV-2 in the Lombard Area. Int. J. Environ. Res. Public Health. 2021, 18, 1226. [Google Scholar] [CrossRef] [PubMed]
- Stufano, A.; Lisco, S.; Bartolomeo, N.; Marsico, A.; Lucchese, G.; Jahantigh, H.; Soleo, L.; Moretti, M.; Trerotoli, P.; De Palma, G.; et al. COVID-19 outbreak in Lombardy, Italy: An analysis on the short-term relationship between air pollution, climatic factors and the susceptibility to SARS-CoV-2 infection. Environ. Res. 2021, 198, 111197. [Google Scholar] [CrossRef] [PubMed]
- Zoran, M.A.; Savastru, R.S.; Savastru, D.M.; Tautan, M.N. Assessing the relationship between ground levels of ozone (O3) and nitrogen dioxide (NO2) with coronavirus (COVID-19) in Milan, Italy. Sci. Total Environ. 2020, 740, 140005. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Roy, P.D.; Elizalde-Martínez, I.; Shruti, V.C. Impacts of the COVID-19 lockdown on air quality and its association with human mortality trends in megapolis Mexico City. Air Qual. Atmos. Health. 2021, 14, 553–562. [Google Scholar] [CrossRef]
- Linares, C.; Culqui, D.; Belda, F.; López-Bueno, J.A.; Luna, Y.; Sánchez-Martínez, G.; Hervella, B.; Díaz, J. Impact of environmental factors and Sahara dust intrusions on incidence and severity of COVID-19 disease in Spain. Effect in the first and second pandemic waves. Environ. Sci. Pollut. Res. Int. 2021, 28, 51948–51960. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Adnan Abukhalaf, A.; Sami, W.; Hoang, T.D. Effect of environmental pollution PM2.5, carbon monoxide, and ozone on the incidence and mortality due to SARS-CoV-2 infection in London, United Kingdom. J. King Saud. Univ. Sci. 2021, 33, 101373. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Abukhalaf, A.A.; Alomar, A.A.; Alessa, O.M.; Sami, W.; Klonoff, D.C. Effect of environmental pollutants PM-2.5, carbon monoxide, and ozone on the incidence and mortality of SARS-COV-2 infection in ten wildfire affected counties in California. Sci. Total Environ. 2021, 757, 143948. [Google Scholar] [CrossRef] [PubMed]
- Persico, C.L.; Johnson, K.R. The effects of increased pollution on COVID-19 cases and deaths. J. Environ. Econ. Manag. 2021, 107, 102431. [Google Scholar] [CrossRef]
- Gujral, H.; Sinha, A. Association between exposure to airborne pollutants and COVID-19 in Los Angeles, United States with ensemble-based dynamic emission model. Environ. Res. 2021, 194, 110704. [Google Scholar] [CrossRef]
- Adhikari, A.; Yin, J. Lag Effects of Ozone, PM2.5, and Meteorological Factors on COVID-19 New Cases at the Disease Epicenter in Queens, New York. Atmosphere 2021, 12, 357. [Google Scholar] [CrossRef]
- Rui, R.; Tian, M.; Tang, M.L.; Ho, G.T.; Wu, C.H. Analysis of the Spread of COVID-19 in the USA with a Spatio-Temporal Multivariate Time Series Model. Int. J. Environ. Res. Public Health. 2021, 18, 774. [Google Scholar] [CrossRef]
- Karimi, S.M.; Majbouri, M.; DuPré, N.; White, K.B.; Little, B.B.; McKinney, W.P. Weather and COVID-19 Deaths During the Stay-at-Home Order in the United States. J. Occup. Environ. Med. 2021, 63, 462–468. [Google Scholar] [CrossRef]
- Kim, H.; Samet, J.M.; Bell, M.L. Association between Short-Term Exposure to Air Pollution and COVID-19 Mortality: A Population-Based Case-Crossover Study Using Individual-Level Mortality Registry Confirmed by Medical Examiners. Environ. Health Perspect. 2022, 130, 117006. [Google Scholar] [CrossRef]
- Akan, A.P. Transmission of COVID-19 pandemic (Turkey) associated with short-term exposure of air quality and climatological parameters. Environ. Sci. Pollut. Res. 2022, 29, 41695–41712. [Google Scholar] [CrossRef]
- Wiśniewski, O.; Kozak, W.; Wiśniewski, M. The ground-level ozone concentration is inversely correlated with the number of COVID-19 cases in Warsaw, Poland. Air Qual. Atmos. Health. 2021, 14, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, M.-E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. No association of COVID-19 transmission with temperature or, U.V. radiation in Chinese cities. Eur. Respir. J. 2020, 55, 2000517. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rahmandad, H.; Gupta, M.; DiGennaro, C.; Ghaffarzadegan, N.; Amini, H.; Jalali, M.S. Weather, air pollution, and SARS-CoV-2 transmission: A global analysis. Lancet Planet. Health. 2021, 5, e671–e680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Liao, H.; Yang, W.; Lu, C. Effects of short-term and long-term exposure to ambient air pollution and temperature on long recovery duration in COVID-19 patients. Environ. Res. 2023, 216 Pt 4, 114781. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2022, 203, 111930. [Google Scholar] [CrossRef]
- Andrée, B.P.J. Incidence of COVID-19 and connections with air pollution exposure: Evidence from the Netherlands. MedRxiv 2020. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F. Air Microbiome and Pollution: Composition and Potential Effects on Human Health, Including SARS Coronavirus Infection. J. Environ. Public Health. 2020, 2020, 1646943. [Google Scholar] [CrossRef]
- Azuma, K.; Kagi, N.; Kim, H.; Hayashi, M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ. Res. 2020, 190, 110042. [Google Scholar] [CrossRef]

| Study and Author | Country | Study Design | Study Quality |
|---|---|---|---|
| 1 [25] To et al. | Canada | Time series analysis | Excellent |
| 2 [26] Bilal et al. | Germany | Retrospective observational | Good |
| 3 [27] Isphording et al. | Germany | Retrospective observational | Excellent |
| 4 [28] Dragone et al. | Italy | Retrospective observational | Good |
| 5 [29] Stufano et al. | Italy | Retrospective observational | Good |
| 6 [30] Zoran et al. | Italy | Time series analysis | Fair |
| 7 [31] Kutralam-Muniasamy et al. | Mexico | Retrospective observational | Fair |
| 8 [32] Linares et al. | Spain | Time series analysis | Good |
| 9 [33] Meo et al. | UK | Retrospective observational | Good |
| 10 [34] Meo et al. | USA | Time series analysis | Good |
| 11 [35] Persico et al. | USA | Retrospective observational | Good |
| 12 [36] Gujral et al. | USA | Retrospective observational | Excellent |
| 13 [37] Adhikari et al. | USA | Time series analysis | Good |
| 14 [38] Rui et al. | USA | Time series analysis | Good |
| 15 [39] Karimi et al. | USA | Longitudinal analysis | Good |
| 16 [40] Kim et al. | USA | Case-crossover analysis | Excellent |
| 17 [41] Akan et al. | Turkey | Retrospective observational | Excellent |
| 18 [42] Wiśniewski et al. | Poland | Retrospective observational | Fair |
| Study and Author | Other Pollutants | Study Outcome | Statistical Model |
|---|---|---|---|
| 1 [25] To et al. | NR | Daily incidence of cases; reproductive number | Generalized linear models using restricted maximum likelihood. |
| 2 [26] Bilal et al. | ; ; | Total cases; total deaths; prevalence of cases; number of recovered patients | Spearman correlation; wavelet transform coherence approach. |
| 3 [27] Isphording et al. | Daily incidence of cases; daily deaths | Instrumental variable for air pollution using region-specific daily variation in wind direction. | |
| 4 [28] Dragone et al. | ; ; NO; ; ; ; | Daily incidence of cases; daily prevalence rate; growth factor | Pearson correlation; time series analysis for each province separately |
| 5 [29] Stufano et al. | ; , ; | Daily incidence of cases | Univariable mixed model with a logarithm transformation. |
| 6 [30] Zoran et al. | Daily incidence of cases; total number of cases; daily deaths | Pearson correlation | |
| 7 [31] Kutralam-Muniasamy et al. | ; ; ; ; | Daily incidence of cases; daily deaths | Correlation analysis (not specified) |
| 8 [32] Linares et al. | ; ; Saharan dust | Daily incidence of cases; rate of emergency admission | Generalized linear models with Poisson link. |
| 9 [33] Meo et al. | ; | Daily incidence of cases; daily deaths | Spearman correlation; Poisson regression analysis. |
| 10 [34] Meo et al. | ; | Daily incidence of cases; daily deaths | Spearman correlation; Poisson Regression Analysis; Binary Logistic Regression. |
| 11 [35] Persico et al. | Daily and weekly incidence of cases; daily deaths | Difference in differences with fixed effects. | |
| 12 [36] Gujral et al. | ; | Daily incidence | Generalized additive models and machine learning ensemble-based dynamic emission model. |
| 13 [37] Adhikari et al. | Daily incidence of cases; daily deaths | Negative binomial regression model and hurdle regression. | |
| 14 [38] Rui et al. | NR | Daily incidence of cases | Spatio–temporal multivariate time series models |
| 15 [39] Karimi et al. | Daily deaths | linear regression modeling with covariates | |
| 16 [40] Kim et al. | Daily deaths | Case-crossover analysis | |
| 17 [41] Akan et al. | ; | Daily incidence of cases; daily deaths | Spearman correlation |
| 18 [42] Wiśniewski et al. | NR | Daily incidence of cases | Spearman correlation |
| Study and Author | Results | Interpretation |
|---|---|---|
| 1 [25] To et al. | O3 risk for hospitalized patients (OR: 1.06 *, 95% CI: 1.00–1.13) O3 risk for the general population (OR: 1.00, 95% CI: 0.98–1.03) | Ozone is not significantly associated with incidence nor reproductive number, but it is positively associated with incidence in institutional settings like long-term care homes, hospitals, and jails. A one-unit increase in average weekly ozone is close to being significant for institutional outbreaks but not for the general population. |
| 2 [26] Bilal et al. | O3 coefficient for cases: 0.214 * O3 coefficient for recoveries: 0.216 * O3 coefficient for active cases: 0.467 * O3 coefficient for deaths: 0.215 * | PM10 and O3 are positively associated with total and active cases. The results for PM2.5, NO2, and cases are mixed depending on whether the outcome is based on active or total cases. O3 and NO2 are significantly positively associated with COVID-19 deaths. PM2.5 is negatively associated with deaths. There is no significant association between PM10 and deaths. |
| 3 [27] Isphording et al. | O3—no significance PM10 1 μg/m3 increase: RR = 1.00042 * | There are significant positive effects of acute exposure to PM10 on COVID-19 cases for all individuals and for deaths in those over 60 years old. Similar results were observed for ozone, but the effects were quantitatively non-significant. Among male patients aged 60–79 years, a one μg/m3 increase in PM10 two to four days after the onset of illness is associated with 0.042 additional deaths per 100,000 individuals. A one-SD increase in air pollution corresponds to an approximately 24 percent of a standard deviation increase in the fatality rate within this demographic. |
| 4 [28] Dragone et al. | PM10 > 50 μg/m3 PM2.5 > 50 μg/m3 75%< RH < 85% 4 °C < AT < 8 °C −0.5 < NAA < 0.5 | Based on a spatial analysis, the results indicate that PM2.5, PM10, NH3, and CO are strongly correlated with COVID-19. On the other hand, NO and NO2 show weak correlations, while O3 and SO2 show almost no correlation. However, it is important to note that none of these results reached statistical significance based on the z score values presented in the table. |
| 5 [29] Stufano et al. | NR | In general, there is no evident relationship observed between pollutants and COVID-19 cases. The relationship between the two variables is inconsistent, with both positive and negative associations observed depending on the specific lag period considered. |
| 6 [30] Zoran et al. | O3 coefficient for cases: 0.640 * O3 coefficient for deaths: 0.690 * | NO2 is negatively and statistically significantly associated with total cases, incidence, and total deaths. On the other hand, O3 is positively and statistically significantly associated with total cases, incidence, and total deaths. |
| 7 [31] Kutralam-Muniasamy et al. | PM10 coefficient for deaths: −0.380 * CO coefficient for deaths: 0.860 * O3 coefficient for deaths: 0.490 * | PM2.5, NO2, and SO2 did not exhibit significant associations with cases or deaths. However, PM10 displayed a negative association with both cases and deaths. On the other hand, CO and O3 showed positive associations with cases and deaths. These findings suggest that higher levels of CO and O3 were linked to increased cases and deaths related to COVID-19. The associations observed for PM10, CO, and O3 were statistically significant. |
| 8 [32] Linares et al. | O3 incidence risk (RR: 1.007 *, 95% CI: 1.004–1.009) | In all eight regions analyzed, NO2 showed a positive association with COVID-19 cases in terms of incidence rates. Additionally, in six out of the eight regions, NO2 displayed a positive association with hospitalizations. Similarly, PM10 exhibited a positive association with cases in six regions and hospitalizations in three regions. Furthermore, O3 demonstrated a positive association with cases in four regions and hospitalizations in three regions. These findings indicate that air pollutants, especially NO2, are closely linked to both the incidence and severity of COVID-19. |
| 9 [33] Meo et al. | O3 incidence risk (RR: 1.008 *) O3 death risk (RR: 1.044 *) | A 1 μm increase in PM2.5 was found to be significantly associated with a 1.1% increase in cases and a 2.3% increase in deaths. Similarly, a 1-unit increase in the CO level is significantly associated with a 21.3% increase in cases and a 21.8% increase in deaths. Furthermore, a 1-unit rise in O3 is significantly associated with a 0.8% increase in cases and a 4.4% increase in deaths. |
| 10 [34] Meo et al. | O3 incidence risk (RR: 1.025 *) O3 coefficient for cases: 0.158 * O3 coefficient for deaths: 0.034 | The analysis revealed positive associations between PM2.5 and CO with both COVID-19 cases and deaths. Additionally, O3 was found to have a positive association with cases, but the association with deaths was not statistically significant. Moreover, the results of a Poisson regression indicated that a 1 μm increase in PM2.5 resulting from wildfires led to a 0.4% increase in the number of deaths. |
| 11 [35] Persico et al. | NR | Both PM2.5 and O3 show positive associations with both cases of and deaths due to COVID-19. Specifically, an 11.8 percent increase in PM2.5, corresponding to an increase of 0.778 mg/m3, is associated with a 53 percent increase in cases. Similarly, a 5 percent increase in ozone is associated with a 10 percent increase in deaths due to COVID-19. These findings highlight the potential impact of air pollution, particularly PM2.5 and O3, on the incidence and severity of COVID-19 cases. |
| 12 [36] Gujral et al. | O3 incidence risk (OR: 4.66, 95% CI: 0.85–8.47) | An increase of one unit in PM2.5, PM10, and O3 is correlated with a decrease of 4.51%, a decrease of 1.62%, and an increase of 4.66% in daily COVID-19 cases, respectively. These findings indicate that higher levels of PM2.5 are associated with a decrease in COVID-19 cases, while higher levels of O3 are linked to an increase in cases. The effects of PM10 on cases is relatively smaller, with a slight decrease observed. |
| 13 [37] Adhikari et al. | O3 incidence risk (OR: 10.51 *, 95% CI: 7.47–13.63) | A one-unit increase in the moving average of PM2.5 was associated with a 33.11% decrease in daily COVID-19 incidence. On the other hand, a one-unit increase in the moving average of ozone was associated with a 10.51% increase in incidence. Regarding COVID-19 deaths, there was no significant association found with either PM2.5 or ozone (O3) based on the analysis. |
| 14 [38] Rui et al. | NR | The density of total atmospheric ozone is negatively associated with the incidence of cases. |
| 15 [39] Karimi et al. | O3 death risk (OR: 2.0, 95% CI: 0.10–3.60) | The analysis did not find a significant association between PM2.5 and COVID-19-related deaths. However, a one ppb increase in the average ozone concentration was associated with a 2.0% decrease in COVID-19-related deaths. |
| 16 [40] Kim et al. | O3 death risk (OR: 29.0 *, 95% CI: 9.9–51.5) | A high percentage of the population (98.9%) had ozone (O3) levels below the maximum 8 h national ambient air quality standard (NAAQS) of 35.7 μg/m3 or 70 parts per billion. An IQR increase in 3-day O3 exposure (8.2 μg/m3) was associated with a 29.0% increase in the risk of COVID-19 mortality. The associations varied depending on demographics, race/ethnicity, and comorbid conditions, indicating potential modifiers of the observed associations. |
| 17 [41] Akan et al. | O3 coefficient for cases: −0.620 * | As the concentration of O3 increases, there tends to be a decrease in the number of reported cases of the particular condition under study. |
| 18 [42] Wiśniewski et al. | O3 coefficient for cases: −0.299 * | As the concentration of O3 increases, there tends to be a decrease in the number of reported cases of the particular condition under study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, I.-M.; Baditoiu, L.M.; Reddy, S.R.; Nalla, A.; Popovici, E.D.; Margan, M.-M.; Anghel, M.; Laitin, S.M.D.; Toma, A.-O.; Herlo, A.; et al. Environmental Factors Influencing the Dynamics and Evolution of COVID-19: A Systematic Review on the Study of Short-Term Ozone Exposure. Healthcare 2023, 11, 2670. https://doi.org/10.3390/healthcare11192670
Popescu I-M, Baditoiu LM, Reddy SR, Nalla A, Popovici ED, Margan M-M, Anghel M, Laitin SMD, Toma A-O, Herlo A, et al. Environmental Factors Influencing the Dynamics and Evolution of COVID-19: A Systematic Review on the Study of Short-Term Ozone Exposure. Healthcare. 2023; 11(19):2670. https://doi.org/10.3390/healthcare11192670
Chicago/Turabian StylePopescu, Irina-Maria, Luminita Mirela Baditoiu, Sandhya Rani Reddy, Akhila Nalla, Emilian Damian Popovici, Madalin-Marius Margan, Mariana Anghel, Sorina Maria Denisa Laitin, Ana-Olivia Toma, Alexandra Herlo, and et al. 2023. "Environmental Factors Influencing the Dynamics and Evolution of COVID-19: A Systematic Review on the Study of Short-Term Ozone Exposure" Healthcare 11, no. 19: 2670. https://doi.org/10.3390/healthcare11192670
APA StylePopescu, I.-M., Baditoiu, L. M., Reddy, S. R., Nalla, A., Popovici, E. D., Margan, M.-M., Anghel, M., Laitin, S. M. D., Toma, A.-O., Herlo, A., Fericean, R. M., Baghina, N., & Anghel, A. (2023). Environmental Factors Influencing the Dynamics and Evolution of COVID-19: A Systematic Review on the Study of Short-Term Ozone Exposure. Healthcare, 11(19), 2670. https://doi.org/10.3390/healthcare11192670