Effects of Moderate Exercise Training on Cancer-Induced Muscle Wasting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Histology
2.3. Tissue Analysis
2.4. Statistical Analysis
3. Results
3.1. Morphometric Analysis of Gastrocnemius and Soleus Muscles
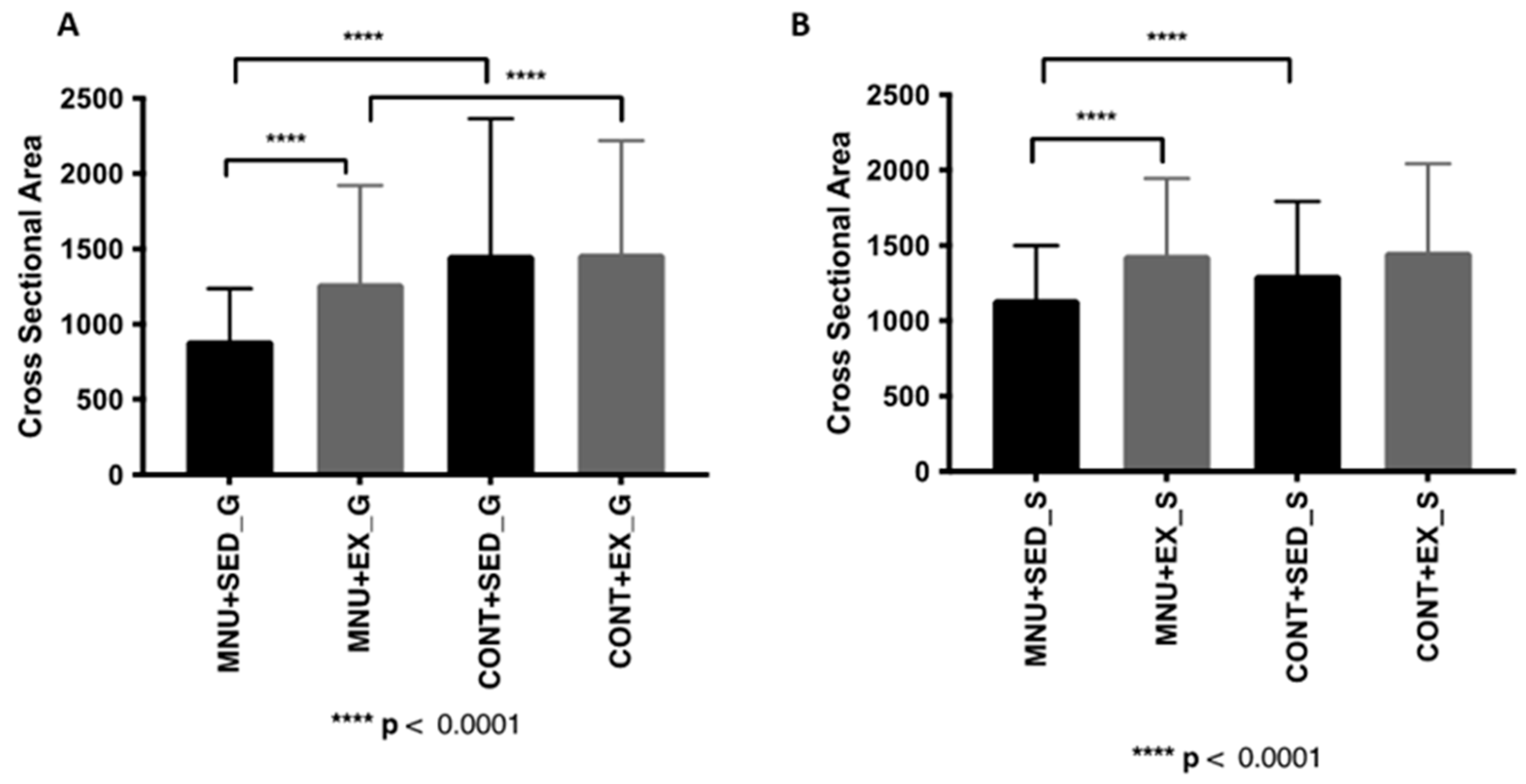
3.1.1. Cross-Sectional Area
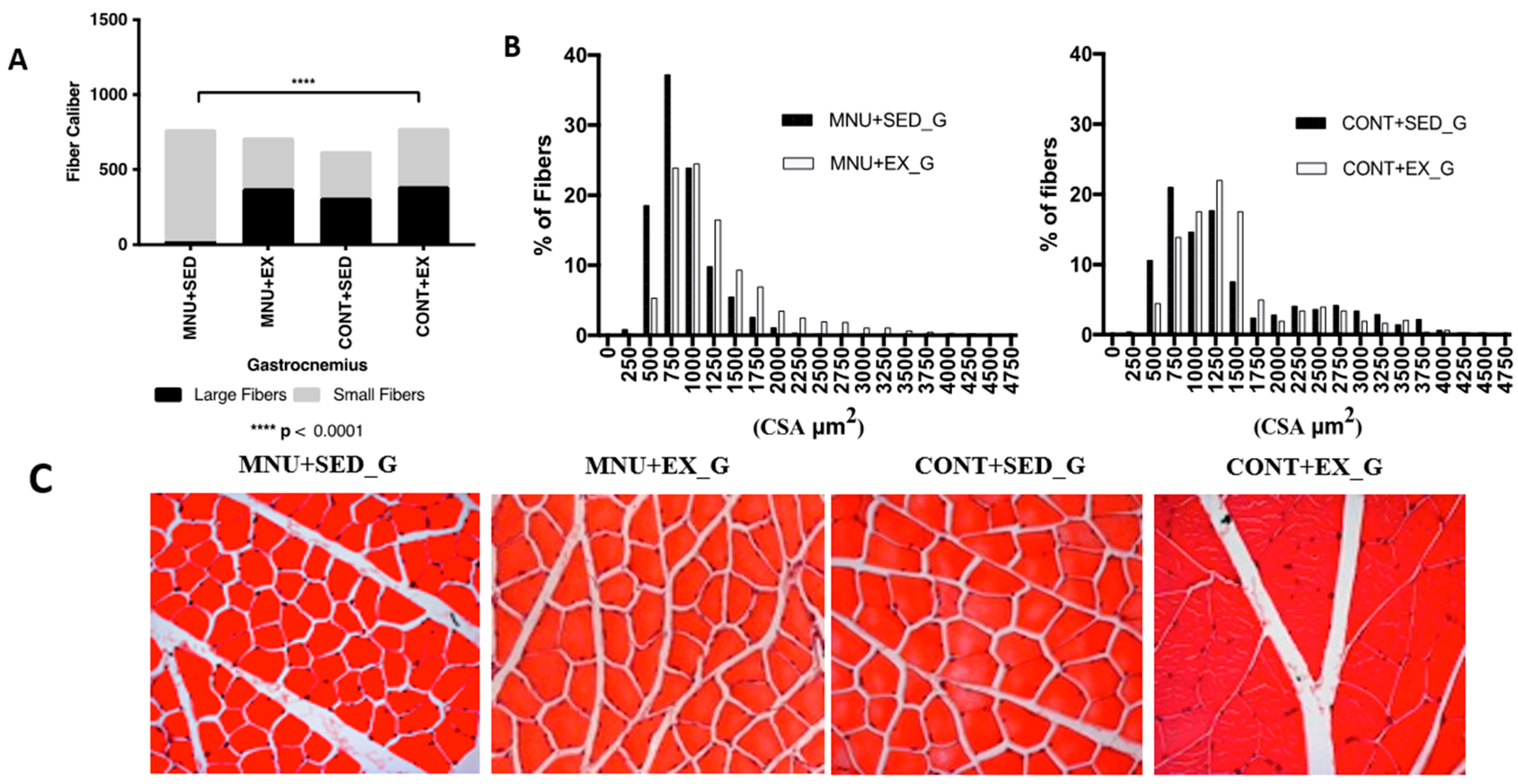
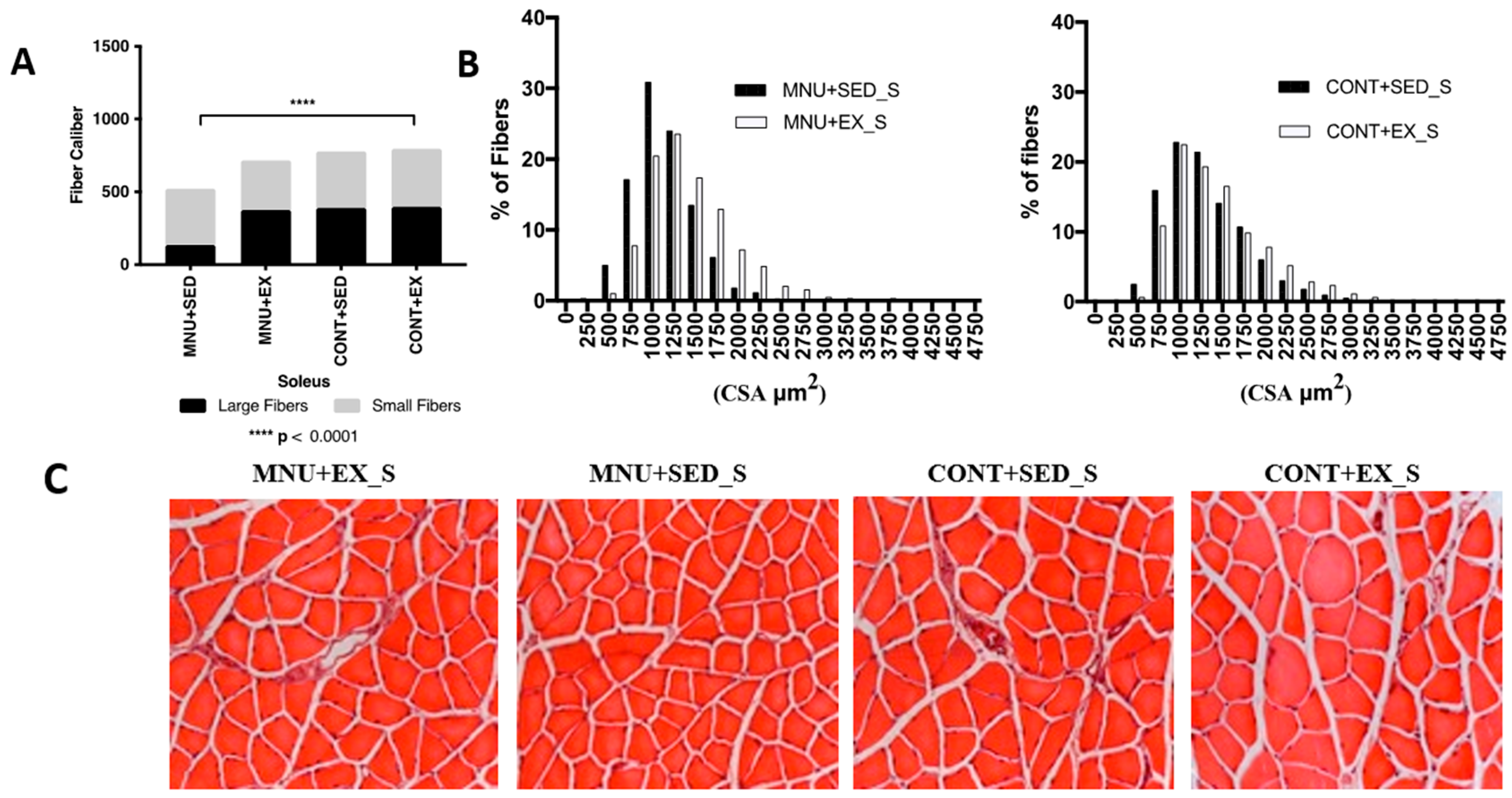
3.1.2. Fiber-Size Distribution
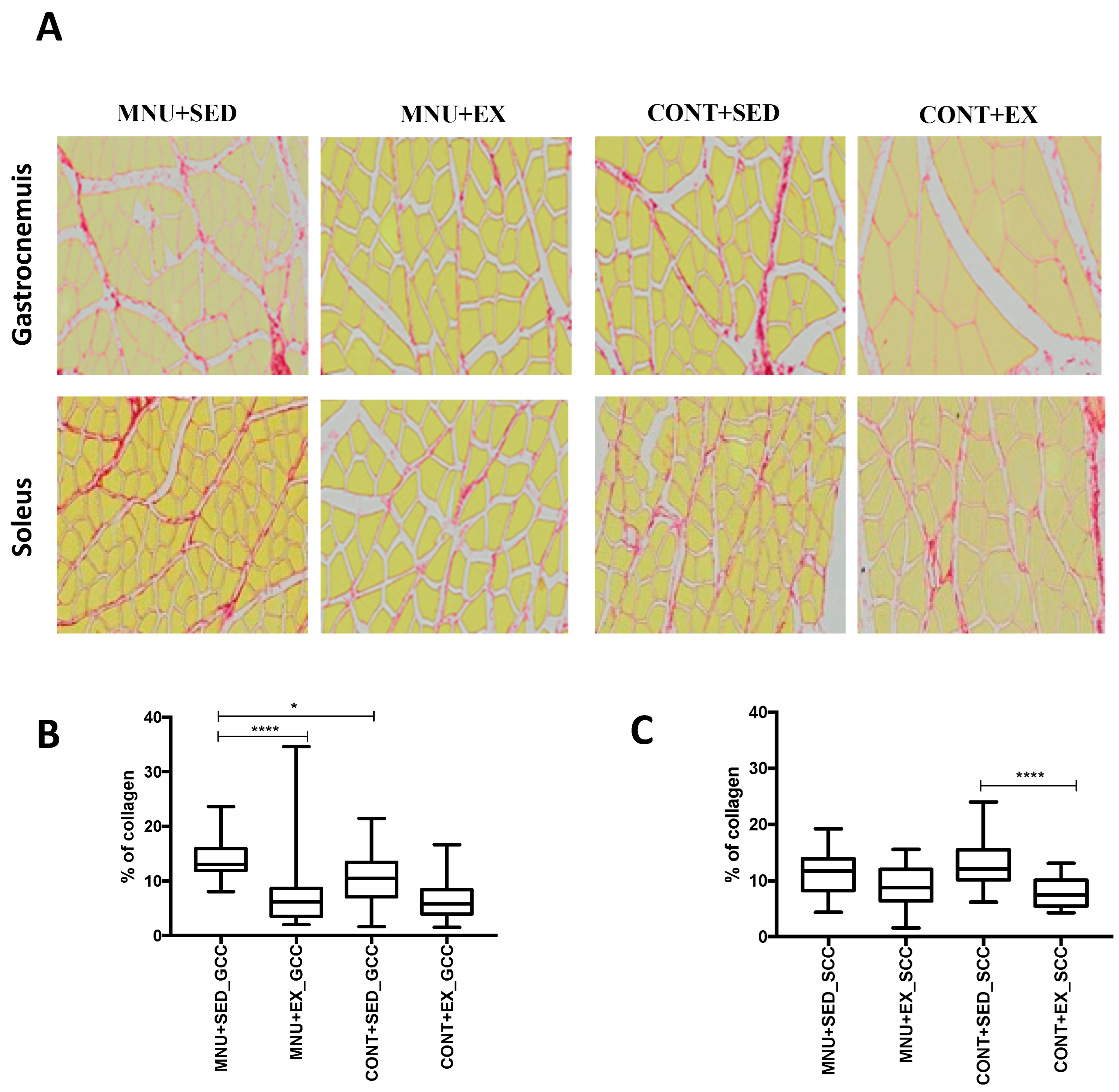
3.1.3. Muscle Collagen Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrenborg, E.; Krook, A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol. Rev. 2009, 61, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Sui, X.; Lobelo, F.; Morrow, J.R., Jr.; Jackson, A.W.; Sjöström, M.; Blair, S.N. Association between muscular strength and mortality in men: Prospective cohort study. BMJ 2008, 337, a439. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Musa, I.; Holm, L.; Lai, Y.C. Recent advances in measuring and understanding the regulation of exercise-mediated protein degradation in skeletal muscle. Am. J. Physiol. Cell Physiol. 2021, 321, C276–C287. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Hawke, T.J. Wasting away: AJP-Cell Physiology initiates thematic reviews on skeletal muscle wasting. Am. J. Physiol. Cell Physiol. 2021, 321, C38–C39. [Google Scholar] [CrossRef]
- Mader, T.; Chaillou, T.; Alves, E.S.; Jude, B.; Cheng, A.J.; Kenne, E.; Mijwel, S.; Kurzejamska, E.; Vincent, C.T.; Rundqvist, H.; et al. Exercise reduces intramuscular stress and counteracts muscle weakness in mice with breast cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 1151–1163. [Google Scholar] [CrossRef]
- Skipworth, R.J.; Stewart, G.D.; Ross, J.A.; Guttridge, D.C.; Fearon, K.C. The molecular mechanisms of skeletal muscle wasting: Implications for therapy. Surg. J. R. Coll. Surg. E. 2006, 4, 273–283. [Google Scholar] [CrossRef]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef]
- Argiles, J.M.; Lopez-Soriano, F.J.; Busquets, S. Mechanisms to explain wasting of muscle and fat in cancer cachexia. Curr. Opin. Support. Palliat. Care 2007, 1, 293–298. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Scott, A.M.; Hoogenraad, N.J.; Osellame, L.D. Mediators and clinical treatment for cancer cachexia: A systematic review. JCSM Rapid Commun. 2021, 4, 166–186. [Google Scholar] [CrossRef]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Tisdale, M.J. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 2010, 6, 503–513. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle dysfunction in cancer patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Villasenor, A.; Ballard-Barbash, R.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; McTiernan, A.; Neuhouser, M.L. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: The HEAL Study. J. Cancer Surviv. 2012, 6, 398–406. [Google Scholar] [CrossRef]
- Teixeira, E.; Duarte, J.A. Skeletal Muscle Loading Changes its Regenerative Capacity. Sports Med. 2016, 46, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.L.; Wagner, K.R. Regeneration versus fibrosis in skeletal muscle. Curr. Opin. Rheumatol. 2011, 23, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Clementi, E.; Manfredi, A.A.; Rovere-Querini, P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: Cellular and molecular players. Cell. Mol. Life Sci. 2015, 72, 2135–2156. [Google Scholar] [CrossRef]
- Michele, D.E. Mechanisms of skeletal muscle repair and regeneration in health and disease. FEBS J. 2022, 289, 6460–6462. [Google Scholar] [CrossRef]
- Al-Majid, S.; Waters, H. The biological mechanisms of cancer-related skeletal muscle wasting: The role of progressive resistance exercise. Biol. Res. Nurs. 2008, 10, 7–20. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Lopez-Soriano, F.J.; Costelli, P.; Penna, F. Are there any benefits of exercise training in cancer cachexia? J. Cachexia Sarcopenia Muscle 2012, 3, 73–76. [Google Scholar] [CrossRef]
- Acharyya, S.; Ladner, K.J.; Nelsen, L.L.; Damrauer, J.; Reiser, P.J.; Swoap, S.; Guttridge, D.C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Investig. 2004, 114, 370–378. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Acharyya, S.; Butchbach, M.E.; Sahenk, Z.; Wang, H.; Saji, M.; Carathers, M.; Ringel, M.D.; Skipworth, R.J.; Fearon, K.C.; Hollingsworth, M.A.; et al. Dystrophin glycoprotein complex dysfunction: A regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 2005, 8, 421–432. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Davis, J.M.; White, J.P.; Carson, J.A. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Archiv. Eur. J. Appl. Physiol. 2009, 457, 989–1001. [Google Scholar]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [PubMed]
- Diffee, G.M.; Kalfas, K.; Al-Majid, S.; McCarthy, D.O. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am. J. Physiol. Cell Physiol. 2002, 283, C1376–C1382. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Powers, S.K.; Hammeren, J.; Martin, A.D. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med. Sci. Sports Exerc. 1993, 25, 1259–1264. [Google Scholar] [CrossRef]
- Guarnier, F.A.; Cecchini, A.L.; Suzukawa, A.A.; Maragno, A.L.; Simao, A.N.; Gomes, M.D.; Cecchini, R. Time course of skeletal muscle loss and oxidative stress in rats with Walker 256 solid tumor. Muscle Nerve 2010, 42, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.L.; Ryan, A.M.; Reynolds, J.V. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol. Res. Pract. 2011, 2011, 601434. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic criteria for the classification of cancer-associated weight loss. Am. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef]
- Ferreira, R.; Vitorino, R.; Neuparth, M.J.; Appell, H.J.; Duarte, J.A.; Amado, F. Proteolysis activation and proteome alterations in murine skeletal muscle submitted to 1 week of hindlimb suspension. Eur. J. Appl. Physiol. 2009, 107, 553–563. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Steenberg, D.E.; Hostrup, M.; Birk, J.B.; Larsen, J.K.; Santos, A.; Kjøbsted, R.; Hingst, J.R.; Schéele, C.C.; Murgia, M.; et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat Commun. 2021, 12, 304. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef]
- Talmadge, R.J. Myosin heavy chain isoform expression following reduced neuromuscular activity: Potential regulatory mechanisms. Muscle Nerve 2000, 23, 661–679. [Google Scholar] [CrossRef]
- Klingler, W.; Jurkat-Rott, K.; Lehmann-Horn, F.; Schleip, R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012, 31, 184–195. [Google Scholar] [PubMed]
- Acharyya, S.; Guttridge, D.C. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin. Cancer Res. 2007, 13, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free. Radic. Biol. Med. 2016, 98, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Henrot, P.; Blervaque, L.; Dupin, I.; Zysman, M.; Esteves, P.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Cellular interplay in skeletal muscle regeneration and wasting: Insights from animal models. J. Cachexia Sarcopenia Muscle 2023, 14, 745–757. [Google Scholar] [CrossRef]
- Abreu, P.; Kowaltowski, A.J. Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption. J. Cachexia Sarcopenia Muscle 2020, 11, 1661–1676. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Eichelberger, E.J.; Neves, W.; Ribeiro, M.A.C.; Bechara, L.R.G.; Voltarelli, V.A.; Almeida, N.R.; Hagen, L.; Sharma, A.; Ferreira, J.C.B.; et al. Cancer-induced muscle atrophy is determined by intrinsic muscle oxidative capacity. FASEB J. 2021, 35, e21714. [Google Scholar] [CrossRef]
- Yu, Z.; Li, P.; Zhang, M.; Hannink, M.; Stamler, J.S.; Yan, Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS ONE 2008, 3, e2086. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Al-Majid, S.; McCarthy, D.O. Cancer-induced fatigue and skeletal muscle wasting: The role of exercise. Biol. Res. Nurs. 2001, 2, 186–197. [Google Scholar] [CrossRef] [PubMed]
| MNU Groups | Control Groups | |||
|---|---|---|---|---|
| Sedentary | Exercised | Sedentary | Exercised | |
| Body weight | ||||
| Initial (g) | 187.7 ± 14.3 | 179.5 ± 11.7 | 159.4 ± 7.2 | 154.3 ± 9.83 |
| At sacrifice (g) | 287.1 ± 13.4 | 294.3 ± 25.0 | 298.3 ± 14.4 | 298.2 ± 24.3 |
| Acurate (g) | 272.5 ± 13.9 | 281.8 ± 31.6 | - | - |
| Gain (g) | 84.8 ± 13.9 | 102.3 ± 31.6 | 138.9 ± 14.4 | 171.7 ± 48.7 |
| Gastrocnemius weight (g) | 3.62 ± 0.3 | 3.70 ± 0.6 | 3.92 ± 0.22 | 4.16 ± 0.13 |
| Gastrocnemius CSA (μm2) | 831–[658–1042] *¥ | 1084–[841–1472] ** | 1178–[792–1964] | 1284–[977–1616] |
| Gastrocnemius CC (%) | 13.01–[11.9–15.9] *¥ | 8.1–[5.4–9.25] | 10.47–[7.1–13.4] | 5.78–[3.9–8.4] |
| Soleus weight (g) | 0.19 ± 0.03 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.23 ± 0.02 |
| Soleus CSA (μm2) | 1104–[903–1350] *¥ | 1347–[1078–1703] | 1220–[950–1589] | 1320–[1030–1751] |
| Soleus CC (%) | 11.77–[8.3–13.9] | 8.77–[6.4–12.0] | 12.09–[10.2–15.5] ** | 7.47–[5.4–10.1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, A.C.C.; Pereira, A.; Leitão, L.; Ferreira, R.; Oliveira, P.A.; Duarte, J.A. Effects of Moderate Exercise Training on Cancer-Induced Muscle Wasting. Healthcare 2023, 11, 2652. https://doi.org/10.3390/healthcare11192652
Figueira ACC, Pereira A, Leitão L, Ferreira R, Oliveira PA, Duarte JA. Effects of Moderate Exercise Training on Cancer-Induced Muscle Wasting. Healthcare. 2023; 11(19):2652. https://doi.org/10.3390/healthcare11192652
Chicago/Turabian StyleFigueira, Ana Cristina Corrêa, Ana Pereira, Luís Leitão, Rita Ferreira, Paula A. Oliveira, and José Alberto Duarte. 2023. "Effects of Moderate Exercise Training on Cancer-Induced Muscle Wasting" Healthcare 11, no. 19: 2652. https://doi.org/10.3390/healthcare11192652
APA StyleFigueira, A. C. C., Pereira, A., Leitão, L., Ferreira, R., Oliveira, P. A., & Duarte, J. A. (2023). Effects of Moderate Exercise Training on Cancer-Induced Muscle Wasting. Healthcare, 11(19), 2652. https://doi.org/10.3390/healthcare11192652








