Cessation of Rectal Screening for Vancomycin-Resistant Enterococci: Experience from a Tertiary Care Hospital from Türkiye
Abstract
1. Introduction
2. Material and Methods
2.1. Setting and Patients
2.2. Infection Prevention and Control Measures for VRE
2.3. Definitions
2.4. Bacterial Isolation and Molecular Analysis
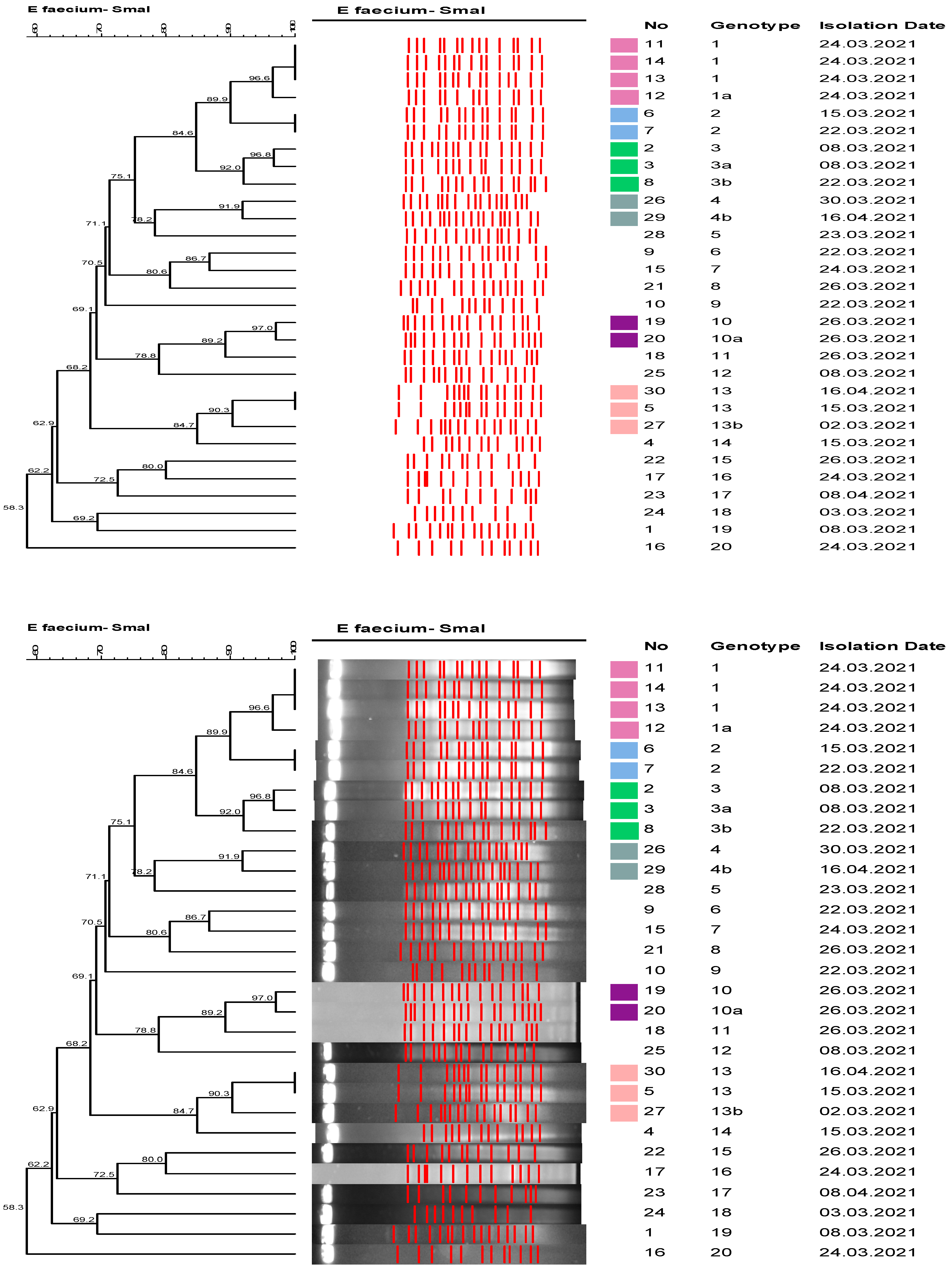
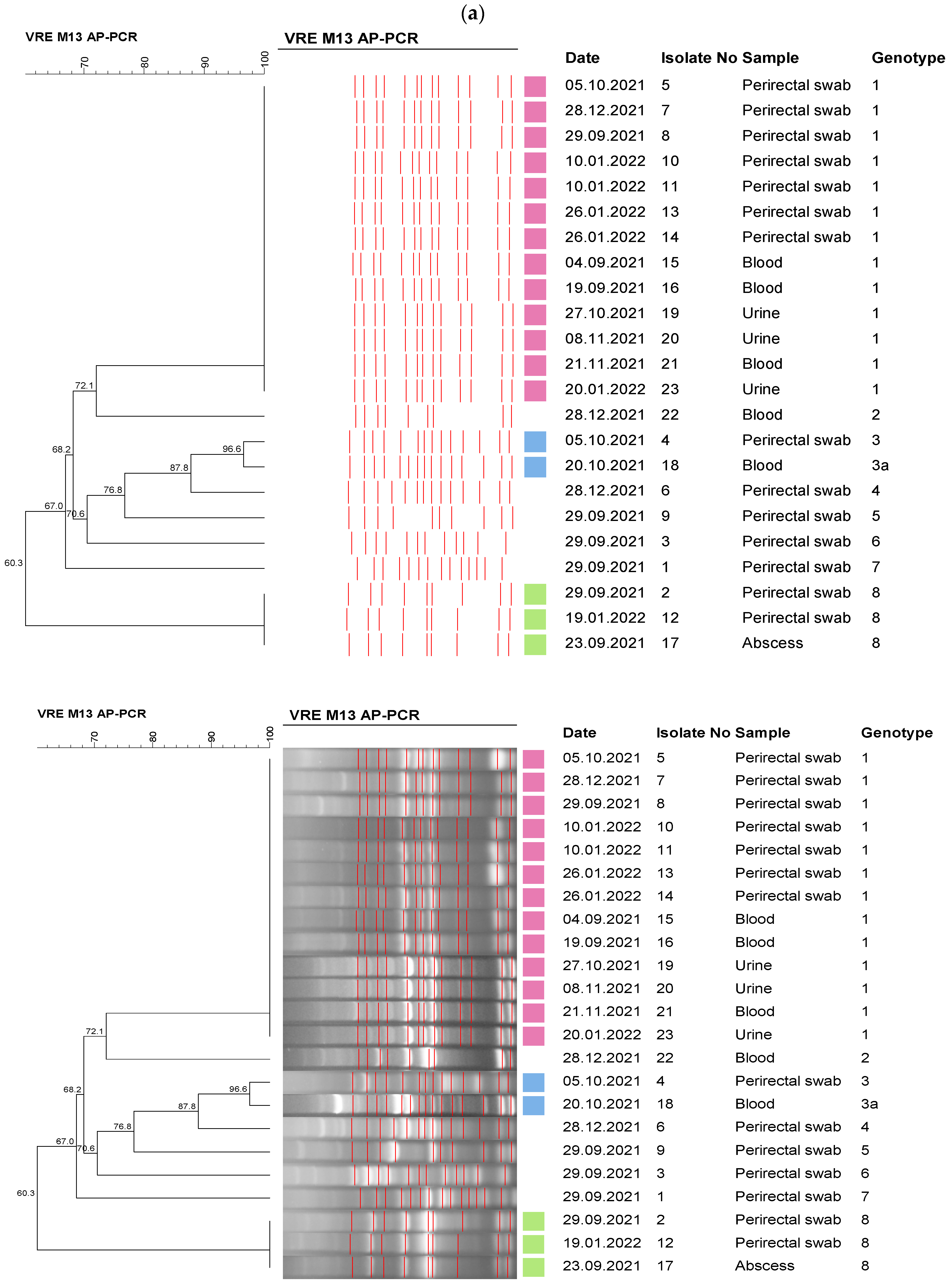
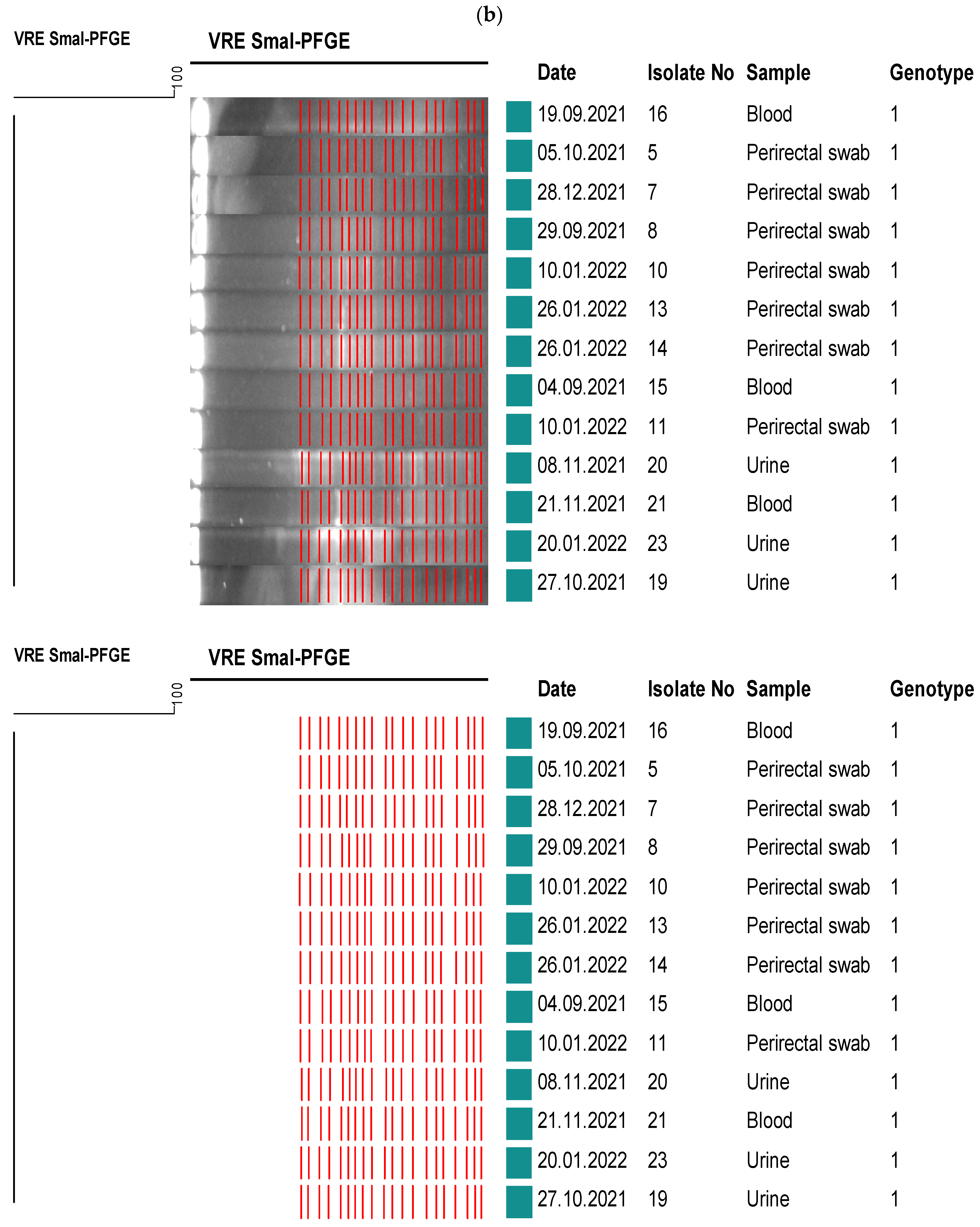
2.5. Molecular Epidemiological Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, J. Antimicrobial Resistance, from bench-to-publicside. Microbes Infect. Chemother. 2021, 1, e1182. [Google Scholar] [CrossRef]
- Raza, T.; Ullah, S.R.; Mehmood, K.; Andleeb, S. Vancomycin resistant Enterococci: A brief review. J. Pak. Med. Assoc. 2018, 68, 768–772. [Google Scholar]
- Almyroudis, N.G.; Osawa, R.; Samonis, G.; Wetzler, M.; Wang, E.S.; McCarthy, P.L.; Segal, B.H. Discontinuation of Systematic Surveillance and Contact Precautions for Vancomycin-Resistant Enterococcus (VRE) and Its Impact on the Incidence of VRE faecium Bacteremia in Patients with Hematologic Malignancies. Infect. Control. Hosp. Epidemiol. 2016, 37, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Shie, S.S.; Cheng, C.W.; Yang, J.H.; Huang, P.Y.; Wu, T.S.; Lee, M.H.; Huang, C.T. Clinical characteristics and treatment outcomes of vancomycin-resistant Enterococcus faecium bacteremia. J. Microbiol. Immunol. Infect. 2018, 51, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Twilla, J.D.; Finch, C.K.; Usery, J.B.; Gelfand, M.S.; Hudson, J.Q.; Broyles, J.E. Vancomycin-resistant Enterococcus bacteremia: An evaluation of treatment with linezolid or daptomycin. J. Hosp. Med. 2012, 7, 243–248. [Google Scholar] [CrossRef]
- McKinnell, J.A.; Patel, M.; Shirley, R.M.; Kunz, D.F.; Moser, S.A.; Baddley, J.W. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol. Infect. 2011, 139, 1342–1350. [Google Scholar] [CrossRef]
- Han, S.H.; Chin, B.S.; Lee, H.S.; Jeong, S.J.; Choi, H.K.; Kim, C.O.; Yong, D.; Choi, J.Y.; Song, Y.G.; Lee, K.; et al. Vancomycin-resistant enterococci bacteremia: Risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J. Infect. 2009, 58, 182–190. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Hekimoğlu, C.H.B.E.; Yıldırım Gözel, E.; Altun, D. USHIESA Ozet Rapor 2021. Available online: https://www.researchgate.net/publication/364113964_ULUSAL_SAGLIK_HIZMETI_ILISKILI_ENFEKSIYONLAR_SURVEYANS_AGI_USHIESA_OZET_RAPORU_2021 (accessed on 1 June 2022).
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Health Care Infection Control Practices Advisory, C. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Muto, C.A.; Jernigan, J.A.; Ostrowsky, B.E.; Richet, H.M.; Jarvis, W.R.; Boyce, J.M.; Farr, B.M.; SHEA. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect. Control Hosp. Epidemiol. 2003, 24, 362–386. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Healthcare Infection Control Practices Advisory, C. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 2007, 35, S165–S193. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, K.; Nishiki, S.; Kaga, Y.; Harada, T.; Kawahara, R.; Takahashi, H.; Ueda, E.; Koshimo, N.; Ito, H.; Matsui, T.; et al. Effectiveness of patient and staff cohorting to reduce the risk of vancomycin-resistant enterococcus (VRE) acquisition: A retrospective cohort study during a VRE outbreak in Japan. J. Hosp. Infect. 2023, 134, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, C.; Gardam, M.; Evans, G.; John, M.; Suh, K.N.; vanWalraven, C.; Vicencio, E.; Coulby, C.; Roth, V.; Hota, S. Longitudinal Multicenter Analysis of Outcomes After Cessation of Control Measures for Vancomycin-Resistant Enterococci. Infect. Control Hosp. Epidemiol. 2017, 38, 24–30. [Google Scholar] [CrossRef]
- Popiel, K.Y.; Miller, M.A. Evaluation of vancomycin-resistant enterococci (VRE)-associated morbidity following relaxation of VRE screening and isolation precautions in a tertiary care hospital. Infect. Control Hosp. Epidemiol. 2014, 35, 818–825. [Google Scholar] [CrossRef]
- Martin, E.M.; Russell, D.; Rubin, Z.; Humphries, R.; Grogan, T.R.; Elashoff, D.; Uslan, D.Z. Elimination of Routine Contact Precautions for Endemic Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus: A Retrospective Quasi-Experimental Study. Infect. Control Hosp. Epidemiol. 2016, 37, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Bearman, G.; Abbas, S.; Masroor, N.; Sanogo, K.; Vanhoozer, G.; Cooper, K.; Doll, M.; Stevens, M.P.; Edmond, M.B. Impact of Discontinuing Contact Precautions for Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus: An Interrupted Time Series Analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 676–682. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, H.M.; Chung, D.R.; Choi, J.R.; Lee, M.A.; Huh, H.J.; Lee, N.Y.; Huh, K.; Kang, C.I.; Peck, K.R. The impact of vancomycin-resistant Enterococcus (VRE) screening policy change on the incidence of healthcare-associated VRE bacteremia. Infect. Control Hosp. Epidemiol. 2022, 43, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.; Shing, E.; Saedi, A.; Adomako, K.; Li, Y.; Brown, K.A.; Garber, G. Discontinuing Contact Precautions for Vancomycin-Resistant Enterococcus (VRE) Is Associated With Rising VRE Bloodstream Infection Rates in Ontario Hospitals, 2009-2018: A Quasi-experimental Study. Clin. Infect. Dis. 2020, 71, 1756–1759. [Google Scholar] [CrossRef]
- Metan, G.; Ilbay, A.; Eser, O.K.; Unal, S.; Zarakolu, P. A Silent Epidemic of Colistin- and Carbapenem-Resistant Enterobacteriaceae at a Turkish University Hospital. Infect. Control Hosp. Epidemiol. 2017, 38, 254–257. [Google Scholar] [CrossRef][Green Version]
- Metan, G.; Zarakolu, P.; Otlu, B.; Tekin, I.; Aytac, H.; Bolek, E.C.; Metin, B.C.; Arsava, E.M.; Unal, S. Emergence of colistin and carbapenem-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii (CCR-Acb) complex in a neurological intensive care unit followed by successful control of the outbreak. J. Infect. Public Health 2020, 13, 564–570. [Google Scholar] [CrossRef]
- Wayne, P. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2009. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for İnterpretation of MICs and Zone Diameters. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 1 September 2016).
- Facklam, R.R.S.; Teixeira, L.M. Enterococcus. In Manual of Clinical Microbiology; Murray, P.R.B.M., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1999; pp. 297–305. [Google Scholar]
- Wayne, P. Methods for Dilution Antimicrobial Susceptibility Tests Forbacteria that Grow Aerobically, Approved standard M7-A5, 5th ed.; NCCLS: Wayne, NJ, USA, 2000. [Google Scholar]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Paule, S.M.; Trick, W.E.; Tenover, F.C.; Lankford, M.; Cunningham, S.; Stosor, V.; Cordell, R.L.; Peterson, L.R. Comparison of PCR assay to culture for surveillance detection of vancomycin-resistant enterococci. J. Clin. Microbiol. 2003, 41, 4805–4807. [Google Scholar] [CrossRef] [PubMed]
- Kuzucu, C.; Durmaz, R.; Otlu, B.; Aktas, E.; Gulcan, H.; Cizmeci, Z. Species distribution, antifungal susceptibility and clonal relatedness of Candida isolates from patients in neonatal and pediatric intensive care units at a medical center in Turkey. New Microbiol. 2008, 31, 401–408. [Google Scholar] [PubMed]
- Ulu-Kilic, A.; Ozhan, E.; Altun, D.; Percin, D.; Gunes, T.; Alp, E. Is it worth screening for vancomycin-resistant Enterococcus faecium colonization?: Financial burden of screening in a developing country. Am. J. Infect. Control 2016, 44, e45–e49. [Google Scholar] [CrossRef] [PubMed]
- Bryce, E.; Grant, J.; Scharf, S.; Dempster, L.; Lau, T.T.; Laing, F.; Shajari, S.; Forrester, L. Horizontal infection prevention measures and a risk-managed approach to vancomycin-resistant enterococci: An evaluation. Am. J. Infect. Control 2015, 43, 1238–1243. [Google Scholar] [CrossRef]
- Martin, E.M.; Colaianne, B.; Bridge, C.; Bilderback, A.; Tanner, C.; Wagester, S.; Yassin, M.; Pontzer, R.; Snyder, G.M. Discontinuing MRSA and VRE contact precautions: Defining hospital characteristics and infection prevention practices predicting safe de-escalation. Infect. Control Hosp. Epidemiol. 2022, 43, 1595–1602. [Google Scholar] [CrossRef]
- Chhatwal, P.; Ebadi, E.; Thol, F.; Koenecke, C.; Beutel, G.; Ziesing, S.; Schluter, D.; Bange, F.C.; Baier, C. Prospective infection surveillance and systematic screening for vancomycin-resistant enterococci in hematologic and oncologic patients—Findings of a German tertiary care center. J. Glob. Antimicrob. Resist. 2020, 22, 102–105. [Google Scholar] [CrossRef]
- Karakecılı, F.D.C.B.; Karagoz, A.; Jeffrrıes, M.; Cıkman, A.; Akalın, H.; Ozakın, C. Molecular subtyping of vancomycın resistant enterococcus: A comparison of two molecular methods. Acta Medica Mediterr. 2016, 32, 1797–1803. [Google Scholar]
| Weekly Screening (VRE-Positive Patients/Number of Screened Patients) (2006–2013) | Point Prevalence Screening (VRE-Positive Patients/Number of Screened Patients) (2015–2017) | p Value | |
|---|---|---|---|
| Adult Hospital | 506/10,171 5% | 54/569 9.5% | <0.001 |
| Oncology Hospital | 105/1642 6.4% | 17/142 12% | 0.02 |
| VRE-BSI Rate/1000 Patient Days (95% Confidence Interval) (2006–2012) | VRE-BSI Rate/1000 Patient Days (95% Confidence Interval) (2013–2021) | p Value | |
|---|---|---|---|
| Oncology Hospital | 0.033 (0.014–0.077) | 0.042 (0.025–0.072) | 0.66 |
| HSCT Unit | 0 | 0.121 (0.047–0.313) | 0.06 |
| Hospital-Wide | 0.011 (0.006–0.018) | 0.022 (0.016–0.031) | 0.015 |
| Hospital-Wide Without Medical ICU | 0.011 (0.006–0.018) | 0.016 (0.01–0.024) | 0.22 |
| Medical ICU | 0.248 (0.08–0.579) | 0.61 (0.382–0.923) | 0.06 |
| Surgical ICU | 0.3 (0.097–0.7) | 0.081 (0.009–0.291) | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telli Dizman, G.; Metan, G.; Zarakolu, P.; Tanrıverdi, E.S.; Hazırolan, G.; Aytaç Ak, H.; Kılınçarslan, D.; Uzun, M.; Çelik Kavaklılar, B.; Arık, Z.; et al. Cessation of Rectal Screening for Vancomycin-Resistant Enterococci: Experience from a Tertiary Care Hospital from Türkiye. Healthcare 2023, 11, 2641. https://doi.org/10.3390/healthcare11192641
Telli Dizman G, Metan G, Zarakolu P, Tanrıverdi ES, Hazırolan G, Aytaç Ak H, Kılınçarslan D, Uzun M, Çelik Kavaklılar B, Arık Z, et al. Cessation of Rectal Screening for Vancomycin-Resistant Enterococci: Experience from a Tertiary Care Hospital from Türkiye. Healthcare. 2023; 11(19):2641. https://doi.org/10.3390/healthcare11192641
Chicago/Turabian StyleTelli Dizman, Gülçin, Gökhan Metan, Pınar Zarakolu, Elif Seren Tanrıverdi, Gülşen Hazırolan, Hanife Aytaç Ak, Dilek Kılınçarslan, Mertcan Uzun, Başak Çelik Kavaklılar, Zafer Arık, and et al. 2023. "Cessation of Rectal Screening for Vancomycin-Resistant Enterococci: Experience from a Tertiary Care Hospital from Türkiye" Healthcare 11, no. 19: 2641. https://doi.org/10.3390/healthcare11192641
APA StyleTelli Dizman, G., Metan, G., Zarakolu, P., Tanrıverdi, E. S., Hazırolan, G., Aytaç Ak, H., Kılınçarslan, D., Uzun, M., Çelik Kavaklılar, B., Arık, Z., Otlu, B., & Ünal, S. (2023). Cessation of Rectal Screening for Vancomycin-Resistant Enterococci: Experience from a Tertiary Care Hospital from Türkiye. Healthcare, 11(19), 2641. https://doi.org/10.3390/healthcare11192641







