The Influence of Food Regimes on Oxidative Stress: A Permutation-Based Approach Using the NPC Test
Abstract
1. Introduction
- -
- Section 1.1 presents the topic of oxidative stress in general and the possible links with dietary habits;
- -
- Section 1.2 refers to the scientific background, focusing on the main contributions related to oxidative stress parameters;
- -
- Section 2 describes the materials and methods; in detail, the sample is described, and the non-parametric combination (NPC) methodology is defined, with an overview of scientific papers that illustrate the different fields in which this methodology can be applied;
- -
- Section 3 illustrates the results of the application of NPC methodology; more specifically, we compared two dietary regimens (omnivorous vs. semi-vegetarian), stratifying patients according to some confounding variables, such as sex, age, BMI, TSH, FT4, diagnosis of Hashimoto’s thyroiditis, and physical activity;
- -
- Section 4 presents a discussion of the obtained results;
- -
- Section 5 describes the conclusions and possible future developments of the covered topic.
1.1. Oxidative Stress: Generality and Relationship with the Modern Lifestyle
1.2. Scientific Background
- -
- -
- Markers of oxidative stress, such as advanced glycation end products (AGEs) and their receptor (RAGE), advanced oxidation protein products (AOPPs), derived reactive oxygen (d-ROMS), malondialdehyde (MDA), oxidized-low density lipoprotein (ox-LDL), total oxidant status (TOS), and oxidative stress index (OSI), to mention a few [3,14,15,19,20,21,22,23,24,25,26].
2. Materials and Methods
2.1. NPC: Methodological Issue
- Property of similarity, as whatever the distribution underlying the data, the probability of rejection under the null hypothesis is invariant with respect to the set of data actually observed, and this is whatever the method of disclosure of the data;
- For any level of significance α, for any underlying distribution, and for all possible observed datasets, if under the alternative, the distribution dominates the null or is dominated by the null, then there is an undistorted conditional test, in the sense that the probability of rejection of the null hypothesis is always lower than the significance level α.
- -
- In the first step, the decomposition of a multivariate hypothesis system into one-dimensional sub-hypotheses occurs; for each sub-hypothesis, a partial permutation test is applied, enabling the examination of the marginal contribution of every variable in comparison between the groups (partial tests) [41,42];
- -
- In the second step, the non-parametric combination of partial tests allows us to obtain a second-order test called the multivariate hypothesis (combined tests) [43].
2.2. The Data
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 1993, 26, 351–357. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrida, G.; Casciaro, M.; Barbalace, M.C.; Hrelia, S.; Trimarchi, F.; Cannavò, S.; Gangemi, S. Oxidative stress as a key feature of autoimmune thyroiditis: An update. Minerva Endocrinol. 2020, 45, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; D’ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Procaccini, C.; Carbone, F.; Galgani, M.; La Rocca, C.; De Rosa, V.; Cassano, S.; Matarese, G. Obesity and susceptibility to autoimmune diseases. Expert Rev. Clin. Immunol. 2011, 7, 287–294. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Geagea, A.G.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Richards, J.L.; Yap, Y.-A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Hirshberg, J.; Lyle, D.; Freij, J.B.; Caturegli, P. Reactive oxygen species in organ-specific autoimmunity. Autoimmun. Highlights 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative Stress and Advanced Glycation End Products in Hashimoto’s Thyroiditis. Thyroid 2016, 26, 504–511. [Google Scholar] [CrossRef]
- Ates, I.; Yilmaz, F.M.; Altay, M.; Yilmaz, N.; Berker, D.; Güler, S. The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur. J. Endocrinol. 2015, 173, 791–799. [Google Scholar] [CrossRef]
- Ahsan, H.; Ali, A.; Ali, R. Oxygen free radicals and systemic autoimmunity. Clin. Exp. Immunol. 2003, 131, 398–404. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Autoimmunity and oxidatively modified autoantigens. Autoimmun. Rev. 2008, 7, 567–573. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Baser, H.; Can, U.; Baser, S.; Yerlikaya, F.H.; Aslan, U.; Hidayetoglu, B.T. Assesment of oxidative status and its association with thyroid autoantibodies in patients with euthyroid autoimmune thyroiditis. Endocrine 2015, 48, 916–923. [Google Scholar] [CrossRef]
- Reddy, V.; Gouroju, S.; Suchitra, M.; Suresh, V.; Sachan, A.; Rao, P.S.; Bitla, A. Antioxidant defense in overt and subclinical hypothyroidism. Horm. Metab. Res. 2013, 45, 754–758. [Google Scholar] [CrossRef]
- Korkmaz, H.; Tabur, S.; Ozkaya, M.; Oguz, E.; Elboga, U.; Aksoy, N.; Akarsu, E. Paraoxonase and arylesterase levels in autoimmune thyroid diseases. Redox Rep. 2016, 21, 227–231. [Google Scholar] [CrossRef][Green Version]
- Ruggeri, R.M.; Cristani, M.; Vicchio, T.M.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; Gangemi, S. Increased serum interleukin-37 (IL-37) levels correlate with oxidative stress parameters in Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2019, 42, 199–205. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Barbalace, M.C.; Cristani, M.T.; Alibrandi, A.; Giovinazzo, S.; Giuffrida, G.; Trimarchi, F.; Cannavò, S.; Campennì, A. Serum levels of advanced glycation end products (AGEs) are increased and their soluble receptor (sRAGE) reduced in Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2020, 43, 1337–1342. [Google Scholar] [CrossRef]
- Rostami, R.; Aghasi, M.; Mohammadi, A.; Nourooz-Zadeh, J. Enhanced oxidative stress in Hashimoto’s thyroiditis: Inter-relationships to biomarkers of thyroid function. Clin. Biochem. 2013, 46, 308–312. [Google Scholar] [CrossRef]
- Ates, I.; Arikan, M.F.; Altay, M.; Yilmaz, F.M.; Yilmaz, N.; Berker, D.; Guler, S. The effect of oxidative stress on the progression of Hashimoto’s thyroiditis. Arch. Physiol. Biochem. 2018, 124, 351–356. [Google Scholar] [CrossRef]
- Giannakou, M.; Saltiki, K.; Mantzou, E.; Loukari, E.; Philippou, G.; Terzidis, K.; Lili, K.; Stavrianos, C.; Kyprianou, M.; Alevizaki, M. RAGEpolymorphisms and oxidative stress levels in Hashimoto’s thyroiditis. Eur. J. Clin. Investig. 2017, 47, 341–347. [Google Scholar] [CrossRef]
- Prasad, C.; Davis, K.E.; Imrhan, V.; Juma, S.; Vijayagopal, P. Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification. Am. J. Lifestyle Med. 2017, 13, 384–404. [Google Scholar] [CrossRef]
- Pesarin, F. Permutation Testing of Multidimensional Hypotheses; Cleup Editrice: Padova, Italy, 1997. [Google Scholar]
- Pesarin, F. Multivariate Permutation Tests, with Applications in Biostatistics; Wiley: England, UK, 2001. [Google Scholar]
- Pesarin, F.; Salmaso, L. Permutation Tests for Complex Data: Theory, Applications and Software; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Pesarin, F. Extending Permutation Conditional Inference to Unconditional Ones Statistical Methods & Applications, Società Italiana di Statistica; Springer: Berlin/Heidelberg, Germany, 2002; Volume 11, pp. 161–173. [Google Scholar]
- Ludbrook, J.; Dudley, H. Why Permutation Tests are Superior to t and F Tests in Biomedical Research. Am. Stat. 1998, 52, 127–132. [Google Scholar] [CrossRef]
- Lehmann, E.L.; Romano, J.P. Testing Statistical Hypotheses; Springer Science & Business Media: Philadelphia, PA, USA, 2006. [Google Scholar]
- Arboretti, R.; Ceccato, R.; Salmaso, L. Permutation testing for goodness-of-fit and stochastic ordering with multivariate mixed variables. J. Stat. Comput. Simul. 2021, 91, 876–896. [Google Scholar] [CrossRef]
- Giancristofaro, R.A.; Brombin, C. Overview of NonParametric Combination-based permutation tests for Multivariate multi-sample problems. Statistica 2014, 74, 233–246. [Google Scholar] [CrossRef]
- Arboretti, R.; Bathke, A.C.; Carrozzo, E.; Pesarin, F.; Salmaso, L. Multivariate permutation tests for two sample testing in presence of nondetects with application to microarray data. Stat. Methods Med. Res. 2020, 29, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Brombin, C.; Salmaso, L. Multi-aspect permutation tests in shape analysis with small sample size. Comput. Stat. Data Anal. 2009, 53, 3921–3931. [Google Scholar] [CrossRef]
- Bonnini, S.; Corain, L.; Marozzi, M.; Salmaso, L. Nonparametric hypothesis testing: Rank and permutation methods with applications in R. John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Corain, L.; Salmaso, L. Improving power of multivariate combination-based permutation tests. Stat. Comput. 2015, 25, 203–214. [Google Scholar] [CrossRef]
- Corain, L.; Salmaso, L. Multivariate and multistrata nonparametric tests: The nonparametric combination method. J. Mod. Appl. Stat. Methods 2004, 3, 443–461. [Google Scholar] [CrossRef]
- Finos, L.; Salmaso, L. Weighted methods controlling the multiplicity when the number of variables is much higher than the number of observations. J. Nonparametric Stat. 2006, 18, 245–261. [Google Scholar] [CrossRef]
- Folks, J.L. 6 Combination of Independent Tests; Handbook of Statistics: North-Holland, Amsterdam, 1984; Volume 4, pp. 113–121. [Google Scholar] [CrossRef]
- Caughey, D.; Dafoe, A.; Seawright, J. Nonparametric combination (NPC): A framework for testing elaborate Theories. J. Politics 2017, 79, 688–701. [Google Scholar] [CrossRef]
- Pesarin, F. On a nonparametric combination method for dependent permutation tests with applications. Psychother. Psychosom. 1990, 54, 172–179. [Google Scholar] [CrossRef]
- Pesarin, F. A resampling procedure for nonparametric combination of several dependent tests. J. Ital. Stat. Soc. 1992, 1, 87–101. [Google Scholar] [CrossRef]
- Brombin, C.; Midena, E.; Salmaso, L. Robust non-parametric tests for complex-repeated measures problems in ophthalmology. Stat. Methods Med. Res. 2013, 22, 643–660. [Google Scholar] [CrossRef]
- Brombin, C.; Di Serio, C. Evaluating treatment effect within a multivariate stochastic ordering framework: NPC methodology applied to a study on multiple sclerosis. Stat. Methods Med. Res. 2016, 25, 366–384. [Google Scholar] [CrossRef]
- Giacalone, M.; Agata, Z.; Cozzucoli, P.C.; Alibrandi, A. Bonferroni-Holm and permutation tests to compare health data: Methodological and applicative issues. BMC Med. Res. Methodol. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Zirilli, A.; Alibrandi, A. A permutation solution to compare two hepatocellular carcinoma markers. JP J. Biostat. 2011, 5, 109–119. [Google Scholar] [CrossRef]
- Zirilli, A.; Alibrandi, A. The alteration of t,t-muconic acid and s-phenilmercapturic acid levels due to benzene exposure: An application of NPC test. JP J. Biostat. 2012, 7, 91–104. [Google Scholar]
- Arboretti, R.; Fontana, R.; Pesarin, F.; Salmaso, L. Nonparametric combination tests for comparing two survival curves with informative and non-informative censoring. Stat. Methods Med. Res. 2018, 27, 3739–3769. [Google Scholar] [CrossRef]
- Winkler, A.M.; Webster, M.A.; Brooks, J.C.; Tracey, I.; Smith, S.M.; Nichols, T.E. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp. 2016, 37, 1486–1511. [Google Scholar] [CrossRef]
- Alfieri, R.; Bonnini, S.; Brombin, C.; Castoro, C.; Salmaso, L. Iterated combination-based paired permutation tests to determine shape effects of chemotherapy in patients with esophageal cancer. Stat. Methods Med. Res. 2016, 25, 598–614. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P.; Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; et al. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F. Iodine nutrition optimization: Are there risks for thyroid autoimmunity? J. Endocrinol. Investig. 2021, 44, 1827–1835. [Google Scholar] [CrossRef]
- Mansournia, N.; Mansournia, M.A.; Saeedi, S.; Dehghan, J. The association between serum 25OHD levels and hypothyroid Hash-imoto’s thyroiditis. J. Endocrinol. Investig. 2014, 37, 473–476. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Ihnatowicz, P.; Wątor, P.; Drywień, M.E. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2021, 28, 558–568. [Google Scholar] [CrossRef]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef]
- Giannakou, M.; Saltiki, K.; Mantzou, E.; Loukari, E.; Philippou, G.; Terzidis, K.; Stavrianos, C.; Kyprianou, M.; Psaltopoulou, T.; Karatzi, K.; et al. The effect of obesity and dietary habits on oxidative stress in Hashimoto’s thyroiditis. Endocr. Connect. 2018, 7, 990–997. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, e4556. [Google Scholar] [CrossRef]
- Bellastella, G.; Scappaticcio, L.; Caiazzo, F.; Tomasuolo, M.; Carotenuto, R.; Caputo, M.; Arena, S.; Caruso, P.; Maiorino, M.I.; Esposito, K. Mediterranean Diet and Thyroid: An Interesting Alliance. Nutrients 2022, 14, 4130. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Saniabadi, A.R. Review article: Diet and inflammatory bowel disease--epidemiology and treatment. Aliment Pharmacol. Ther. 2009, 30, 99–112. [Google Scholar] [CrossRef]
- Willebrand, R.; Kleinewietfeld, M. The role of salt for immune cell function and disease. Immunology 2018, 154, 346–353. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 5459. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord.-Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Godny, L.; Reshef, L.; Fischler, T.S.; Elial-Fatal, S.; Pfeffer-Gik, T.; Raykhel, B.; Rabinowitz, K.; Levi-Barda, A.; Perets, T.; Barkan, R.; et al. Increasing adherence to the Mediterranean diet and lifestyle is associated with reduced fecal calprotectin and intra-individual changes in microbial composition of healthy subjects. Gut Microbes 2022, 14, 2120749. [Google Scholar] [CrossRef]
- Phan, C.; Touvier, M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Wolkenstein, P.; Chosidow, O.; Ezzedine, K.; Sbidian, E. Association Between Mediterranean Anti-inflammatory Dietary Profile and Severity of Psoriasis: Results From the NutriNet-Santé Cohort. JAMA Dermatol. 2018, 154, 1017–1024. [Google Scholar] [CrossRef]
- Öztürk, Y.E.; Helvaci, E.M.; Kaya, P.S.; Terzi, M. Is Mediterranean diet associated with multiple sclerosis related symptoms and fatigue severity? Nutr. Neurosci. 2023, 26, 228–234. [Google Scholar] [CrossRef]
- de Toda, I.M.; González-Sánchez, M.; Cerro, E.D.-D.; Valera, G.; Carracedo, J.; Guerra-Pérez, N. Sex differences in markers of oxidation and inflammation. Implications for ageing. Mech. Ageing Dev. 2023, 211, 111797. [Google Scholar] [CrossRef]
- D’Addezio, L.; Capriotti, M.; Pettinelli, A.; Turrini, A. L’indagine Nazionale sui Consumi Alimentari in Italia INRAN-SCAI 2005-06. In Parte C: I Risultati dei Questionari sulle Abitudini Alimentari; Osservatorio Consumi Alimentari, INRAN: Rome, Italy, 2011. [Google Scholar]
- Istituto Nazionale di Statistica. 14° Censimento Generale della Popolazione e delle Abitazioni del 2001; ISTAT: Rome, Italy, 2001. [Google Scholar]
- Leclercq, C.; Berardi, D.; Sorbillo, M.R.; Lambe, J. Intake of saccharin, aspartame, acesulfame K and cyclamate in Italian teenagers: Present levels and projections. Food Addit Contam 1999, 16, 99–109. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A.; INRAN-SCAI 2005-06 Study Group. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef]
- Leclercq, C.; Piccinelli, R.; Arcella, D.; Le Donne, C. Food consumption and nutrient intake in a sample of Italian secondary school students. Results from the INRAN-RM-2001 food survey. Int. J. Food. Sci. Nutr. 2004, 55, 265–277. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Holtermann, A.; Marott, J.L.; Gyntelberg, F.; Søgaard, K.; Suadicani, P.; Mortensen, O.S.; Prescott, E.; Schnohr, P. Occupational and leisure time physical activity: Risk of all-cause mortality and myocardial infarction in the Copenhagen City Heart Study. A prospective cohort study. BMJ Open 2012, 2, e000556. [Google Scholar] [CrossRef]
- Holtermann, A.; Schnohr, P.; Nordestgaard, B.G.; Marott, J.L. The physical activity paradox in cardiovascular disease and all-cause mortality: The contemporary Copenhagen General Population Study with 104 046 adults. Eur. Heart J. 2021, 42, 1499–1511. [Google Scholar] [CrossRef]
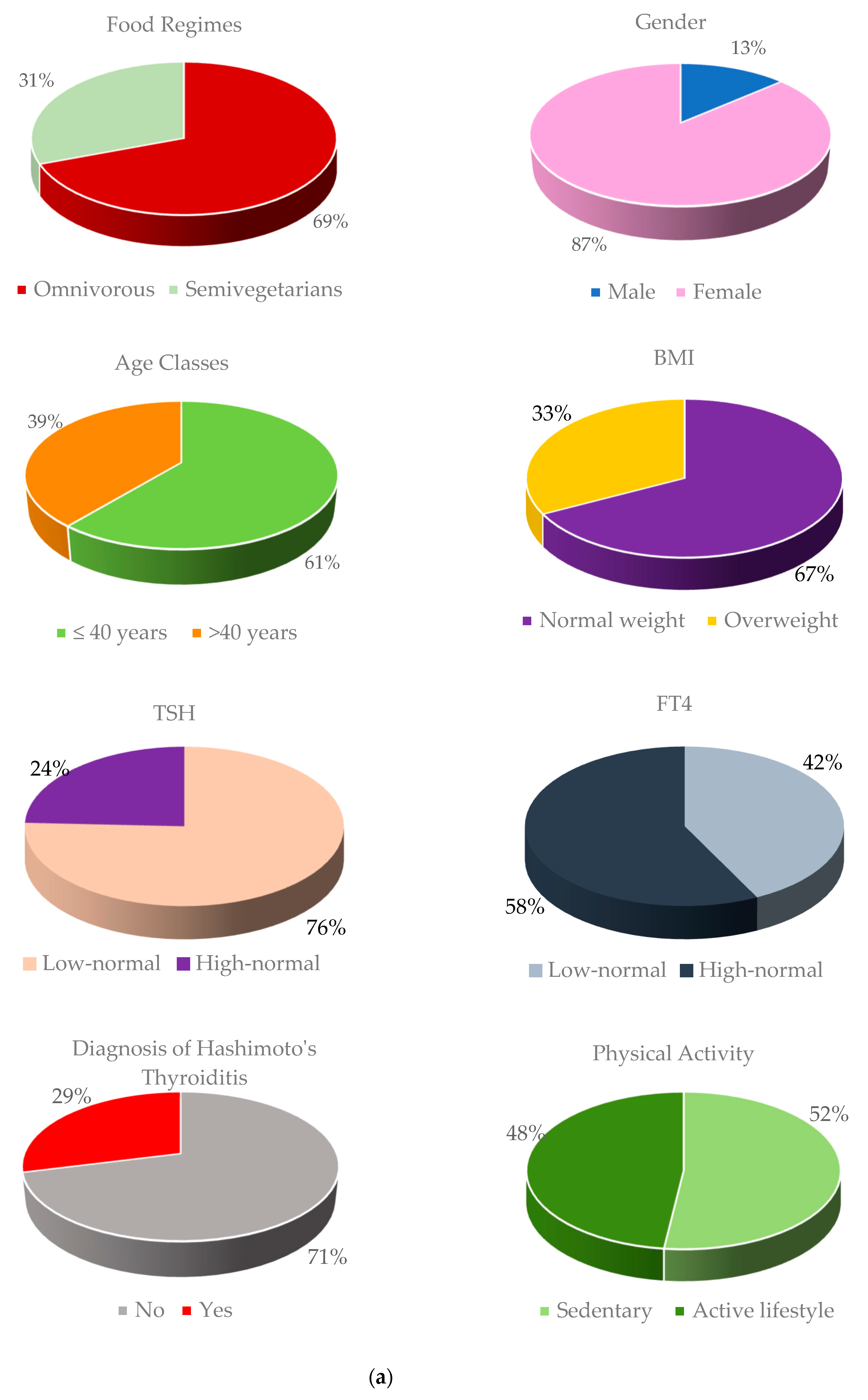







| Stratum 1: Male | Stratum 2: Female | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.92 ± 0.86 | 4.74 ± 0.42 | 0.535 | 4.87 ± 0.96 | 4.86 ± 0.74 | 0.960 |
| 5.32 (4.22–5.67) | 4.59 (4.32–4.90) | 4.77 (4.17–5.68) | 4.90(4.42–4.51) | |||
| GPx | 0.58 ± 0.01 | 0.62 ± 0.01 | 0.068 | 0.58 ± 0.10 | 0.63 ± 0.01 | 0.016 |
| 0.58 (0.54–0.64) | 0.64 (0.58–0.70) | 0.59 (0.52–0.65) | 0.61 (0.56–0.66) | |||
| TRxR | 2.17 ± 0.87 | 2.55 ± 0.48 | 0.202 | 1.87 ± 0.71 | 2.33 ± 0.99 | 0.001 |
| 1.97 (1.45–2.41) | 2.72 (2.08–2.91) | 1.86 (1.34–2.22) | 2.25 (1.85–2.76) | |||
| GR | 70.30 ± 21.95 | 63.84 ± 19.53 | 0.459 | 70.29 ± 16.68 | 69.62 ± 12.15 | 0.800 |
| 72 (57.6–84.7) | 64.39 (48.06–74.93) | 71.52 (65.12–74.93) | 70.39 (63.51–78.61) | |||
| AGEs | 152.5 ± 50.04 | 126.3 ± 30.82 | 0.104 | 126.5 ± 51.01 | 122.4 ± 41.66 | 0.606 |
| 154.3 (111.6–192.2) | 127.4 (114.8–151.1) | 125.2 (107.2–135.8) | 123.4 (91.6–134.4) | |||
| AOPPs | 1.18 ± 0.32 | 1.10 ± 0.01 | 0.455 | 1.06 ± 0.25 | 1.08 ± 0.28 | 0.640 |
| 1.17 (1.02–1.36) | 1.08 (1.06–1.09) | 1.03 (0.93–1.07) | 1.07 (0.95–1.13) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.380 | 0.132 | |||||
| Stratum 1: Age ≤ 40 years | Stratum 2: Age > 40 Years | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.80 ± 1.01 | 4.77 ± 0.69 | 0.853 | 5.01 ± 0.83 | 4.91 ± 0.70 | 0.576 |
| 4.74 (4.16–5.49) | 4.78 (4.38–5.31) | 5.24 (4.43–5.78) | 4.90 (4.43–5.51) | |||
| GPx | 0.57 ± 0.11 | 0.62 ± 0.01 | 0.026 | 0.60 ± 0.01 | 0.65 ± 0.01 | 0.030 |
| 0.57 (0.53–0.64) | 0.61 (0.56–0.64) | 0.59 (0.52–0.64) | 0.64 (0.59–0.74) | |||
| TRxR | 1.91 ± 0.78 | 2.40 ± 0.93 | 0.005 | 1.88 ± 0.65 | 2.34 ± 0.93 | 0.012 |
| 1.88 (1.30–2.19) | 2.59 (1.95–2.91) | 1.90 (1.39–2.44) | 2.21 (1.71–2.76) | |||
| GR | 72.31 ± 15.06 | 71.53 ± 10.69 | 0.789 | 66.32 ± 20.44 | 65.76 ± 15.84 | 0.890 |
| 71.52 (67.26–75.72) | 70.63 (66.52–77.61) | 71.52 (53.99–74.93) | 70.39 (50.49–81.58) | |||
| AGEs | 118.8 ± 43.02 | 119.9 ± 38.89 | 0.899 | 149.9 ± 59.60 | 126.2 ± 36.89 | 0.042 |
| 114.8 (99.75–127.4) | 120.7 (81.9–127.4) | 145.8 (118.1–186.1) | 127.4 (114.8–186.6) | |||
| AOPPs | 1.12 ± 0.34 | 1.03 ± 0.23 | 0.096 | 1.15 ± 0.30 | 1.05 ± 0.14 | 0.063 |
| 1.11 (0.95–1.17) | 1.03 (0.91–1.07) | 1.14 (1.03–1.43) | 1.07 (1.03–1.26) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.250 | 0.009 | |||||
| Stratum 1: Normal Weight | Stratum 2: Overweight | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 5.04 ± 0.96 | 4.92 ± 0.65 | 0.456 | 4.65 ± 0.91 | 4.22 ± 0.70 | 0.227 |
| 5.01 (4.37–5.75) | 4.90 (4.43–5.47) | 4.62 (3.84–5.42) | 4.33 (3.23–4.85) | |||
| GPx | 0.57 ± 0.01 | 0.62 ± 0.01 | 0.006 | 0.63 ± 0.01 | 0.67 ± 0.01 | 0.302 |
| 0.59 (0.52–0.64) | 0.61 (0.56–0.65) | 0.62 (0.58–0.69) | 0.65 (0.64–0.75) | |||
| TRxR | 1.88 ± 0.81 | 2.40 ± 0.89 | 0.001 | 1.90 ± 0.62 | 2.15 ± 1.24 | 0.381 |
| 1.79 (1.29–2.31) | 2.62 (1.90–2.88) | 1.91 (1.38–2.36) | 2.08 (0.71–3.72) | |||
| GR | 72.90 ± 16.65 | 67.94 ± 13.31 | 0.071 | 66.64 ± 17.52 | 73.50 ± 17.19 | 0.334 |
| 71.52 (66.53–76.73) | 69.39 (62.41–77.27) | 71.52 (58.68–74.93) | 75.43 (50.49–91.87) | |||
| AGEs | 123.9 ± 35.08 | 126.1 ± 38.17 | 0.735 | 136.9 ± 67.53 | 100.2 ± 25.53 | 0.140 |
| 124.1 (119.4–127.4) | 125.2 (109.6–135.3) | 137.4(105.3–162.3) | 91.12 (78.28–127–4) | |||
| AOPPs | 1.06 ± 0.20 | 0.99 ± 0.16 | 0.047 | 1.27 ± 0.52 | 1.17 ± 0.33 | 0.495 |
| 1.07 (1.06–1.09) | 1.03 (0.88–1.07) | 1.28 (0.82–2.02) | 1.16 (1.02–1.34) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.005 | 0.336 | |||||
| Stratum 1: Low-Normal | Stratum 2: High-Normal | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.84 ± 1.03 | 4.49 ± 0.75 | 0.829 | 5.00 ± 0.68 | 4.78 ± 0.39 | 0.352 |
| 4.77 (4.28–5.65) | 4.50 (4.34–5.46) | 4.98 (4.08–5.79) | 4.85 (4.61–5.32) | |||
| GPx | 0.59 ± 0.01 | 0.63 ± 0.01 | 0.044 | 0.59 ± 0.01 | 0.62 ± 0.01 | 0.047 |
| 0.58 (0.54–0.64) | 0.61 (0.56–0.68) | 0.55 (0.51–0.66) | 0.61 (0.56–0.67) | |||
| TRxR | 1.89 ± 0.77 | 2.37 ± 0.97 | 0.005 | 1.92 ± 0.61 | 2.47 ± 0.77 | 0.028 |
| 1.89 (1.37–2.11) | 2.23 (1.85–2.72) | 1.84 (1.36–2.51) | 2.93 (2.41–3.18) | |||
| GR | 71.46 ± 16.99 | 71.40 ± 44.20 | 0.984 | 66.66 ± 17.63 | 60.32 ± 14.85 | 0.334 |
| 71.52 (64.09–74.93) | 70.39 (63.88–78.28) | 67.52 (64.79–74.93) | 61.0 (53.7–69.9) | |||
| AGEs | 125.8 ± 51.04 | 128.7 ± 37.62 | 0.776 | 140.1 ± 51.64 | 105.5 ± 34.57 | 0.066 |
| 126.4 (108.2–148.2) | 127.8 (116.1–128.4) | 127.4 (78.28–138.18) | 114.8 (111.2–127.4) | |||
| AOPPs | 1.12 ± 0.25 | 1.03 ± 0.22 | 0.076 | 1.19 ± 0.34 | 0.96 ± 0.18 | 0.052 |
| 1.11 (1.04–1.15) | 1.03 (0.92–1.07) | 1.17 (0.82–1.29) | 1.03 (0.98–1.07) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.053 | 0.059 | |||||
| Stratum 1: Low-Normal | Stratum 2: High-Normal | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.69 ± 0.96 | 4.99 ± 0.66 | 0.120 | 4.98 ± 0.94 | 4.66 ± 0.69 | 0.094 |
| 4.74 (3.85–5.41) | 5.07 (4.37–5.51) | 4.93 (4.28–5.78) | 4.71 (4.43–5.23) | |||
| GPx | 0.58 ± 0.01 | 0.63 ± 0.01 | 0.012 | 0.59 ± 0.11 | 0.63 ± 0.11 | 0.125 |
| 0.59 (0.53–0.63) | 0.61 (0.59–0.65) | 0.60 (0.56–0.70) | 0.64 (0.52–0.66) | |||
| TRxR | 1.95 ± 0.74 | 2.39 ± 0.93 | 0.019 | 1.93 ± 0.79 | 2.32 ± 0.94 | 0.056 |
| 1.91 (1.40–2.32) | 2.25 (1.85–2.72) | 1.81 (1.34–2.34) | 2.69 (2.08–2.94) | |||
| GR | 68.89 ± 15.93 | 68.97 ± 12.67 | 0.982 | 71.12 ± 18.00 | 78.12 ± 15.15 | 0.422 |
| 71.52 (64.86–72.53) | 70.39 (62.41–78.61) | 71.52 (63.49–77.06) | 76.07 (56.87–77.27) | |||
| AGEs | 120.4 ± 39.13 | 124.4 ± 27.86 | 0.609 | 134.7 ± 57.00 | 121.5 ± 47.27 | 0.266 |
| 119.5 (101.1–127.4) | 124.7 (120.3–128.2) | 127.4 (114.9–149.8) | 122.4 (77.99–127–4) | |||
| AOPPs | 1.11 ± 0.18 | 1.01 ± 0.19 | 0.031 | 1.10 ± 0.29 | 1.06 ± 0.33 | 0.451 |
| 1.10 (0.96–1.19) | 1.03 (0.88–1.07) | 1.08 (0.96–1.13) | 1.07 (0.82–1.27) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.037 | 0.130 | |||||
| Stratum 1: No HT Diagnosis | Stratum 2: Yes HT Diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.82 ± 1.08 | 4.87 ± 0.71 | 0.782 | 4.97 ± 0.71 | 4.56 ± 0.28 | 0.217 |
| 4.78 (3.87–5.68) | 4.90 (3.34–5.46) | 4.88 (4.42–5.60) | (4.43 (4.43–4.75) | |||
| GPx | 0.58 ± 0.01 | 0.61 ± 0.01 | 0.036 | 0.64 ± 0.01 | 0.65 ± 0.11 | 0.851 |
| 0.59 (0.52–0.65) | 0.61 (0.57–0.64) | 0.64 (0.56–0.74) | 0.64 (0.53–0.74) | |||
| TRxR | 1.96 ± 0.77 | 2.42 ± 0.94 | 0.003 | 1.80 ± 0.67 | 1.85 ± 0.52 | 0.880 |
| 1.91 (1.34–2.89) | 2.66 (1.87–2.91) | 1.82 (1.39–2.08) | 1.90 (1.51–2.08) | |||
| GR | 71.51 ± 16.95 | 68.19 ± 14.25 | 0.219 | 68.30 ± 17.66 | 73.00 ± 4.32 | 0.562 |
| 71.53 (65.01–74.02) | 69.39 (59.60–78.61) | 74.93 (58.29–74.93) | 73.93 (70.11–74.93) | |||
| AGEs | 114.7 ± 42.4 | 124.3 ± 37.64 | 0.168 | 153.0 ± 56.02 | 109.5 ± 39.97 | 0.094 |
| 114.9 (94.23–127.4) | 124.2 (114.7–127.4) | 147.3 (127.4–187.6) | 110.7(82.73–117.8) | |||
| AOPPs | 1.09 ± 0.25 | 1.04 ± 0.27 | 0.269 | 1.12 ± 0.28 | 1.01 ± 0.15 | 0.321 |
| 1.07 (0.94–1.15) | 1.03 (0.92–1.07) | 1.07 (1.01–1.20) | 1.02 (0.90–1.08) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.026 | 0.614 | |||||
| Stratum 1: Sedentary | Stratum 2: Active Lifestyle | |||||
|---|---|---|---|---|---|---|
| Variables | Omniv. | Semi-Veg. | p-Value | Omniv. | Semi-Veg. | p-Value |
| SOD | 4.87 ± 0.95 | 4.71 ± 0.48 | 0.451 | 4.88 ± 0.96 | 4.91 ± 0.78 | 0.896 |
| 4.88 (4.16–5.74) | 4.67 (4.43–4.84) | 4.78 (4.22–5.49) | 4.95 (4.31–5.51) | |||
| GPx | 0.58 ± 0.01 | 0.63 ± 0.01 | 0.041 | 0.58 ± 0.10 | 0.63 ± 0.11 | 0.043 |
| 0.59 (0.54–0.63) | 0.61 (0.56–0.66) | 0.59 (0.52–0.66) | 0.61 (0.56–0.68) | |||
| TRxR | 1.96 ± 0.66 | 2.39 ± 0.93 | 0.018 | 1.81 ± 0.83 | 2.36 ± 0.94 | 0.003 |
| 1.90 (1.52–2.46) | 2.33 (1.89–2.72) | 1.71 (1.31–2.02) | 2.60 (1.87–2.91) | |||
| GR | 68.92 ± 18.17 | 68.61 ± 14.30 | 0.941 | 72.31 ± 15.69 | 68.56 ± 13.64 | 0.225 |
| 71.53 (62.02–74.93) | 70.39 (64.59–76.77) | 71.53 (66.83–75.72) | 70.39 (58.66–80.50) | |||
| AGEs | 126.0 ± 47.18 | 116.9 ± 25.34 | 0.398 | 134.2 ± 57.11 | 126.3 ± 42.74 | 0.456 |
| 127.3 (101.5–149.5) | 126.6 (101.1–127.6) | 129.1 (81.78–131.8) | 127.1 (114.8–135.4) | |||
| AOPPs | 1.06 ± 0.24 | 1.01 ± 0.14 | 0.426 | 1.09 ± 0.30 | 1.12 ± 0.30 | 0.635 |
| 1.03 (0.92–1.07) | 1.02 (0.91–1.22) | 1.03 (0.98–1.07) | 1.11 (0.96–1.18) | |||
| Combined p-value | ↓ | ↓ | ||||
| 0.479 | 0.112 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirilli, A.; Ruggeri, R.M.; Barbalace, M.C.; Hrelia, S.; Giovanella, L.; Campennì, A.; Cannavò, S.; Alibrandi, A. The Influence of Food Regimes on Oxidative Stress: A Permutation-Based Approach Using the NPC Test. Healthcare 2023, 11, 2263. https://doi.org/10.3390/healthcare11162263
Zirilli A, Ruggeri RM, Barbalace MC, Hrelia S, Giovanella L, Campennì A, Cannavò S, Alibrandi A. The Influence of Food Regimes on Oxidative Stress: A Permutation-Based Approach Using the NPC Test. Healthcare. 2023; 11(16):2263. https://doi.org/10.3390/healthcare11162263
Chicago/Turabian StyleZirilli, Agata, Rosaria Maddalena Ruggeri, Maria Cristina Barbalace, Silvana Hrelia, Luca Giovanella, Alfredo Campennì, Salvatore Cannavò, and Angela Alibrandi. 2023. "The Influence of Food Regimes on Oxidative Stress: A Permutation-Based Approach Using the NPC Test" Healthcare 11, no. 16: 2263. https://doi.org/10.3390/healthcare11162263
APA StyleZirilli, A., Ruggeri, R. M., Barbalace, M. C., Hrelia, S., Giovanella, L., Campennì, A., Cannavò, S., & Alibrandi, A. (2023). The Influence of Food Regimes on Oxidative Stress: A Permutation-Based Approach Using the NPC Test. Healthcare, 11(16), 2263. https://doi.org/10.3390/healthcare11162263






