A Systematic Review to Manage Avoidant/Restrictive Food Intake Disorders in Pediatric Gastroenterological Practice
Abstract
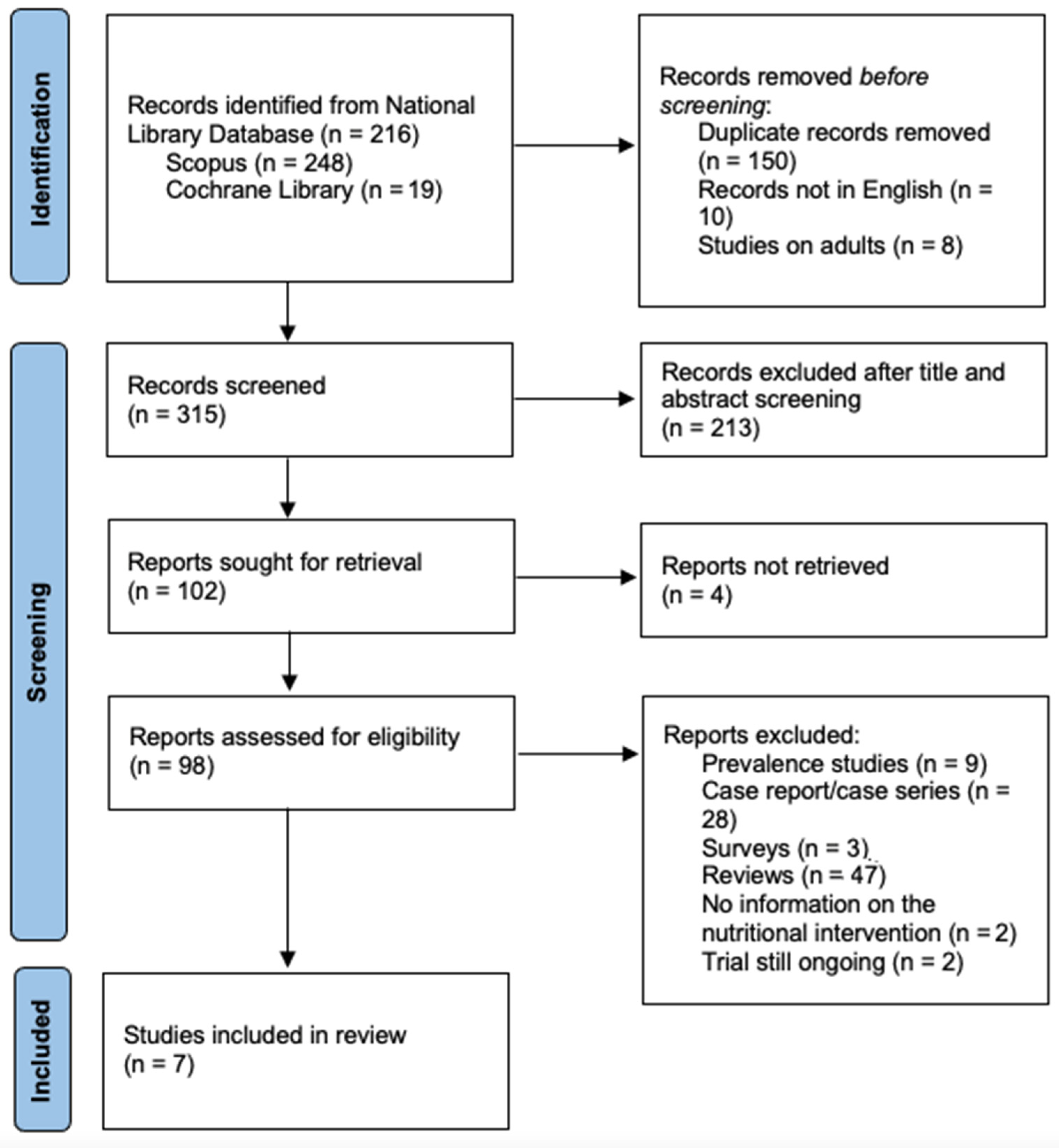
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Study Selection
2.3. Analyses and Endpoints
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Characteristics of Nutritional Interventions
3.3. Refeeding-Related Adverse Events
3.4. Co-Interventions and Criteria for Discharge
4. Discussion
- ≤75% of IBW for age and sex;
- Dehydration or electrolyte disturbance (hypokalemia, hyponatremia, hypophosphatemia);
- Electrocardiogram abnormalities (e.g., prolonged QTc or severe bradycardia);
- Severe bradycardia (heart rate < 50 beats/min daytime); hypotension (<90/45 mm Hg); hypothermia (body temperature < 35.6 °C); orthostatic increase in pulse (>20 beats/min) or decrease in blood pressure (>20 mm Hg systolic or >10 mm Hg diastolic);
- Arrested growth and development;
- Failure of outpatient treatment;
- Acute food refusal;
- Uncontrollable bingeing and purging;
- Acute medical complications of malnutrition (e.g., syncope, seizures, cardiac failure, pancreatitis, and so forth);
- Comorbid psychiatric or medical condition that prohibits or limits appropriate outpatient treatment (e.g., severe depression, suicidal ideation, obsessive-compulsive disorder, type 1 diabetes mellitus).
5. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Iron-Segev, S.; Best, D.; Arad-Rubinstein, S.; Efron, M.; Serur, Y.; Dickstein, H.; Stein, D. Feeding, Eating, and Emotional Disturbances in Children with Avoidant/Restrictive Food Intake Disorder (ARFID). Nutrients 2020, 12, 3385. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics, 11th Revision; World Health Organization: Geneva, Switzerland, 2018; Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281 (accessed on 10 May 2023).
- Goday, P.S.; Huh, S.Y.; Silverman, A.; Lukens, C.T.; Dodrill, P.; Cohen, S.S.; Delaney, A.L.; Feuling, M.B.; Noel, R.J.; Gisel, E.; et al. Pediatric Feeding Disorder: Consensus Definition and Conceptual Framework. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Salatto, A.; Riccio, M.P.; Garotti, R.; Bravaccio, C.; Spagnuolo, M.I. Pitfalls and Risks of “New Eating Disorders”: Let the Expert Speak! Nutrients 2023, 15, 1307. [Google Scholar] [CrossRef] [PubMed]
- Krom, H.; van Oers, H.A.; van der Sluijs Veer, L.; van Zundert, S.M.C.; Otten, M.G.M.; Haverman, L.; Benninga, M.A.; Kindermann, A. Health-Related Quality of Life and Distress of Parents of Children with Avoidant Restrictive Food Intake Disorder. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 115–124. [Google Scholar] [CrossRef]
- Sharp, W.G.; Stubbs, K.H. Avoidant/restrictive food intake disorder: A diagnosis at the intersection of feeding and eating disorders necessitating subtype differentiation. Int. J. Eat. Disord 2019, 52, 398–401. [Google Scholar] [CrossRef]
- Norris, M.L.; Spettigue, W.; Katzman, D.K. Update on eating disorders: Current perspectives on avoidant/restrictive food intake disorder in children and youth. Neuropsychiatr. Dis. Treat. 2016, 12, 213–218. [Google Scholar] [CrossRef]
- Murray, H.B.; Bailey, A.P.; Keshishian, A.C.; Silvernale, C.J.; Staller, K.; Eddy, K.T.; Thomas, J.J.; Kuo, B. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin. Gastroenterol. Hepatol. 2020, 18, 1995–2002.e1. [Google Scholar] [CrossRef]
- Thomas, J.J.; Lawson, E.A.; Micali, N.; Misra, M.; Deckersbach, T.; Eddy, K.T. Avoidant/Restrictive Food Intake Disorder: A Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Curr. Psychiatr. Rep. 2017, 19, 54. [Google Scholar] [CrossRef]
- Eddy, K.T.; Harshman, S.G.; Becker, K.R.; Bern, E.; Bryant-Waugh, R.; Hilbert, A.; Katzman, D.K.; Lawson, E.A.; Manzo, L.D.; Menzel, J.; et al. Radcliffe ARFID Workgroup: Toward operationalization of research diagnostic criteria and directions for the field. Int. J. Eat. Disord. 2019, 52, 361–366. [Google Scholar] [CrossRef]
- Estrem, H.H.; Park, J.; Thoyre, S.; McComish, C.; McGlothen-Bell, K. Mapping the gaps: A scoping review of research on pediatric feeding disorder. Clin. Nutr. ESPEN 2022, 48, 45–55. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 30 April 2023).
- Kurotori, I.; Shioda, K.; Abe, T.; Kato, R.; Ishikawa, S.; Suda, S. An Inpatient Observational Study: Characteristics and Outcomes of Avoidant/Restrictive Food Intake Disorder (ARFID) in Children and Adolescents in Japan. Neuropsychiatr. Dis. Treat. 2019, 15, 3313–3321. [Google Scholar] [CrossRef] [PubMed]
- Makhzoumi, S.H.; Schreyer, C.C.; Hansen, J.L.; Laddaran, L.A.; Redgrave, G.W.; Guarda, A.S. Hospital course of underweight youth with ARFID treated with a meal—Based behavioral protocol in an inpatient-partial hospitalization program for eating disorders. Int. J. Eat. Disord. 2019, 52, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Minami, K.; Tsuda, Y.; Tsujimoto, H.; Ichikawa, T.; Mizumoto, K.; Suzuki, H. Characteristics and outcomes of avoidant/restrictive food intake disorder in Japanese elementary-school students on total parenteral nutrition. Pediatr. Investig. 2021, 5, 293–298. [Google Scholar] [CrossRef]
- Strandjord, S.E.; Sieke, E.H.; Richmond, M.; Rome, E.S. Avoidant/restrictive food intake disorder: Illness and hospital course in patients hospitalized for nutritional insufficiency. J. Adolesc. Health 2015, 57, 673–678. [Google Scholar] [CrossRef]
- Peebles, R.; Lesser, A.; Park, C.C.; Heckert, K.; Timko, C.A.; Lantzouni, E.; Liebman, R.; Weaver, L. Outcomes of an inpatient medical nutritional rehabilitation protocol in children and adolescents with eating disorders. J. Eat. Disord. 2017, 5, 7. [Google Scholar] [CrossRef]
- Maginot, T.R.; Kumar, M.M.; Shiels, J.; Kaye, W.; Rhee, K.E. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa: Rady Children’s Hospital San Diego/University of California, San Diego. J. Eat. Disord. 2017, 5, 1. [Google Scholar] [CrossRef]
- Tsang, K.K.; Hayes, L.C.; Bujoreanu, S.; Samsel, C.B.; Ibeziako, P.I. Characterization Study of Patients Presenting to an Acute Care Pediatric Hospital Identified with Avoidant/Restrictive Food Intake Disorder. Hosp. Pediatr. 2020, 10, 600–607. [Google Scholar] [CrossRef]
- Society for Adolescent Health and Medicine; Golden, N.H.; Katzman, D.K.; Sawyer, S.M.; Ornstein, R.M.; Rome, E.S.; Garber, A.K.; Kohn, M.; Kreipe, R.E. Position Paper of the Society for Adolescent Health and Medicine: Medical management of restrictive eating disorders in adolescents and young adults. J. Adolesc. Health 2015, 56, 121–125. [Google Scholar]
- Yager, J.; Devlin, M.J.; Halmi, K.A.; Herzog, D.B.; Michell, J.E.; Powers, P.; Zerbe, K.J. Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am. J. Psychiatry 2006, 163, 4–54. [Google Scholar]
- American Dietetic Association. Position of the American Dietetic Association: Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J. Am. Diet. Assoc. 2006, 106, 2073–2082. [Google Scholar]
- Nitsch, A.; Knopf, E.; Manwaring, J.; Mehler, P.S. Avoidant/Restrictive Food Intake Disorder (ARFID): Its Medical Complications and Their Treatment—An Emerging Area. Curr. Pediatr. Rep. 2021, 9, 21–29. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cucinotta, U.; Romano, C. Acute Malnutrition in Children: Pathophysiology, Clinical Effects and Treatment. Nutrients 2020, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Keane-Miller, C.; Sainani, K.L.; Kapphahn, C.J. Higher Caloric Intake in Hospitalized Adolescents with Anorexia Nervosa is Associated with Reduced Length of Stay and No Increased Rate of Refeeding Syndrome. J. Adolesc. Health 2013, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.K.; Mauldin, K.; Michihata, N.; Buckelew, S.M.; Shafer, M.A.; Moscicki, A.B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized patients with anorexia nervosa. J. Adolesc. Health 2013, 53, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Madden, S.; Miskovic-Wheatley, J.; Clarke, S.; Touyz, S.; Hay, P.; Kohn, M.R. Outcomes of rapid refeeding protocol in adolescent anorexia nervosa. J. Eat. Disord. 2015, 3, 8. [Google Scholar] [CrossRef]
- Garber, A.K.; Sawyer, S.M.; Golden, N.H.; Guarda, A.S.; Katzman, D.K.; Kohn, M.R.; Le Grange, D.; Madden, S.; Whitelaw, M.; Redgrave, G.W. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int. J. Eat. Disord. 2016, 49, 293–310. [Google Scholar] [CrossRef]
- Homan, M.; Hauser, B.; Romano, C.; Tzivinikos, C.; Torroni, F.; Gottrand, F.; Hojsak, I.; Dall’Oglio, L.; Thomson, M.; Bontems, P.; et al. Percutaneous Endoscopic Gastrostomy in Children: An Update to the ESPGHAN Position Paper. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 415–426. [Google Scholar] [CrossRef]
- Findlay, S.M.; Toews, H.; Grant, C. Use of gastrostomy tubes in children and adolescents with eating disorders and related illnesses. J. Adolesc. Health 2011, 48, 625–629. [Google Scholar] [CrossRef]
- Brigham, K.S.; Manzo, L.D.; Eddy, K.T.; Thomas, J.J. Evaluation and Treatment of Avoidant/Restrictive Food Intake Disorder (ARFID) in Adolescents. Curr. Pediatr. Rep. 2018, 6, 107–113. [Google Scholar] [CrossRef]
- Lock, J.; Robinson, A.; Sadeh-Sharvit, S.; Rosania, K.; Osipov, L.; Kirz, N.; Derenne, J.; Utzinger, L. Applying family-based treatment (FBT) to three clinical presentations of avoidant/restrictive food intake disorder: Similarities and differences from FBT for anorexia nervosa. Int. J. Eat. Disord. 2019, 52, 439–446. [Google Scholar]
- Thomas, J.J.; Becker, K.R.; Kuhnle, M.C.; Jo, J.H.; Harshman, S.G.; Wons, O.B.; Keshishian, A.C.; Hauser, K.; Breithaupt, L.; Liebman, R.E.; et al. Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: Feasibility, acceptability, and proof-of-concept for children and adolescents. Int. J. Eat. Disord. 2020, 53, 1636–1646. [Google Scholar] [PubMed]
- Lock, J.; Sadeh-Sharvit, S.; L’Insalata, A. Feasibility of conducting a randomized clinical trial using family-based treatment for avoidant/restrictive food intake disorder. Int. J. Eat. Disord. 2019, 52, 746–751. [Google Scholar] [PubMed]
- Nicely, T.A.; Lane-Loney, S.; Masciulli, E.; Hollenbeak, C.S.; Ornstein, R.M. Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. J. Eat. Disord. 2014, 2, 21. [Google Scholar] [PubMed]
| References | Design and Patients | Clinical Characteristics | Nutritional Intervention | Adverse Events | Outcomes/Other Findings |
|---|---|---|---|---|---|
| Strandjord et al. (2015) [18] USA | Retrospective To compare patients with different EDs hospitalized for acute medical stabilization (n = 244) in terms of presentation, treatment response and 1-year outcomes. ARFID patients (n = 41), 85% female, mean age 16 years. | Mean %IBW on admission 78% Patients with ARFID had less weight loss, comorbidity, and bradycardia than AN patients at admission. | Initial caloric intake between 1500 and 2300 kcal/day orally. Increase of 200 kcal per day. Goal: 0.2 kg weight gain per day. Unconsumed calories: replaced with high-calorie supplement drinks. If refused orally, given via NG tube. | No patient experienced RS During refeeding n = 2 experienced hypokalemia n = 1 hypomagnesemia n = 1 hypophosphatemia | Mean increase in %IBW during hospitalization: 15% ARFID and AN patients had similar outcomes 1 year after initial admission ARFID patients required more EN and longer hospitalizations than AN |
| Maginot et al. (2017) [20] USA | Retrospective Patients with EDs hospitalized for medical rehabilitation (n = 87) ARFID patients (n = 10), 100% female, mean age 14.6 years | Mean %IBW on admission 78.7% 29% of the entire cohort was severely malnourished (<75% IBW) | Initial caloric intake between 1000 and 3000 kcal/day orally (3 meals ± 3 snacks). ~1200 kcal/day if patient severely malnourished. Then adjusted to goal: 0.15–0.3 kg weight gain/day. If refused orally, NG tube. | Up to 57% of patients experienced hypophosphatemia and up to 52% hypomagnesemia during the first 72 h after admission | Increase in %IBW during hospitalization between 5% and 6.7% A higher calorie regimen was not associated with increased risk of hypophosphatemia, hypomagnesemia or hypokalemia |
| Peebles et al. (2017) [19] USA | Retrospective Patients with EDs admitted for a first hospitalization (n = 215), 88% female, mean age 15.3 years. ARFID patients n = 9 | 84.2% of the entire cohort met criteria for severe malnutrition (<75% IBW) | Initial caloric intake between 900 and 2800 kcal/day via 7 days rotating menus. Increase of 200–400 kcal/day until goal calories. If repetitively refused orally, given via NG tube. | No patient experienced RS 14% received phosphorus supplementation for refeeding hypophosphatemia, 4% potassium supplementation and 3% magnesium supplementation. | Mean increase in %IBW during hospitalization: 5% Patients averaged 100.9 %IBW at 4-weeks follow-up. Just 3.8% were rehospitalised in the 30 days after discharge. |
| Makhzoumi et al. (2019) [16] USA | Retrospective Patients with EDs admitted to an integrated hospital-based treatment programme (n = 275, 86% female) ARFID patients (n = 27, mean age 19.1) | Mean BMI at admission: 16.5 (−2 z-score) More common GI symptoms at admission: abdominal pain, GERD, vomiting. 78% of ARFID presented with an aversive subtype | 3 varied meals/day started on 1200–1500 kcal/day. Caloric increases every 2 days (target calories 3500–4000 kcal/day by day 10–12). Calories above 2500/day administered via nutritional supplements. | N.A. | Mean BMI at admission: 16.5 Mean BMI at discharge: 18.9 Mean inpatient weight gain rate: 1.36 kg/week |
| Kurotori et al. (2019) [15] Japan | Retrospective Patients with EDs hospitalized for nutritional rehabilitation (n = 92). ARFID patients (n = 13, 85% females, mean age 10.7 years) | Mean %IBW on admission 74% 8 (61.5%) were severely malnourished (<75% expected BW) 92% of ARFID presented with an aversive subtype | Meals administered orally (no data available on the caloric intake). Enteral nutrition via NG tube in case of persistent food refusal. | N.A. | Mean increase in %IBW during hospitalization: 4.9% |
| Tsang et al. (2020) [21] USA | Retrospective ARFID patients hospitalized for nutritional rehabilitation (n = 38, 68% females, mean age 12.8 years). | Average %IBW on admission 85.9. Mean BMI z-score on admission: −1.66. Most reported GI symptoms: abdominal pain, nausea, vomiting. | Almost half of patients (47.4%) required enteral feeds (i.e., via nasogastric, nasojejunal, or gastrostomy tube). No data on the caloric intake. | N.A. | Average %IBW on admission 85.9, Average %IBW on discharge 87.6. |
| Tamura et al. (2021) [17] Japan | Retrospective Elementary-school children hospitalized for refractory EDs started on TPN (n = 22). ARFID patients (n = 9), 78% females, mean age 11.5 years | Mean BMI z-score at admission −2.2 89% of ARFID presented an aversive subtype | All patients started on TPN with 2090 kcal/day for the first week, then increased every week (by increase of PN and introduction of oral feeding) to ensure acceptable weight gain. Enteral nutrition via NG tube if persistent oral intake refusal. | N.A. | Mean BMI z-score at admission −2.2; at discharge −1.1. No significant differences in weight gain between ARFID patients and AN patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucinotta, U.; Romano, C.; Dipasquale, V. A Systematic Review to Manage Avoidant/Restrictive Food Intake Disorders in Pediatric Gastroenterological Practice. Healthcare 2023, 11, 2245. https://doi.org/10.3390/healthcare11162245
Cucinotta U, Romano C, Dipasquale V. A Systematic Review to Manage Avoidant/Restrictive Food Intake Disorders in Pediatric Gastroenterological Practice. Healthcare. 2023; 11(16):2245. https://doi.org/10.3390/healthcare11162245
Chicago/Turabian StyleCucinotta, Ugo, Claudio Romano, and Valeria Dipasquale. 2023. "A Systematic Review to Manage Avoidant/Restrictive Food Intake Disorders in Pediatric Gastroenterological Practice" Healthcare 11, no. 16: 2245. https://doi.org/10.3390/healthcare11162245
APA StyleCucinotta, U., Romano, C., & Dipasquale, V. (2023). A Systematic Review to Manage Avoidant/Restrictive Food Intake Disorders in Pediatric Gastroenterological Practice. Healthcare, 11(16), 2245. https://doi.org/10.3390/healthcare11162245







