Abstract
The development of abnormal scars has a great impact on people’s well-being, and improving scarring outcomes after surgery is a field that currently lacks consensus. This review aims to identify newly researched approaches to improving the quality of surgical scars. A systematic search of PubMed, Scopus, Web of Science, and ScienceDirect was conducted between 13 May 2023 and 17 May 2023, in accordance with the recommendations of the PRISMA Statement. Study selection and analysis of methodological quality were performed in parts, independently and blindly, based on eligibility criteria. The 21 prospective, comparative, and randomized studies reviewed included 1057 subjects and studied approaches such as topical applications of creams with herbal extracts and silicone gels, growth factors, negative pressure dressings, oligonucleotides, intralesional injection of compounds such as botulinum toxin, skin closure techniques such as suturing and tissue adhesive, and laser treatments. There are recent research techniques that generate good results and are really promising to improve the results of surgical scars; however, the available evidence is extremely limited in some cases, and it is necessary to deepen its analysis to obtain reliable action protocols in each type of surgery.
1. Introduction
Scarring is the physiological response to skin damage. During the wound healing process, a phase of inflammation occurs, with an induction of the hemostatic cascade, leukocytes recruitment, and accumulation of fibrin [1,2]. This is followed by a stage of proliferation of fibroblasts that differentiate into myofibroblast precursors to generate local tissue adhesion, as well as keratinocyte proliferation and epithelization and the deposition of collagen fibers [1,3]. Finally, in the third stage, there is a remodeling of tissue until the final appearance is achieved. During this process, reepithelization ends, and the damaged dermis loses elastic fibers and hardens its collagen, generating greater firmness in the scar area [4,5,6].
Although scars are a part of the healing process, sometimes abnormalities such as hypertrophy or scar atrophy are generated, with the consequent increase in recovery time and physical and psychological impact for people [7]. To avoid these unwanted effects and improve clinical practice in the management of scars, scholars have tried to discover the biological mechanisms and factors that influence the recovery process [7,8,9]. Scar quality is influenced by factors such as age, infection, immune function, tissue oxygenation, nutrition, tobacco use, and particular circumstances such as the presence of diabetes, radiation, or chemotherapy [10].
For the treatment of abnormal scars and the improvement of the healing process, numerous investigations have been conducted on intervention or revision methods, and there are clinical management recommendations based on the available evidence. These include topical applications, intralesional medication (such as hyaluronic acid, corticosteroids, antimitotics), cryotherapy, laser applications, make-up camouflage, micro needling, radiofrequency, or oral agents [11,12,13,14,15].
Nevertheless, in wounds caused by surgical interventions, it is essential to know and meticulously plan the incisions, as well as the application of therapeutic measures that help reduce complications and improve the surgical outcome. These techniques include the surgical revision of the scars and subsequent treatments that promote proper healing. Based on previous scientific evidence, surgical revision of scars seems to show more reliable results, although there are countless advances in topical medications and treatments that could facilitate the therapeutic approach of surgical scars [16,17,18].
However, the application of treatments that improve the scarring outcome in surgical incisions is a field that still lacks consensus and is constantly updated by scientific progress and novel therapies that could bring benefits. This systematic review aims to identify approaches that are currently being investigated to improve the quality of surgical scars.
2. Materials and Methods
A systematic review of the scientific literature was conducted between 13 May and 17 May 2023, following the recommendations of the PRISMA Statement [19]. It began by formulating a research question (Table 1) in PIO format [20]. The electronic versions of the following databases were consulted: Pubmed, Web of Science, Scopus, and ScienceDirect.

Table 1.
PIO format: keywords.
To answer the question, different search strategies were used, adapted to the particularities of each database. Medical Subjects Headings (MeSH) and free text terms combined with Boolean operators AND/OR/NOT were included (Table 2).

Table 2.
Search strategy used, adapted to each of the databases.
We selected those original articles with a prospective longitudinal methodology, comparative or controlled, published in the last 5 years, carried out with humans, whose results evaluated the quality of scars caused by surgical interventions. Clinical case reports, scientific letters, bibliography reviews, those that analyzed other populations (animals) or etiologies, studies that do not answer the research question or were not related with the main objective of the review, and scientific reports of low quality were excluded.
Additionally, we carried out a manual reverse search, known as snowball-searching, in the bibliographic references of the studies included in the review, to identify possible relevant studies that had not previously been found through search engines.
The selection and evaluation of the methodological quality of the studies was carried out in pairs, blindly and independently. Discrepancies were solved by consensus, or failing that, through the participation of a third evaluator. The PEDro scale [21] was used to assess the methodological quality of the studies, considering a cut-off point of 8 points to accept the inclusion of each study in the review (Table 3). Each study was evaluated in terms of eligibility criteria specified, random allocation, concealed allocation, similarity of groups at the baseline, subjects blinding, therapists blinding, assessor blinding, less than 15% dropouts, intention to treat analysis, between-group statistical comparisons, and point measures and variability data.

Table 3.
Results of the PEDro quality assessment of the studies.
A standardized data extraction form was designed in order to guarantee the homogeneity of the collected information, including the following aspects of the selected articles: principal investigator, year of publication, characteristics and sample size, implemented interventions, evaluation tools, and main results obtained, along with the results of their scientific quality evaluation.
3. Results
Of the 1738 studies initially identified, 21 were selected for systematic review after several phases of screening by automation, manual, and critical reading (Figure 1). The main characteristics of the selected studies are available in Table 4.
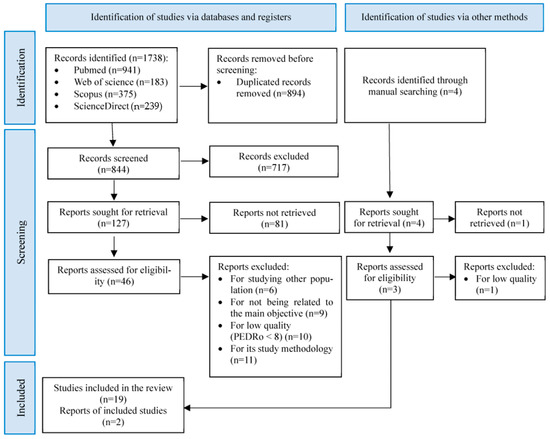
Figure 1.
PRISMA flow diagram of search results and included studies.

Table 4.
Characteristics of the studies included in the systematic review.
The studies comprised a total of 1057 subjects, with a range between 12 and 142. The female gender was predominant, with 702 women; however, some studies were designed only for one gender (studies within only females n = 4, and only male participants n = 1). Subjects of all ages were found as most of the reports analyzed an adult population, but four of them included children and teenager populations. All studies were comparative, with a control group (n = 12) or with other approaches and protocols (n = 9). A placebo was used in four of the studies. Follow-up time varied in selected studies from 1 to 12 months.
To assess the quality of the scars, standardized scales, clinical analyses, and measuring devices were used. The Patient Observer Scar Assessment Scale Score (POSAS) was used in 15 selected articles. It contains 2 domains with several subscales: Observer measure (vascularity, pigmentation, thickness, relief, pliability, and surface area), and Patient measure (pain, itchiness, color, stiffness, thickness, and relief). The Modified Stony Brook Scar Evaluation Scale (mSBSES) was used in 2 selected articles and includes width, height, color suture mark, overall appearance, and total score. The Vancouver Scar Scale (VSS) was used in 5 articles to assess the vascularity, pigmentation, pliability, and height. The Manchester Scar Scale (MSS) was used in 2 articles to assess color, matte/shiny, contour, distortion, and texture. The Hollander Wound Evaluation Score (HWES) was used in 1 trial, and includes step-off borders, contour irregularities, margin separation, edge inversion, excessive distortion, overall appearance, and total score. The Visual Analogue Scale (VAS) was used in 3 investigations to assess pain. Some of selected studies also assessed scar width (n = 1), patient satisfaction (n = 1), the skin elasticity coefficient (n = 1), and evaluations of elasticity, firmness, color, and composition with cutometer (n = 2), durometer (n = 1), mexameter (n = 1), and biopsies (n = 2), respectively.
According to these parameters, the characteristics that define the quality of the scars are their signs and symptoms. The selected studies had a large variability in the results (Table 4). Some reported improvements in total score [22,26,27,30,37,38,40,42,43], while others did so on specific subscales. Great variability was also observed in the type of surgery and in the interventions performed. The surgeries which subjects underwent were chest surgeries (n = 4), abdominoplasty (n = 2), surgical excisions (n = 3), tumor resections (n = 3), thyroidectomy (n = 3), epicanthoplasty (n = 1), trauma surgeries (n = 2), lumpectomy (n = 1), sternotomy (n = 1), and carpal tunnel relay (n = 1).
Among the studies analyzed, different suture materials were found. For the subcuticular closure, absorbable filaments were used, such as monocryl [28,29,32,36], polydioxanone [23], and vicryl [25,30,31,35,38,40]. Also, the skin sutures were made with nylon [24,32,35,39], Ethicon [23,28,29,30], and monocryl [36,40]. However, not all studies specified the materials used.
The implemented techniques include approaches which are carried out prior or at the moment of the original surgical procedure, such as botulinum toxin [24,35], tissue adhesive or sutures [25,31,33,39], laser treatments [30,32], and off-loading devices [41]; those which were implemented at the time of the intervention and are prolonged during the formation of the scar: off-loading devices [23], topical compounds [38,42], and negative pressure wounds [40] or those treatments that occur during the formation of the scar: botulinum toxin [22,27], topical compounds [26], off-loading devices [28], injected compounds [29], and laser treatments [36,37].
According to this, a great variability was observed at the time of implementation of the treatments, which are topical applications such as offloading devices, silicone, herbal extracts, pressure dressing or growth factors (n = 8); intralesional injections like botulinum toxin or oligonucleotides (n = 5); laser applications such as non-ablative fractional laser (NAFL), fractional carbon dioxide (FACL), pulse diode laser (PDL), or photobiomodulation (n = 4); and skin closure methods, like suturing techniques or tissue adhesive (n = 4).
Among the selected studies, research was conducted that analyzed different topical applications of creams, gel, and silicone sheets. Pangkanon et al. [34] studied the efficacy of a silicone gel with onion and aloe vera extracts, compared to silicone sheets to prevent the development of scar hypertrophy, without finding improvements in any of the variables analyzed, except in pliability (p = 0.009). Surakunprapha et al. [38] studied the effectiveness of a silicone gel with herbal extracts (which also includes onion extract) in sternotomy patients. At 6 months, the gel enriched with herbal extracts achieved improvements in vascularity, pigmentation, and overall opinion (p = 0.013; p = 0.000; p = 0.018) and the silicone group in vascularity and pigmentation (p = 0.046; p = 0.000), although intergroup comparisons are not known.
Other studies looked at topical application of compounds, such as that conducted by Dolynchuk et al. [26], who studied the topical use of 1,4-Diaminobutane (1,4DAB) compared to a topical control treatment (which does not contain 1,4DAB). A higher concentration of 1.4DAB was obtained in the biopsy analysis of the experimental group compared to the control, along with better results in firmness and scar quality (p < 0.05 each). On the other hand, Zoumalan et al. [42] found significant improvements compared to the control group with the cream enriched with growth factors in the measurements of the investigators, patients, and independent evaluators (p < 0.0001; p <0.001; p < 0.0001, respectively).
Furthermore, one of the selected studies investigated the efficacy of incisional negative pressure wound therapy on mastectomy scars. Timmermans et al. [40] found significant improvements at 3 months compared to the control group in total score, vascularity, and overall cosmesis, although the benefit was not maintained until 12 months by physician assessment. Patient assessment maintained improvements in color, pliability, thickness, and overall score at 12 months.
Other studies used adhesive devices on the skin. Chen et al. [23] found significant improvements in intragroup comparisons of the experimental group for width (p = 0.0025) and significant intergroup differences for scar irregularity (p = 0.0145) with a tissue adhesive zipper in children with surgical excision on the face. Ilori et al. [28] studied a heterogeneous group (excision tumors, open reduction fixation of fractures, osteotomies, arthroplasties) and found significant improvements in the type of scar, height, and width in the group treated with microporous tape over the scar compared to the control group (p < 0.0001 each), without having reported differences between the types of surgery. Zhang et al. [41] tested a discharge device to improve skin elasticity and promote healing in patients with a history of scarring hypertrophy with benign skin excisions. They found that there were no significant differences between the group that wore the device preoperatively and postoperatively, and those who wore it only after surgery; although there are significant improvements with respect to the control group (they did not use device) in terms of width, color, and overall opinion.
Another method found in the selected trials was the intralesional injection of compounds. Botulinum toxin was used by several authors with different results. Abedini et al. [22] recorded significant improvements at 3 and 6 months (p < 0.001) between their control (saline) and experimental (botulinum toxin) groups in mammoplasty and abdominoplasty patients; however, in intragroup comparisons, neither group improved significantly in width. In contrast, Chen et al. [24] found between-group differences in width and visibility (p < 0.01), getting better results with a high dose of botulinum toxin in patients with tumor resection. Huang et al. [27] found improvements after 1, 3, and 6 months (p = 0.034; p < 0.001; p < 0.001, respectively) over the control group (placebo) in their experimental group of botulinum toxin in epicanthoplasty patients. On the other hand, Phillips et al. [35] investigated the effect of botulinum toxin in thyroidectomy and found no significant differences between groups over time, although significant improvements were observed in POSAS (p = 0.012) and VSS (p = 0.021) between patients with a history of healing problems and those with a history of normal healing.
In studies with other injectable substances, Jensen et al. [29] performed well on mammoplasty scars after 24 weeks with their intervention using injectable anti-CTGF oligonucleotide compared to the control group in the evaluations of investigators (vascularity, pigmentation, thickness, relief, pliability, surface area, and overall opinion) and patients (color, stiffness, thickness, surface area, and overall opinion).
Another method found in the selected studies was the comparison of skin closure techniques. In one of the studies, Suwannaphisit et al. [39] compared the running subcuticular technique and the Donati technique of suture in patients with open carpal tunnel release, without finding significant differences between the two groups at 6 and 12 weeks.
In the same way, several selected studies analyzed the differences between suture and tissue adhesive. Musham et al. [33] found that tissue adhesive produced less pain than subcuticular suture (p < 0.01), but there was no significant difference in scar outcome at 1 and 3 months between groups in patients who underwent thyroidectomy. Kong et al. [31] also found no significant differences between groups in patients with bilateral hip arthroplasty, although from the point of view of patients, tissue adhesive was statistically better (p = 0.004).
However, Chung et al. [25] compared tissue adhesive application, suturing, and early non-ablative laser treatment (NAFL) in three experimental groups with thyroidectomy patients. Tissue adhesive was applied to the first group, which did not obtain any significantly better parameters in the intergroup comparisons. The second group received suturing and laser treatment, and significant improvements were observed for multiple subscales according to the researchers (thickness relief, pliability, surface area, and overall cosmesis); and the third group was treated with tissue adhesive and laser treatment, which also did not obtain significant improvements compared to the other protocols. However, patients reported greater satisfaction in the two laser-treated groups in all parameters except pigmentation.
On the other hand, the satisfaction of laser treatment was also observed by Ramos et al. [36] (PSAS p = 0.0047), who also found improvements in evaluator assessment (p = 0.0034) and VSS (p = 0.0065) with photobiomodulation after abdominoplasty. In the same way, Karmisholt et al. [30] found significant improvements in patients who underwent surgical excision with three sessions of NAFL in comparison with the control group (p < 0.001) and also noted that the best results with this technique were obtained in the chest area (p < 0.001). Safra et al. [37] also reported benefits in their experimental group of fractional ablative CO2 laser (FACL) with respect to the control group in lumpectomy patients (p < 0.001). Lin et al. [32] also studied the effects of applying FACL before suture or after the removal of sutures in patients with excision of skin cancer in extremities and found no significant differences in any of the scores, nor in biopsy analysis.
4. Discussion
From a global perspective, this systematic review aimed to explore current investigations of approaches to improve the quality of post-surgical scars. This review found that very promising approaches are currently being carried out to promote the correct healing process.
Among the methods found in the reviewed articles, topical applications of compounds, bandages, or off-loading devices stand out. Two of the articles applied silicone gel with herbal extracts with onion, compared to silicone gel [34,38], obtaining moderate improvements. A previous meta-analysis studied the effects of onion extract on healing, finding that onion gel increases adverse effects and has no benefit; however, onion extract in silicone gel generates improvements in healing and could be the optimal solution. Accordingly, the scientific evidence for silicone gel with herbal extracts for surgical scar enhancement is very scarce, and further research is needed to determine appropriate parameters such as doses and dosages that generate maximum benefit without harmful effects [44].
Other studies investigated the adhesion of devices to discharge mechanical stress from the surgical incision [23,28,41]. These mechanisms have been shown in animals to be associated with transcriptional downregulation of inflammatory pathways [45] and have obtained benefits in other similar research, helping to heal and improving their aesthetic appearance [46,47].
Similarly, a study using incisional negative pressure wound therapy was selected [40], achieving prolonged improvements in the opinion of the subjects, although according to the evaluation of the researchers the effect was not maintained until 12 months. According to a previous systematic review [48], this is a technique with very limited research and uncertain results, although it shows promise in preventing complications and improving healing in surgical incisions; therefore, it is necessary to expand this field of knowledge to establish intervention protocols if the results are favorable.
Other topical applications used 1,4DAB or putrescine [26] in the treatment of breast reduction scars; these generate their action by inhibiting transglutaminase and apoptosis of fibroblasts [49]. In the selected study, improvements were found at 6 and 12 weeks in the firmness and characteristics of the scar, which coincides with the claims of a previous review [50].
Growth factors were also used in one of the studies [42], obtaining good results in topical application. This is consistent with systematic reviews and meta-analyses conducted with growth factors applied to other etiologies [51,52]; however, there is little rigorous research on its application to surgical incision healing.
In contrast, one of the studies applied injectable anti-connective tissue growth factor, obtaining very good results at 12 and 24 weeks [29]. However, this is a preliminary study, so it is not known if it is the optimal dose and if it is a cost-effective treatment. Because of this, no additional research has been found with this compound in the literature consulted.
Another intervention that stands out in the selected studies was the injection of botulinum toxin, having been analyzed in four of the studies. This substance has a proven effectiveness in the treatment of other therapeutic conditions such as spasticity or neuropathic pain [53,54,55]. However, it is still under constant review for its application in the healing process, especially in a preventive way. Its action inhibits the production of fibroblasts, and consequently collagen, being able to stop the appearance of scarring complications such as hypertrophy or keloids [56,57].
In the articles included in this review, it was applied to scars of surgical etiology in the first 10 days after the intervention, with a concentration of 5 U in all cases [22,27,35], except in the dose comparison of Chen et al. [24], which found better results in appearance when using 8 U. This dosage and the results obtained are consistent with the conclusions of the limited evidence available in this regard [57,58]. However, one of the investigations [35] also found that the effect had significant differences between patients with a history of hypertrophic scars, which was not found in the previous literature consulted for this comparison.
On the other hand, some studies analyzed the effect of the skin closure method on the surgical scar. One study compared the Donati technique and the subcuticular suture technique, with better short-term assessment by patients towards the subcuticular technique, but no significant differences were obtained for observers [39]. These results are consistent with a previous systematic review, according to which subcuticular sutures may be preferable to interrupted sutures; however, the available research still has many limitations for establishing particular recommendations [59,60].
Three of the studies conducted comparisons between skin closure with suture and with tissue adhesive [25,31,33]. Significant improvements with tissue adhesive were found in healing time, postoperative pain levels, and patient satisfaction [31], but none of them found significant differences in scar characteristics. This contrasts with the literature found, which claims the improvement of scarring appearance with tissue adhesive [61].
Chung et al. [25] also performed a combination of the two methods of skin closure with non-ablative laser treatment, finding that the best results were offered by the combination of suture and laser treatment, and that the groups treated with non-ablative laser showed greater satisfaction. Other research included in this review [30,32,36,37] used ablative and non-ablative laser treatments. These results agree with a previous systematic review [62], according to which, the treatment of surgical scars by PDL, radiofrequency, and ultrasound can improve the texture thickness and appearance and relieve contractures. However, the literature consulted does not value the long-term results, as well as the combination with other techniques, and the samples were small, so it is necessary to expand the research to establish more specific treatment protocols.
This review aimed to understand the techniques that are currently being investigated to improve surgical healing. The time frame selected was reasonable to show the trend of current research in this area. However, and because of this, limitations related to the youth of certain experimental treatments were found, such as the lack of standardization of techniques, small samples, and unicentric designs, as well as limited high-quality evidence in some therapeutic methods. Consequently, studies that support these conclusions were located, but nevertheless did not reach the cut-off point established in the methodological quality review.
Other techniques, on the other hand, were supported by previous scientific evidence as can be seen in the discussion, although they are still present in current research work due to the need for an increase in evidence with methodological quality. Another limitation of the study is due to the limitation of the search to scars of surgical etiology. Although in other situations such as burns there is a greater availability of scientific evidence and some techniques may be common, the scarring characteristics cannot always be compared, and, therefore, conclusions cannot be clearly drawn.
Despite all these limitations, it was observed that the improvement of post-surgical healing is a field of high activity, with promising techniques. Although these techniques must be expanded and replicated in order to establish reliable protocols and recommendations, they can generate an improvement in health care and in the quality of life of patients.
5. Conclusions
Improving the quality of surgical scars remains an area of emerging research. In this systematic review, we found numerous current approaches that sought to minimize the signs and symptoms of surgical scarring. Topical application of silicones, enriched creams, tension relief devices, negative pressure bandaging, intradermal applications, skin closure techniques, and laser treatments were reported.
Silicone gels with herbal extracts such as onion should be studied to ensure good results without causing adverse reactions. Scar tension relief devices show benefits in the healing process and appearance. The incisional negative pressure wound therapy, putrescine, growth factors, and anti-connective tissue growth factor show very encouraging results, although the evidence with surgical scars is still very small, and in some cases practically non-existent.
The injection of botulinum toxin generates good results in surgical scars at doses of 5 U, especially in people with a history of scar hypertrophy, and generates better results in scar width with higher doses. Regarding skin closure, patients value the subcuticular suture technique more, although in the long term it does not generate additional benefits. Similarly, tissue adhesive helps with healing time and pain levels, but offers no difference over time compared to suturing. The combination of subcuticular suture and non-ablative laser treatment improves recovery and scarring characteristics and generates patient satisfaction, as well as the use of isolated ablative and non-ablative laser treatments.
However, despite the improvements demonstrated by some approaches, many of the techniques used have not yet consolidated their parameters, times, doses, and specific action protocols. In addition, most existing research is of low quality, with small samples and single-center designs, which may detract from the external validity of the available evidence. It is necessary to generate extensive and quality research on these approaches and establish comparisons that help to converge and determine which technique is the most appropriate in each surgical intervention, so that the impact of scars on the lives of patients can be reduced.
Author Contributions
Conceptualization, R.P.-H., A.B. and E.N.-S.; methodology; R.P.-H., R.d.l.F.-A. and J.J.G.-B.; validation; J.J.G.-B. and R.d.l.F.-A.; investigation, R.P.-H., A.B. and E.N.-S.; writing-original draft preparation, R.P.-H., A.B. and E.N.-S.; writing-review and editing, R.P.-H., A.B. and E.N.-S.; visualization R.P.-H., A.B., E.N.-S., R.d.l.F.-A. and J.J.G.-B.; supervision, J.J.G.-B. and R.d.l.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lingzhi, Z.; Meirong, L.; Xiaobing, F. Biological Approaches for Hypertrophic Scars. Int. Wound J. 2020, 17, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, J.E. Wound Healing. Surg. Clin. N. Am. 1978, 58, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.L.; Roberts, J.L.; Moseley, R.; Griffiths, P.C.; Thomas, D.W. Evaluation of the Physical and Biological Properties of Hyaluronan and Hyaluronan Fragments. Int. J. Pharm. 2011, 420, 84–92. [Google Scholar] [CrossRef]
- Son, D.; Harijan, A. Overview of Surgical Scar Prevention and Management. J. Korean Med. Sci. 2014, 29, 751–757. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin Signaling in Wound Repair. Birth Defects Res. Part C Embryo Today 2012, 96, 248–257. [Google Scholar] [CrossRef]
- Monslow, J.; Sato, N.; MacK, J.A.; Maytin, E.V. Wounding-Induced Synthesis of Hyaluronic Acid in Organotypic Epidermal Cultures Requires the Release of Heparin-Binding EGF and Activation of the EGFR. J. Investig. Dermatol. 2009, 129, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Slemp, A.E.; Kirschner, R.E. Keloids and Scars: A Review of Keloids and Scars, Their Pathogenesis, Risk Factors, and Management. Curr. Opin. Pediatr. 2006, 18, 396–402. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough Treatments for Accelerated Wound Healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Watson, D.; Reuther, M. Scar Revision Techniquespearls and Pitfalls. Facial Plast. Surg. 2012, 28, 487–491. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and Physiology of Wound Healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef]
- David Chang, C.W.; Russell Ries, W. Nonoperative Techniques for Scar Management and Revision. Facial Plast. Surg. 2001, 17, 283–287. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Goldberg, D.J. Update on the Treatment of Scars. J. Drugs Dermatol. 2019, 18, 550–555. [Google Scholar] [PubMed]
- Ogawa, R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2022, 149, 79E–94E. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.E. Internationale Klinische Empfehlungen Zur Narbenbehandlung. Zentralbl. Chir. 2004, 129, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Marchesini, A.; Senesi, L.; Skrami, E.; Gesuita, R.; De Francesco, F. Managing Pathologic Scars by Injecting Auto-Cross-Linked Hyaluronic Acid: A Preliminary Prospective Clinical Study. Aesthet. Plast. Surg. 2019, 43, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Garg, S.; Dahiya, N. Surgical Scar Revision: An Overview. J. Cutan. Aesthet. Surg. 2014, 7, 3. [Google Scholar] [CrossRef]
- Kerwin, L.Y.; El Tal, A.K.; Stiff, M.A.; Fakhouri, T.M. Scar Prevention and Remodeling: A Review of the Medical, Surgical, Topical and Light Treatment Approaches. Int. J. Dermatol. 2014, 53, 922–936. [Google Scholar] [CrossRef]
- Lee Peng, G.; Kerolus, J.L. Management of Surgical Scars. Facial Plast. Surg. Clin. N. Am. 2019, 27, 513–517. [Google Scholar] [CrossRef]
- Urrútia, G.; Bonfill, X. PRISMA Declaration: A Proposal to Improve the Publication of Systematic Reviews and Meta-Analyses. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence Based Medicine: What It Is and What It Isn’t. 1996. Clin. Orthop. Relat. Res. 2007, 455, 3–5. [Google Scholar]
- Sherrington, C.; Herbert, R.D.; Maher, C.G.; Moseley, A.M. PEDro. A Database of Randomized Trials and Systematic Reviews in Physiotherapy. Man. Ther. 2000, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Abedini, R.; Mehdizade Rayeni, N.; Haddady Abianeh, S.; Rahmati, J.; Teymourpour, A.; Nasimi, M. Botulinum Toxin Type A Injection for Mammoplasty and Abdominoplasty Scar Management: A Split-Scar Double-Blinded Randomized Controlled Study. Aesthet. Plast. Surg. 2020, 44, 2270–2276. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Y.; Zou, Y.; Qiu, Y.; Hu, L.; Chang, L.; Chen, H.; Lin, X. Scar Prevention with Prolonged Use of Tissue Adhesive Zipper Immediately after Facial Surgery: A Randomized Controlled Trial. Aesthet. Surg. J. 2022, 42, NP265–NP272. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Pang, R.; Wei, Z.; Zhang, H.; Liu, W.; Li, G. The Effect of Botulinum Toxin Injection Dose on the Appearance of Surgical Scar. Sci. Rep. 2021, 11, 13670. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kim, D.S.; Cheon, J.H.; Yoon, J.M.; Baek, S.K.; Jung, K.Y.; Yoon, E.S.; Park, S.H. Current Protocol for Aesthetic Scar Management in Thyroid Surgery. Laryngoscope 2021, 131, E2188–E2195. [Google Scholar] [CrossRef] [PubMed]
- Dolynchuk, K.N.; Tredget, E.E. A Preliminary Report of the Biochemical and Clinical Effects of 1,4-Diaminobutane on Prevention of Human Hypertrophic Scars. Plast. Reconstr. Surg. 2020, 145, 76e–84e. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Ho, C.K.; Tremp, M.; Xie, Y.; Li, Q.; Zan, T. Early Postoperative Application of Botulinum Toxin Type A Prevents Hypertrophic Scarring after Epicanthoplasty: A Split-Face, Double-Blind, Randomized Trial. Plast. Reconstr. Surg. 2019, 144, 835–844. [Google Scholar] [CrossRef]
- Ilori, O.S.; Oladele, A.O.; Ilori, O.R.; Onilede, D.A. Efficacy of Microporous Tape in the Prevention of Abnormal Post-Surgical Scars among a Black Population. J. Ayub Med. Coll. Abbottabad 2022, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Gentzkow, G.; Berman, G.; Senne, L.; Jewell, M.; Connall, T.P.; Miller, S.R.; Galiano, R.D.; Young, L. Anti-CTGF Oligonucleotide Reduces Severity of Postsurgical Hypertrophic Scars in a Randomized, Double-Blind, within-Subject, Placebo-Controlled Study. Plast. Reconstr. Surg. 2018, 142, 192E–201E. [Google Scholar] [CrossRef]
- Karmisholt, K.E.; Banzhaf, C.A.; Glud, M.; Yeung, K.; Paasch, U.; Nast, A.; Haedersdal, M. Laser Treatments in Early Wound Healing Improve Scar Appearance: A Randomized Split-Wound Trial with Nonablative Fractional Laser Exposures vs. Untreated Controls. Br. J. Dermatol. 2018, 179, 1307–1314. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Cao, Z.; Chen, J.; Chai, W.; Wang, Y. Tissue Adhesive for Wound Closure in Enhanced-Recovery Total Hip Arthroplasty: A Prospective, Randomized and Controlled Study. BMC Musculoskelet. Disord. 2020, 21, 178. [Google Scholar] [CrossRef]
- Lin, M.J.; Dubin, D.P.; Torbeck, R.L.; Bernstein, D.M.; Nabatian, A.; Dolan, C.K.; Bacigalupi, R.; Zade, J.; Zheng, Z.; Desman, G.; et al. Early Fractional Ablative Laser for Skin Cancer Excision Scars: A Randomized Split-Scar Study. Dermatol. Surg. 2023, 49, 338–342. [Google Scholar] [CrossRef]
- Musham, A.; Samuel, E.M.K.; Sahoo, A.K.; Elamurugan, T.P.; Manwar, A.S. Comparison of Tissue Adhesive Glue with Subcuticular Absorbable Suture for Skin Closure Following Thyroid Surgery A Single-Blinded Randomised Controlled Trial. Sultan Qaboos Univ. Med. J. 2023, 23, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Pangkanon, W.; Yenbutra, P.; Kamanamool, N.; Tannirandorn, A.; Udompataikul, M. A Comparison of the Efficacy of Silicone Gel Containing Onion Extract and Aloe Vera to Silicone Gel Sheets to Prevent Postoperative Hypertrophic Scars and Keloids. J. Cosmet. Dermatol. 2021, 20, 1146–1153. [Google Scholar] [CrossRef]
- Phillips, T.J.; Fung, E.; Rigby, M.H.; Burke, E.; Hart, R.D.; Trites, J.R.B.; Gassner, H.G.; Taylor, S.M. The Use of Botulinum Toxin Type A in the Healing of Thyroidectomy Wounds. Plast. Reconstr. Surg. 2019, 143, 375e–381e. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.M.; Burland, M.; Silva, J.B.; Burman, L.M.; Gelain, M.S.; Debom, L.M.; Bec, J.M.; Alirezai, M.; Uebel, C.O.; Valmier, J.; et al. Photobiomodulation Improved the First Stages of Wound Healing Process After Abdominoplasty: An Experimental, Double-Blinded, Non-Randomized Clinical Trial. Aesthet. Plast. Surg. 2019, 43, 147–154. [Google Scholar] [CrossRef]
- Safra, T.; Shehadeh, W.; Koren, A.; Salameh, F.; Friedman, O.; Sprecher, E.; Artzi, O. Early Intervention with Pulse Dye and CO2 Ablative Fractional Lasers to Improve Cutaneous Scarring Post-Lumpectomy: A Randomized Controlled Trial on the Impact of Intervention on Final Cosmesis. Lasers Med. Sci. 2019, 34, 1881–1887. [Google Scholar] [CrossRef]
- Surakunprapha, P.; Winaikosol, K.; Chowchuen, B.; Jenwithaesuk, K.; Jenwitheesuk, K. Adding Herbal Extracts to Silicone Gel on Post-Sternotomy Scar: A Prospective Randomised Double-Blind Study. J. Wound Care 2020, 29, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Suwannaphisit, S.; Aonsong, W.; Suwanno, P.; Yuenyongviwat, V. Comparing the Running Subcuticular Technique versus the Donati Technique in Open Carpal Tunnel Release: A Randomized Controlled Trial. J. Orthop. Surg. Res. 2021, 16, 565. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, F.W.; Mokken, S.E.; Smit, J.M.; Bouman, M.B.; van de Grift, T.C.; Mullender, M.G.; Middelkoop, E. The Impact of Incisional Negative Pressure Wound Therapy on Scar Quality and Patient-Reported Outcomes: A within-Patient-Controlled, Randomised Trial. Wound Repair Regen. 2022, 30, 210–221. [Google Scholar] [CrossRef]
- Zhang, S.; Nabi, O.; Jiang, X. New Strategy of Modulating Incision Tension: A Wound Tension Offloading Device Applied before Surgery. Dermatol. Ther. 2021, 34, e14797. [Google Scholar] [CrossRef] [PubMed]
- Zoumalan, C.I.; Tadayon, S.C.; Roostaeian, J.; Rossi, A.M.; Gabriel, A. Safety and Efficacy of a Scar Cream Consisting of Highly Selective Growth Factors within a Silicone Cream Matrix: A Double-Blinded, Randomized, Multicenter Study. Aesthet. Surg. J. 2019, 39, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Kemaloğlu, C.A.; Özyazgan, İ.; Gönen, Z.B. Immediate Fat and Nanofat-Enriched Fat Grafting in Breast Reduction for Scar Management. J. Plast. Surg. Hand Surg. 2021, 55, 173–180. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, J.; Chen, L.; Wang, L.; Yan, Q.; Zhang, J. Onion Extract Gel Is Not Better than Other Topical Treatments in Scar Management: A Meta-Analysis from Randomised Controlled Trails. Int. Wound J. 2021, 18, 396–409. [Google Scholar] [CrossRef]
- Januszyk, M.; Wong, V.W.; Bhatt, K.A.; Vial, I.N.; Paterno, J.; Longaker, M.T.; Gurtner, G.C. Mechanical Offloading of Incisional Wounds Is Associated with Transcriptional Downregulation of Inflammatory Pathways in a Large Animal Model. Organogenesis 2014, 10, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Beasley, B.; Zepeda, J.; Dauskardt, R.H.; Yock, P.G.; Longaker, M.T.; Gurtner, G.C. A Mechanomodulatory Device to Minimize Incisional Scar Formation. Adv. Wound Care 2013, 2, 185–194. [Google Scholar] [CrossRef]
- Wang, D.; Ding, J.; Jiang, Y.; Liu, Y.; Chen, B. Continuous Tension Reduction Technique in Facial Scar Management: A Comparison of W-Plasty and Straight-Line Closure on Aesthetic Effects in Asian Patients. Int. Wound J. 2022, 19, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Scalise, A.; Calamita, R.; Tartaglione, C.; Pierangeli, M.; Bolletta, E.; Gioacchini, M.; Gesuita, R.; Di Benedetto, G. Improving Wound Healing and Preventing Surgical Site Complications of Closed Surgical Incisions: A Possible Role of Incisional Negative Pressure Wound Therapy. A Systematic Review of the Literature. Int. Wound J. 2016, 13, 1260–1281. [Google Scholar] [CrossRef] [PubMed]
- Armour, A.; Scott, P.G.; Tredget, E.E. Cellular and Molecular Pathology of HTS: Basis for Treatment. Wound Repair Regen. 2007, 15, S6–S17. [Google Scholar] [CrossRef]
- Klotz, T. The Effect of Moisturisers on Scars: A Systematic Review. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2018. [Google Scholar]
- Cheng, J.W.; Cheng, S.W.; Wei, R.L.; Lu, G.C. Anti-Vascular Endothelial Growth Factor for Control of Wound Healing in Glaucoma Surgery. Cochrane Database Syst. Rev. 2016, 2016, CD009782. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; He, J.; Dong, J. Growth Factor Therapy in Patients with Partial-Thickness Burns: A Systematic Review and Meta-Analysis. Int. Wound J. 2016, 13, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Santamato, A.; Cinone, N.; Panza, F.; Letizia, S.; Santoro, L.; Lozupone, M.; Daniele, A.; Picelli, A.; Baricich, A.; Intiso, D.; et al. Botulinum Toxin Type A for the Treatment of Lower Limb Spasticity after Stroke. Drugs 2019, 79, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Santamato, A.; Chemello, E.; Cinone, N.; Cisari, C.; Gandolfi, M.; Ranieri, M.; Smania, N.; Baricich, A. Adjuvant Treatments Associated with Botulinum Toxin Injection for Managing Spasticity: An Overview of the Literature. Ann. Phys. Rehabil. Med. 2019, 62, 291–296. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Lee, B.H.; Sung, H.M.; Park, S.Y.; Ahn, D.K.; Jung, M.S.; Suh, I.S. Effect of Botulinum Toxin Type A on Differentiation of Fibroblasts Derived from Scar Tissue. Plast. Reconstr. Surg. 2015, 136, 171e–178e. [Google Scholar] [CrossRef] [PubMed]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. Botulin Toxin Use in Scars/Keloids Treatment. Open Access Maced. J. Med. Sci. 2019, 7, 2979–2981. [Google Scholar] [CrossRef]
- Arias-Rodríguez, C. Toxina Botulínica: Usos Novedosos y Su Evidencia Botulinum Toxin: Novel Uses and Evidence. Dermatol. Cosmét. Med. Quir. 2022, 20, 448–452. [Google Scholar]
- Ashraf, I.; Butt, E.; Veitch, D.; Wernham, A. Dermatological Surgery: An Update on Suture Materials and Techniques: Part 1. Clin. Exp. Dermatol. 2021, 46, 1400–1410. [Google Scholar] [CrossRef]
- Butt, E.; Ashraf, I.; Veitch, D.; Wernham, A. Dermatological Surgery: An Update on Suture Materials and Techniques: Part 2. Clin. Exp. Dermatol. 2021, 46, 1411–1419. [Google Scholar] [CrossRef]
- Byrne, M.; Aly, A. The Surgical Suture. Aesthet. Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef]
- Chowdhury, B.; Kassir, M.; Salas-Alanis, J.; Nistico, S.; Galadari, H.; Fritz, K.; Salavastru, C.; Blicharz, L.; Goldust, M. Laser in Surgical Scar Clearance: An Update Review. J. Cosmet. Dermatol. 2021, 20, 3808–3811. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).