Self at Risk: Self-Esteem and Quality of Life in Cancer Patients Undergoing Surgical Treatment and Experiencing Bodily Deformities
Abstract
1. Introduction
2. Study 1: Self-Esteem and QoL in Patients with Oral Cancer
2.1. Introduction
2.2. Materials and Methods
2.2.1. Study Design
2.2.2. Research Methods
2.2.3. Study Participants
2.2.4. Statistical Analysis
2.3. Results
2.4. Discussion
3. Study 2: Explicit and Implicit Self-Esteem and Quality of Life in Women with Breast Cancer
3.1. Introduction
3.2. Materials and Methods
3.2.1. Study Design
3.2.2. Research Methods
3.2.3. Study Participants
3.2.4. Statistical Analysis
3.3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avci, I.A.; Kumcagiz, H.; Altinel, B.; Caloglu, A. Turkish female academician self-esteem and health beliefs for breast cancer screening. Asian Pac. J. Cancer Prev. 2014, 15, 155–160. [Google Scholar] [CrossRef][Green Version]
- Abu-Helalah, M.; Al-Hanaqta, M.; Alshraideh, H.; Abdulbaqi, N.; Hijazeen, J. Quality of life and psychological well-being of breast cancer survivors in Jordan. Asian Pac. J. Cancer Prev. 2014, 15, 5927–5936. [Google Scholar] [CrossRef]
- Leite, M.A.; Nogueira, D.A.; Terra Fde, S. Evaluation of self-esteem in cancer patients undergoing chemotherapy treatment. Rev. Lat. Am. Enferm. 2015, 23, 1082–1089. [Google Scholar] [CrossRef]
- Maciel, P.C.; Veiga-Filho, J.; Carvalho, M.P.; Fonseca, F.E.; Ferreira, L.M.; Veiga, D.F. Quality of life and self-esteem in patients submitted to surgical treatment of skin carcinomas: Long-term results. Bras. Dermatol. 2014, 89, 594–598. [Google Scholar] [CrossRef]
- Tachi, T.; Teramachi, H.; Tanaka, K.; Asano, S.; Osawa, T.; Kawashima, A.; Yasuda, M.; Mizui, T.; Nakada, T.; Noguchi, Y.; et al. The impact of outpatient chemotherapy-related adverse events on the quality of life of breast cancer patients. PLoS ONE 2015, 10, e0124169. [Google Scholar] [CrossRef]
- Schipper, H. Quality of life: Principles of the clinical paradigm. J. Psychosoc. Oncol. 1990, 8, 171–185. [Google Scholar] [CrossRef]
- Connell, J.; O’Cathain, A.; Brazier, J. Measuring quality of life in mental health: Are we asking the right questions? Soc. Sci. Med. 2014, 120, 12–20. [Google Scholar] [CrossRef]
- Kossakowski, J.J.; Epskamp, S.; Kieffer, J.M.; van Borkulo, C.D.; Rhemtulla, M.; Borsboom, D. The application of a network approach to Health-Related Quality of Life (HRQoL): Introducing a new method for assessing HRQoL in healthy adults and cancer patients. Qual. Life Res. 2016, 25, 781–792. [Google Scholar] [CrossRef]
- Janz, N.K.; Friese, C.R.; Li, Y.; Graff, J.J.; Hamilton, A.S.; Hawley, S.T. Emotional well-being years post-treatment for breast cancer: Prospective, multi-ethnic, and population-based analysis. J. Cancer Surviv. 2014, 8, 131–142. [Google Scholar] [CrossRef]
- Rohani, C.; Abedi, H.A.; Omranipour, R.; Langius-Eklöf, A. Health-related quality of life and the predictive role of sense of coherence, spirituality and religious coping in a sample of Iranian women with breast cancer: A prospective study with comparative design. Health Qual. Life Outcomes 2015, 13, 40. [Google Scholar] [CrossRef]
- Berterö, C.M. Affected self-respect and self-value: The impact of breast cancer treatment on self-esteem and QoL. Psychooncology 2002, 11, 356–364. [Google Scholar] [CrossRef]
- Morales-Sánchez, L.; Luque-Ribelles, V.; Gil-Olarte, P.; Ruiz-González, P.; Guil, R. Enhancing Self-Esteem and Body Image of Breast Cancer Women through Interventions: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 1640. [Google Scholar] [CrossRef]
- Bowie, J.; Brunckhorst, O.; Stewart, R.; Dasgupta, P.; Ahmed, K. Body image, self-esteem, and sense of masculinity in patients with prostate cancer: A qualitative meta-synthesis. J. Cancer Surviv. 2022, 16, 95–110. [Google Scholar] [CrossRef]
- Bailey, J.A. 2nd. Self-image, self-concept, and self-identity revisited. J. Natl. Med. Assoc. 2003, 95, 383–386. [Google Scholar]
- Orth, U.; Erol, R.Y.; Luciano, E.C. Development of self-esteem from age 4 to 94 years: A meta-analysis of longitudinal studies. Psychol. Bull. 2018, 144, 1045–1080. [Google Scholar] [CrossRef]
- Dargan, S.; MacDonald, K.B.; Schermer, J.A. Exploring Locus-of-Hope: Relational Tendencies, Self-Esteem, Attachment, and Gender. Behav. Sci. 2021, 11, 120. [Google Scholar] [CrossRef]
- Eppel-Meichlinger, J.; Kobleder, A.; Mayer, H. Developing a theoretical definition of self-organization: A principle-based concept analysis in the context of uncertainty in chronic illness. Nurs. Forum 2022, 57, 954–962. [Google Scholar] [CrossRef]
- Niveau, N.; New, B.; Beaudoin, M. How Should Self-Esteem Be Considered in Cancer Patients? Front. Psychol. 2021, 12, 763900. [Google Scholar] [CrossRef]
- Lundberg, T.; Årestedt, K.; Forinder, U.; Olsson, M.; Fürst, C.J.; Alvariza, A. Higher Self-Esteem Associated with Less Symptoms of Anxiety and Depression Among Young Adults After the Loss of a Parent to Cancer-A Longitudinal Study. J. Palliat. Care 2022, 37, 113–119. [Google Scholar] [CrossRef]
- Náfrádi, L.; Nakamoto, K.; Schulz, P.J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS ONE 2017, 12, e0186458. [Google Scholar] [CrossRef]
- Dirksen, S.R. Predicting well-being among breast cancer survivors. J. Adv. Nurs. 2000, 32, 937–943. [Google Scholar] [CrossRef]
- Helgeson, V.S.; Lepore, S.J.; Eton, D.T. Moderators of the benefits of psychoeducational interventions for men with prostate cancer. Health Psychol. 2006, 25, 348–354. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Brockopp, D.Y.; Andrykowski, M.A. Self-transformation as a factor in the self-esteem and well-being of breast cancer survivors. J. Adv. Nurs. 1999, 29, 1402–1411. [Google Scholar] [CrossRef]
- Wojtyna, E.; Życińska, J.; Stawiarska, P. The influence of cognitive-behaviour therapy on quality of life and self-esteem in women suffering from breast cancer. Rep. Pract. Oncol. Radiother. 2007, 12, 109–117. [Google Scholar] [CrossRef]
- Rehse, B.; Pukrop, R. Effects of psychosocial interventions on quality of life in adult cancer patients: Meta analysis of 37 published controlled outcome studies. Patient Educ. Couns. 2003, 50, 179–186. [Google Scholar] [CrossRef]
- Reports|Polish National Cancer Registry. Available online: https://onkologia.org.pl/en/report (accessed on 13 June 2023).
- Dahill, A.; Al-Nakishbandi, H.; Cunningham, K.B.; Humphris, G.M.; Lowe, D.; Rogers, S.N. Loneliness and quality of life after head and neck cancer. Br. J. Oral. Maxillofac. Surg. 2020, 58, 959–965. [Google Scholar] [CrossRef]
- van Beek, F.E.; Jansen, F.; Baatenburg de Jong, R.J.; Langendijk, J.A.; Leemans, C.R.; Smit, J.H.; Takes, R.P.; Terhaard, C.H.J.; Custers, J.A.E.; Prins, J.B.; et al. Psychological Problems among Head and Neck Cancer Patients in Relation to Utilization of Healthcare and Informal Care and Costs in the First Two Years after Diagnosis. Curr. Oncol. 2022, 29, 3200–3214. [Google Scholar] [CrossRef]
- Hammermüller, C.; Hinz, A.; Dietz, A.; Wichmann, G.; Pirlich, M.; Berger, T.; Zimmermann, K.; Neumuth, T.; Mehnert-Theuerkauf, A.; Wiegand, S.; et al. Depression, anxiety, fatigue, and quality of life in a large sample of patients suffering from head and neck cancer in comparison with the general population. BMC Cancer 2021, 21, 94. [Google Scholar] [CrossRef]
- Devins, G.M.; Wong, J.C.; Payne, A.Y.; Lebel, S.; Lee, R.N.; Mah, K.; Irish, J.; Rodin, G. Distancing, self-esteem, and subjective well-being in head and neck cancer. Psychooncology 2015, 24, 1506–1513. [Google Scholar] [CrossRef]
- EORTC Study Group on Quality of Life. Scoring Manual; EORTC: Brussels, Belgium, 1995. [Google Scholar]
- Singer, S.; Arraras, J.I.; Baumann, I.; Boehm, A.; Chie, W.C.; Galalae, R.; Langendijk, J.A.; Guntinas-Lichius, O.; Hammerlid, E.; Pinto, M.; et al. Quality of life in patients with head and neck cancer receiving targeted or multimodal therapy-update of the EORTC QLQ-H&N35, Phase I. Head. Neck 2013, 35, 1331–1338. [Google Scholar] [CrossRef]
- Dzwonkowska, I.; Lachowicz-Tabaczek, K.; Łaguna, M. Samoocena i Jej Pomiar SES. Polska Adaptacja Skali SES M. Rosenberga; Pracownia Testów Psychologicznych: Warszawa, Poland, 2008. [Google Scholar]
- Scandurra, C.; Mangiapia, F.; La Rocca, R.; Di Bello, F.; De Lucia, N.; Muzii, B.; Cantone, M.; Zampi, R.; Califano, G.; Maldonato, N.M.; et al. A cross-sectional study on demoralization in prostate cancer patients: The role of masculine self-esteem, depression, and resilience. Support. Care Cancer 2022, 30, 7021–7030. [Google Scholar] [CrossRef]
- Inci, H.; Inci, F.; Ersoy, S.; Karatas, F.; Adahan, D. Self-esteem, metacognition, and coping strategies in cancer patients: A case-control study. J. Cancer Res. Ther. 2021, 17, 956–962. [Google Scholar] [CrossRef]
- Koole, S.L.; Pelham, B.W. On the nature of implicit self-esteem: The case of the name letter effect. In Motivated Social Perception: The Ontario Symposium; Spencer, S.J., Fein, S., Zanna, M.P., Olson, J.M., Eds.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2003; Volume 9, pp. 93–116. [Google Scholar]
- Baumeister, R.F. Self-Esteem: The Puzzle of Low Self-Regard; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Baumeister, R.F.; Campbell, J.D.; Krueger, J.I.; Vohs, K.D. Does High Self-Esteem Cause Better Performance, Interpersonal Success, Happiness, or Healthier Lifestyles? Psychol. Sci. Public. Interest. 2003, 4, 1–44. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Farnham, S.D. Using the implicit association test to measure self-esteem and self-concept. J. Pers. Soc. Psychol. 2000, 79, 1022–1038. [Google Scholar] [CrossRef]
- Kernis, M.H. Toward a Conceptualization of Optimal Self-Esteem. Psychol. Inq. 2003, 14, 1–26. [Google Scholar] [CrossRef]
- Creemers, D.H.; Scholte, R.H.; Engels, R.C.; Prinstein, M.J.; Wiers, R.W. Damaged Self-Esteem is Associated with Internalizing Problems. Front. Psychol. 2013, 4, 152. [Google Scholar] [CrossRef]
- Fetaini, M.; Hawari, A.; Kaki, F.; Ujaimi, R.; Tashkandi, H.; AbuSanad, A. Impact of breast cancer treatments on body image and quality of life in survivors. IJMDCs 2020, 4, 635–644. [Google Scholar] [CrossRef]
- Harter, S. The Construction of the Self; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Cole, D.A.; Jacquez, F.M.; Maschman, T.L. Social origins of depressive cognitions: A longitudinal study of self-perceived competence in children. Cogn. Ther. Res. 2001, 25, 377–395. [Google Scholar] [CrossRef]
- Mann, M.; Hosman, C.M.; Schaalma, H.P.; de Vries, N.K. Self-esteem in a broad-spectrum approach for mental health promotion. Health Educ. Res. 2004, 19, 357–372. [Google Scholar] [CrossRef]
- Sowislo, J.F.; Orth, U. Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychol. Bull. 2013, 139, 213–240. [Google Scholar] [CrossRef]
- van Tuijl, L.A.; de Jong, P.J.; Sportel, B.E.; de Hullu, E.; Nauta, M.H. Implicit and explicit self-esteem and their reciprocal relationship with symptoms of depression and social anxiety: A longitudinal study in adolescents. J. Behav. Ther. Exp. Psychiatry 2014, 45, 113–121. [Google Scholar] [CrossRef]
- Strack, F.; Deutsch, R. Reflective and impulsive determinants of social behavior. Pers. Soc. Psychol. Rev. 2004, 8, 220–247. [Google Scholar] [CrossRef]
- Bosson, J.K.; Swann, W.B., Jr.; Pennebaker, J.W. Stalking the perfect measure of implicit self-esteem: The blind men and the elephant revisited? J. Pers. Soc. Psychol. 2000, 79, 631–643. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Banaji, M.R. Implicit social cognition: Attitudes, self-esteem, and stereotypes. Psychol. Rev. 1995, 102, 4–27. [Google Scholar] [CrossRef]
- Schröder-Abé, M.; Rudolph, A.; Schütz, A. High implicit self-esteem is not necessarily advantageous: Discrepancies between explicit and implicit self-esteem and their relationship with anger expression and psychological health. Eur. J. Pers. 2007, 21, 319–339. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Nosek, B.A.; Banaji, M.R. Understanding and using the implicit association test: I. An improved scoring algorithm. J. Pers. Soc. Psychol. 2003, 85, 197–216. [Google Scholar] [CrossRef]
- Bar-Anan, Y.; Nosek, B.A. A comparative investigation of seven indirect attitude measures. Behav. Res. Methods 2014, 46, 668–688. [Google Scholar] [CrossRef]
- Krause, S.; Back, M.D.; Egloff, B.; Schmukle, S.C. Reliability of implicit self-esteem measures revisited. Eur. J. Pers. 2011, 25, 239–251. [Google Scholar] [CrossRef]
- Bosson, J.K.; Brown, R.P.; Zeigler-Hill, V.; Swann, W.B. Self-Enhancement Tendencies Among People with High Explicit Self-Esteem: The Moderating Role of Implicit Self-Esteem. Self Identity 2003, 2, 169–187. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does rejection hurt? An FMRI study of social exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef]
- Wojtyna, E.; Popiołek, K. The pain of a heart being broken: Pain experience and use of analgesics by caregivers of patients with Alzheimer’s disease. BMC Psychiatry 2015, 15, 176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayerson, N.H.; Rhodewalt, F. Role of Self-Protective Attributions in the Experience of Pain. J. Soc. Clin. Psychol. 1988, 6, 203–218. [Google Scholar] [CrossRef]
- Fennell, M. Overcoming Social Anxiety and Shyness, 2nd Edition: A Self-Help Guide Using Cognitive Behavioural Techniques; Robinson Press: London, UK, 2022. [Google Scholar]
- Miller, W.R.; Rollnick, S. Motivational Interviewing: Helping People Change and Grow (Applications of Motivational Interviewing), 4th ed.; The Guilford Press: New York, NY, USA, 2023. [Google Scholar]
- Faghani, F.; Choobforoushzadeh, A.; Sharbafchi, M.R.; Poursheikhali, H. Effectiveness of mindfulness-based supportive psychotherapy on posttraumatic growth, resilience, and self-compassion in cancer patients: A pilot study. Wien. Klin. Wochenschr. 2022, 134, 593–601. [Google Scholar] [CrossRef]
- Oliver, J.; Bennett, R. The Mindfulness and Acceptance Workbook for Self-Esteem: Using Acceptance and Commitment Therapy to Move Beyond Negative Self-Talk and Embrace Self-Compassion; New Harbinger Publications: Oakland, CA, USA, 2020. [Google Scholar]
- Bolcato, M.; Fassina, G.; Rodriguez, D.; Russo, M.; Aprile, A. The contribution of legal medicine in clinical risk management. BMC Health Serv. Res. 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n | % | |
|---|---|---|---|
| Gender | Male | 15 | 42.9 |
| Female | 20 | 57.1 | |
| Age | 21–40 years | 6 | 17.1 |
| 41–60 years | 15 | 42.9 | |
| 61–80 years | 14 | 40.0 | |
| Place of residence | City | 22 | 62.9 |
| Village | 13 | 37.1 | |
| Education | Primary | 5 | 14.3 |
| Vocational | 9 | 25.7 | |
| Secondary | 7 | 20.0 | |
| Higher | 14 | 40.0 | |
| Diagnosis | Tongue | 12 | 34.3 |
| Bottom of the mouth | 6 | 17.1 | |
| Gum | 6 | 17.1 | |
| Palate | 5 | 14.3 | |
| Lip | 4 | 11.4 | |
| Buccal mucosa | 2 | 5.7 | |
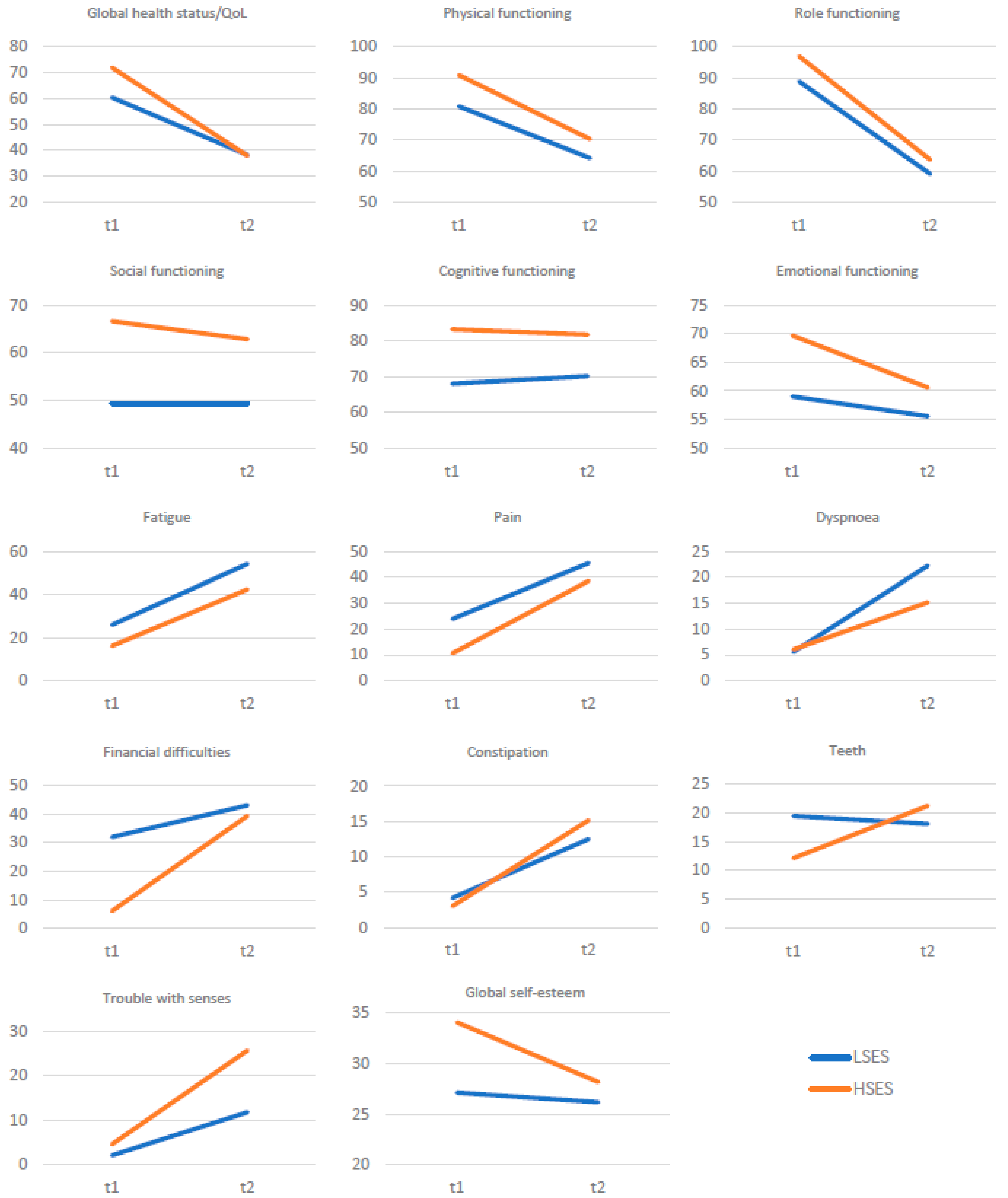
| QoL and Self-Esteem | Before Surgery | After Surgery | p | Cohen’s d | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Global health status/QoL | 64.05 | 16.27 | 38.10 | 22.44 | <0.001 | 1.31 |
| Physical functioning | 84.00 | 17.28 | 66.10 | 26.88 | <0.001 | 0.67 |
| Role functioning | 91.43 | 15.32 | 60.48 | 27.44 | <0.001 | 1.22 |
| Emotional functioning | 54.76 | 27.06 | 53.57 | 25.35 | 0.807 | 0.04 |
| Cognitive functioning | 72.86 | 26.53 | 73.81 | 19.08 | 0.840 | −0.03 |
| Social functioning | 62.38 | 32.42 | 57.14 | 25.97 | 0.299 | 0.18 |
| Fatigue | 22.86 | 22.05 | 50.48 | 30.17 | <0.001 | −0.75 |
| Nausea and vomiting | 1.43 | 4.73 | 16.67 | 24.25 | <0.001 | −0.60 |
| Dyspnoea | 5.71 | 15.09 | 20.00 | 25.82 | 0.002 | −0.58 |
| Insomnia | 40.00 | 36.87 | 57.14 | 32.91 | 0.029 | −0.38 |
| Appetite loss | 37.14 | 40.24 | 50.48 | 39.08 | 0.104 | −0.28 |
| Constipation | 5.71 | 15.09 | 18.10 | 26.00 | 0.017 | −0.42 |
| Diarrhoea | 0.95 | 5.63 | 2.86 | 9.47 | 0.324 | −0.17 |
| Financial difficulties | 20.95 | 30.34 | 22.86 | 23.94 | 0.661 | −0.07 |
| Pain | 19.76 | 20.32 | 43.33 | 19.99 | <0.001 | −0.93 |
| Swallowing | 7.38 | 13.67 | 45.48 | 28.25 | <0.001 | −1.13 |
| Teeth | 17.14 | 23.39 | 19.05 | 30.56 | 0.721 | −0.06 |
| Opening mouth | 23.81 | 29.78 | 56.19 | 36.85 | <0.001 | −0.81 |
| Dry mouth | 24.76 | 24.71 | 60.00 | 26.57 | <0.001 | −1.13 |
| Sticky saliva | 30.48 | 26.04 | 56.19 | 28.89 | <0.001 | −0.69 |
| Senses problems | 2.86 | 8.56 | 16.19 | 20.80 | <0.001 | −0.71 |
| Coughing | 3.81 | 10.76 | 13.33 | 18.44 | 0.006 | −0.50 |
| Felt ill | 23.81 | 29.78 | 41.90 | 31.67 | 0.002 | −0.55 |
| Speech problems | 10.79 | 16.93 | 39.37 | 22.44 | <0.001 | −1.12 |
| Trouble with social eating | 18.33 | 20.29 | 47.62 | 26.86 | <0.001 | −1.02 |
| Trouble with social contact | 16.38 | 20.15 | 36.38 | 22.77 | <0.001 | −0.91 |
| Less sexuality | 36.19 | 39.29 | 54.76 | 40.94 | 0.016 | −0.43 |
| Pain killers | 25.71 | 44.34 | 88.57 | 32.28 | <0.001 | −1.15 |
| Nutritional supplements | 11.43 | 32.28 | 45.71 | 50.54 | 0.002 | −0.58 |
| Feeding tube | 0.00 | 0.00 | 65.71 | 48.16 | <0.001 | −1.36 |
| Weight loss | 28.57 | 45.83 | 57.14 | 50.21 | 0.010 | −0.46 |
| Weight gain | 0.00 | 0.00 | 2.86 | 16.90 | 0.324 | −0.17 |
| Self-esteem | 29.31 | 4.34 | 27.34 | 5.14 | 0.031 | 0.38 |
| Characteristics | n | % | M | SD | |
|---|---|---|---|---|---|
| Age | 51.76 | 6.83 | |||
| Education | Primary | 4 | 4.2 | ||
| Vocational | 21 | 21.9 | |||
| Secondary | 35 | 36.5 | |||
| High | 36 | 37.5 | |||
| Marital status | Single | 12 | 17.4 | ||
| Married or living together | 67 | 69.8 | |||
| Divorced | 11 | 11.5 | |||
| Widowed | 6 | 6.3 | |||
| Self-esteem | Explicit self-esteem | 31.52 | 4.98 | ||
| Implicit self-esteem | 0.42 | 0.63 | |||
| Low explicit and implicit self-esteem | 21 | 21.9 | |||
| High explicit and implicit self-esteem | 32 | 33.3 | |||
| Fragile self-esteem | 43 | 44.8 | |||
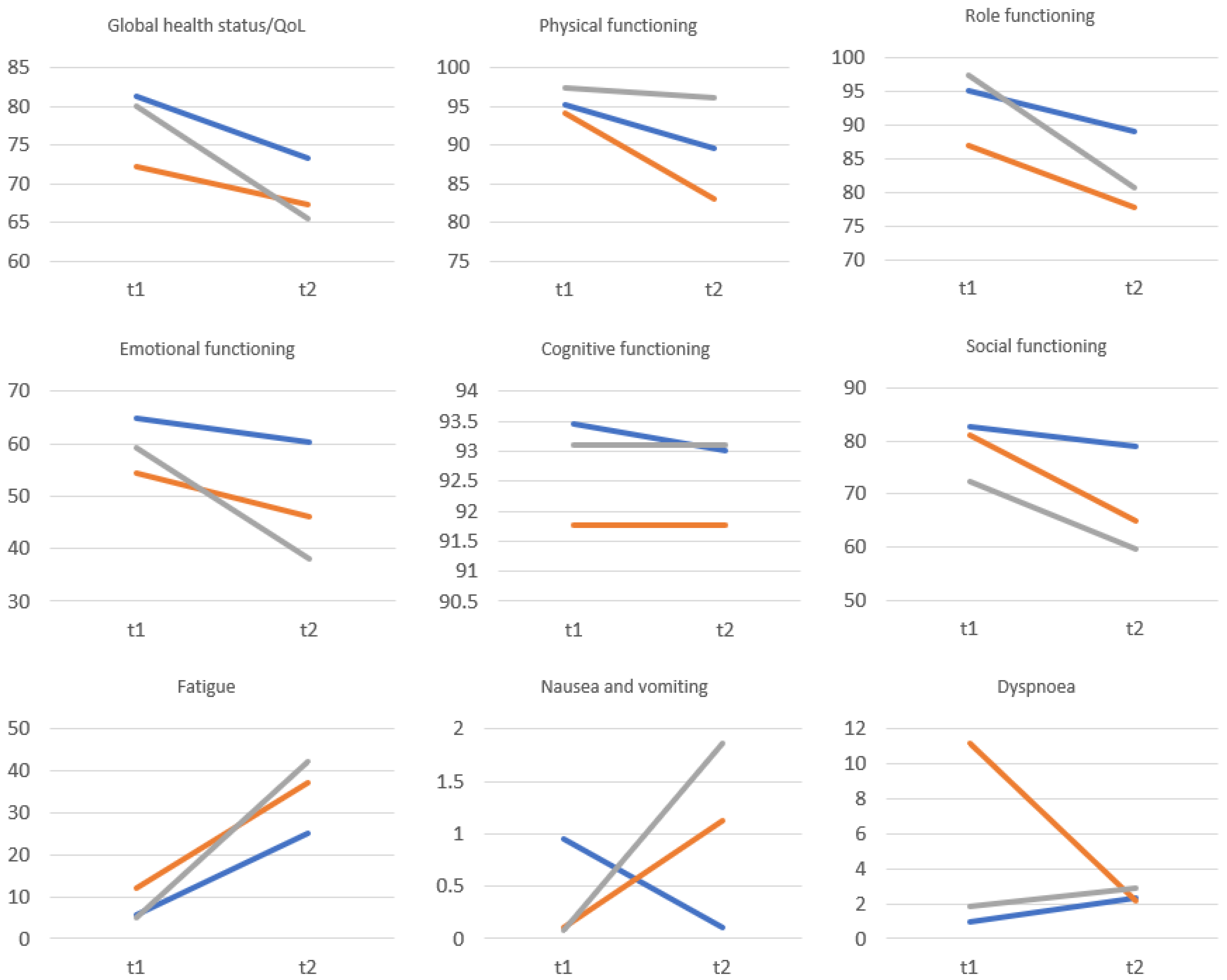
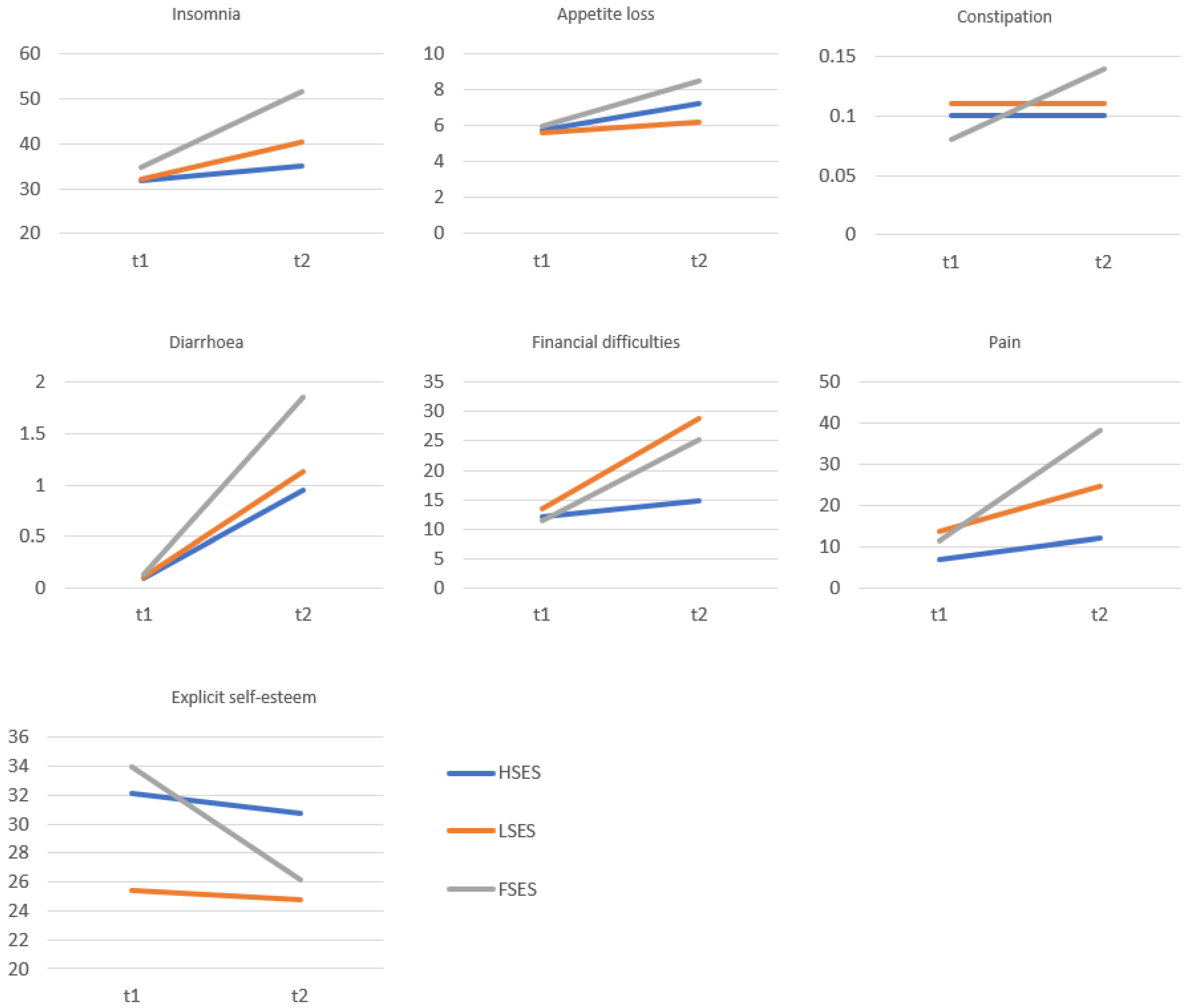
| QoL and Self-Esteem | Before Surgery | After Surgery | p | Cohen’s d | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Global health status/QoL | 78.71 | 15.42 | 68.42 | 20.67 | <0.001 | 0.56 |
| Physical functioning | 96.18 | 16.01 | 89.96 | 21.15 | <0.001 | 0.33 |
| Role functioning | 94.36 | 12.83 | 82.85 | 24.71 | <0.001 | 0.58 |
| Emotional functioning | 59.97 | 23.95 | 47.11 | 21.63 | <0.001 | 0.57 |
| Cognitive functioning | 92.92 | 24.66 | 92.76 | 21.04 | 0.890 | 0.01 |
| Social functioning | 77.87 | 30.27 | 67.23 | 22.75 | <0.001 | 0.40 |
| Fatigue | 6.91 | 21.18 | 35.32 | 28.64 | <0.001 | 1.13 |
| Nausea and vomiting | 0.39 | 3.36 | 1.13 | 5.51 | 0.562 | 0.16 |
| Dyspnoea | 3.61 | 11.92 | 2.55 | 14.77 | 0.713 | 0.08 |
| Insomnia | 33.24 | 32.70 | 43.76 | 31.03 | 0.002 | 0.33 |
| Appetite loss | 5.87 | 19.04 | 7.56 | 16.38 | 0.304 | 0.10 |
| Constipation | 0.09 | 4.13 | 0.12 | 9.56 | 0.875 | 0.01 |
| Diarrhoea | 0.12 | 3.07 | 1.40 | 8.89 | 0.504 | 0.19 |
| Financial difficulties | 12.19 | 18.22 | 22.55 | 13.94 | <0.001 | 0.64 |
| Pain | 10.38 | 19.01 | 26.49 | 17.48 | <0.001 | 0.88 |
| Global self-esteem | 31.52 | 4.98 | 27.36 | 5.02 | <0.001 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtyna, E.; Pasek, M.; Nowakowska, A.; Goździalska, A.; Jochymek, M. Self at Risk: Self-Esteem and Quality of Life in Cancer Patients Undergoing Surgical Treatment and Experiencing Bodily Deformities. Healthcare 2023, 11, 2203. https://doi.org/10.3390/healthcare11152203
Wojtyna E, Pasek M, Nowakowska A, Goździalska A, Jochymek M. Self at Risk: Self-Esteem and Quality of Life in Cancer Patients Undergoing Surgical Treatment and Experiencing Bodily Deformities. Healthcare. 2023; 11(15):2203. https://doi.org/10.3390/healthcare11152203
Chicago/Turabian StyleWojtyna, Ewa, Małgorzata Pasek, Aleksandra Nowakowska, Anna Goździalska, and Małgorzata Jochymek. 2023. "Self at Risk: Self-Esteem and Quality of Life in Cancer Patients Undergoing Surgical Treatment and Experiencing Bodily Deformities" Healthcare 11, no. 15: 2203. https://doi.org/10.3390/healthcare11152203
APA StyleWojtyna, E., Pasek, M., Nowakowska, A., Goździalska, A., & Jochymek, M. (2023). Self at Risk: Self-Esteem and Quality of Life in Cancer Patients Undergoing Surgical Treatment and Experiencing Bodily Deformities. Healthcare, 11(15), 2203. https://doi.org/10.3390/healthcare11152203






