A Systematic Review on the Impact of Seasonality on Severe Mental Illness Admissions: Does Seasonal Variation Affect Coercion?
Abstract
1. Introduction
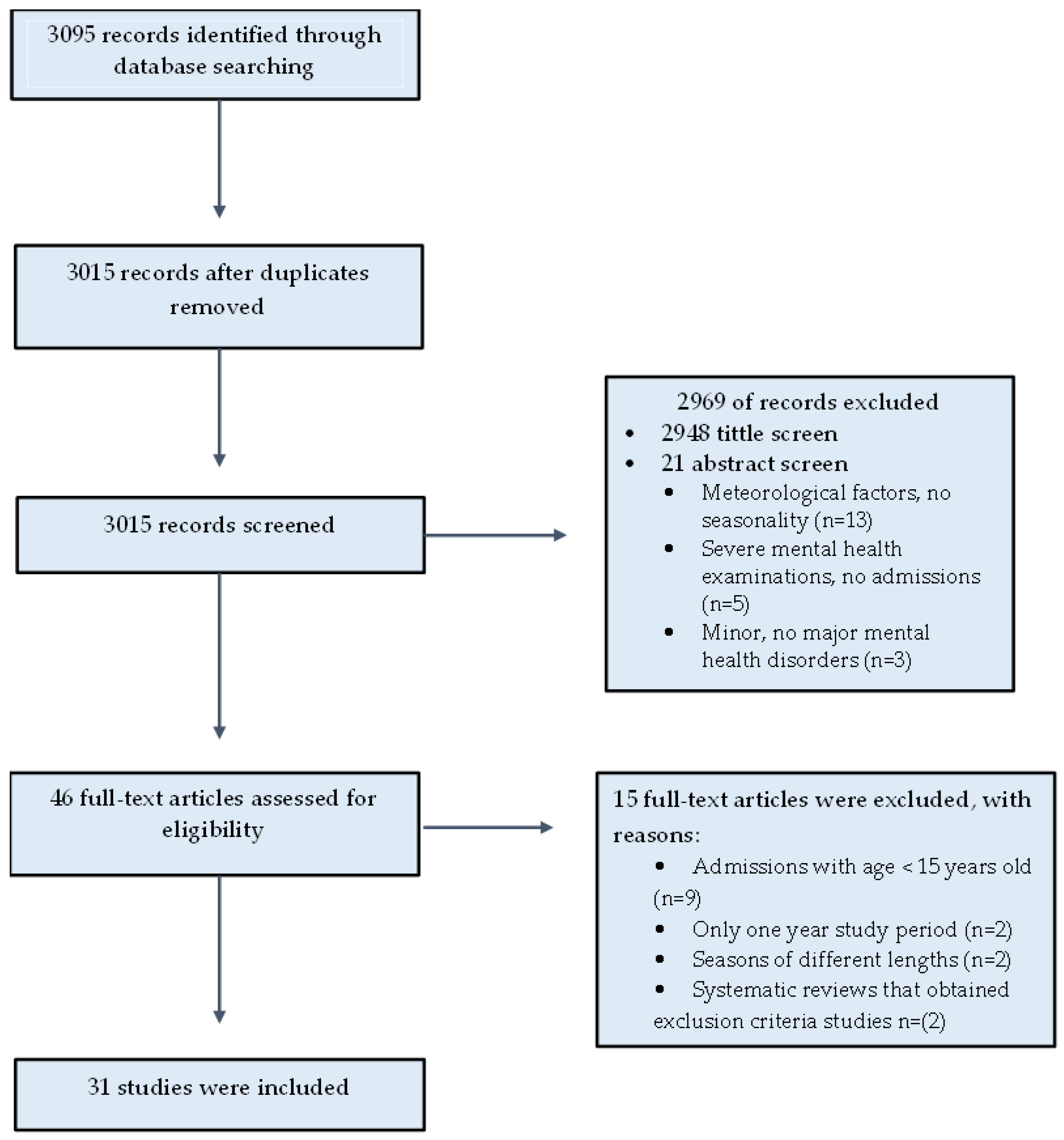
2. Materials and Methods
3. Results
3.1. Affective Disorders
3.1.1. Bipolar Disorder
3.1.2. Hypomanic Episodes
3.1.3. Manic Episodes
3.1.4. Mixed Episodes
3.1.5. Bipolar Depression
3.1.6. Unipolar Depression
3.2. Schizophrenia Spectrum Disorders
3.2.1. Schizophrenia
3.2.2. Schizoaffective Disorder
3.3. Involuntary Admissions
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tountas, Y. The historical origins of the basic concepts of health promotion and education: The role of ancient Greek philosophy and medicine. Health Promot. Int. 2009, 24, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Knappenberger, P.C.; Michaels, P.J.; Novicoff, W.M. Seasonality of climate-human mortality relationships in US cities and Impacts of climate change. Clim. Res. 2004, 26, 61–76. [Google Scholar] [CrossRef]
- Nakaji, S.; Parodi, S.; Fontana, V.; Umeda, T.; Suzuki, K.; Sakamoto, J.; Sugawara, K. Seasonal changes in mortality rates from main causes of death in Japan. Eur. J. Epidemiol. 2004, 19, 905–913. [Google Scholar] [CrossRef]
- Seretakis, D.; Lagiou, P.; Lipworth, L.; Signorello, L.B.; Rothman, K.J.; Trichopoulos, D. Changing seasonality of mortality from coronary heart disease. JAMA 1997, 278, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; Kelleher, C.; Diem, G.; Concin, H.; Ruttmann, E. Estimation of seasonal variations in risk factor profiles and mortality from coronary heart disease. Wien. Klin. Wochenschr. 2004, 116, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Bhatia, S.; Mears, J.; Dibu, G.; Deshmukh, A. Seasonal periodicity of ischemic heart disease and heart failure. Heart Fail. Clin. 2017, 13, 681–689. [Google Scholar] [CrossRef]
- Matizirofa, L.; Ranganai, E. An analysis of recent stroke cases in South Africa: Trend, seasonality and predictors. S. Afr. Med. J. 2020, 110, 92–99. [Google Scholar] [CrossRef]
- Tsementzis, S.A.; Kennet, R.P.; Hitchcock, E.R.; Gill, J.S.; Beevers, D.G. Seasonal variation of cerebrovascular diseases. Acta Neurochir. 1991, 111, 80–83. [Google Scholar] [CrossRef]
- Dowell, S.F.; Ho, M.S. Seasonality of infectious diseases and severe acute respiratory syndrome–what we don’t know can hurt us. Lancet Infect. Dis. 2004, 4, 704–708. [Google Scholar] [CrossRef]
- Fisman, D.N. Seasonality of infectious diseases. Annu. Rev. Public Health 2007, 28, 127–143. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Yamamoto-Honda, R.; Kajio, H.; Kishimoto, M.; Noto, H.; Hachiya, R.; Noda, M. Seasonal variations of severe hypoglycemia in patients with type 1 diabetes mellitus, type 2 diabetes mellitus, and non-diabetes mellitus: Clinical analysis of 578 hypoglycemia cases. Medicine 2014, 93, e148. [Google Scholar] [CrossRef] [PubMed]
- Gruskay, J.; Smith, J.; Kepler, C.K.; Radcliff, K.; Harrop, J.; Albert, T.; Vaccaro, A. The seasonality of postoperative infection in spine surgery. J. Neurosurg. Spine 2013, 18, 57–62. [Google Scholar] [CrossRef]
- Kane, P.; Chen, C.; Post, Z.; Radcliff, K.; Orozco, F.; Ong, A. Seasonality of infection rates after total joint arthroplasty. Orthopedics 2014, 37, e182–e186. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.; Berry, M.; Martin, P.; Rojas-Garcia, A.; Moonesinghe, S.R. Seasonality in surgical outcome data: A systematic review and narrative synthesis. Br. J. Anaesth. 2021, 128, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Belleville, G.; Foldes-Busque, G.; Dixon, M.; Marquis-Pelletier, É.; Barbeau, S.; Poitras, J.; Marchand, A. Impact of seasonal and lunar cycles on psychological symptoms in the ED: An empirical investigation of widely spread beliefs. Gen. Hosp. Psychiatry 2013, 35, 192–194. [Google Scholar] [CrossRef]
- Brandl, E.J.; Lett, T.A.; Bakanidze, G.; Heinz, A.; Bermpohl, F.; Schouler-Ocak, M. Weather conditions influence the number of psychiatric emergency room patients. Int. J. Biometeorol. 2018, 62, 843–850. [Google Scholar] [CrossRef]
- Cervellin, G.; Comelli, I.; Lippi, G.; Comelli, D.; Rastelli, G.; Ossola, P.; Marchesi, C. The number of emergency department visits for psychiatric emergencies is strongly associated with mean temperature and humidity variations. Results of a nine year survey. Emerg. Care J. 2014, 10, 48–53. [Google Scholar] [CrossRef]
- Corcuera Hotz, I.; Hajat, S. The effects of temperature on accident and emergency department attendances in London: A time-series regression analysis. Int. J. Environ. Res. Public Health 2020, 17, 1957. [Google Scholar] [CrossRef]
- Rollnik, J.D.; Dimsdale, J.E.; Ng, B. Variation of psychiatric emergencies across seasons in San Diego county. Depress. Anxiety 2000, 11, 48–49. [Google Scholar] [CrossRef]
- De Graaf, R.; van Dorsselaer, S.; Ten Have, M.; Schoemaker, C.; Vollebergh, W.A. Seasonal variations in mental disorders in the general population of a country with a maritime climate: Findings from the Netherlands mental health survey and incidence study. Am. J. Epidemiol. 2005, 162, 654–661. [Google Scholar] [CrossRef]
- Heboyan, V.; Stevens, S.; McCall, W.V. Effects of seasonality and daylight savings time on emergency department visits for mental health disorders. Am. J. Emerg. Med. 2019, 37, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.C.; Shaman, J.; Washburn, I.J.; Vuchinich, S.; Neppl, T.K.; Capaldi, D.M.; Conger, R.D. Two longterm studies of seasonal variation in depressive symptoms among community participants. J. Affect. Disord. 2013, 151, 837–842. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Lajnef, M.; Bellivier, F.; Jamain, S.; Gard, S.; Kahn, J.P.; Etain, B. Genetic association study of circadian genes with seasonal pattern in bipolar disorders. Sci. Rep. 2015, 5, 10232. [Google Scholar] [CrossRef] [PubMed]
- Spasova, Z. Seasonal variations of schizophrenic patients in emergency departments in Sofia, Bulgaria. South East. Eur. J. Public Health 2015, 1, 32–39. [Google Scholar] [CrossRef]
- Aguglia, A.; Serafini, G.; Solano, P.; Giacomini, G.; Conigliaro, C.; Salvi, V.; Mencacci, C.; Romano, M.; Aguglia, E.; Amore, M. The role of seasonality and photoperiod on the lethality of suicide attempts: A case-control study. J. Affect. Disord. 2019, 246, 895–901. [Google Scholar] [CrossRef]
- Christodoulou, C.; Douzenis, A.; Papadopoulos, F.C.; Papadopoulou, A.; Bouras, G.; Gournellis, R.; Lykouras, L. Suicide and seasonality. Acta Psychiatr. Scand. 2012, 125, 127–146. [Google Scholar] [CrossRef]
- Murray, G.; Lam, R.W.; Beaulieu, S.; Sharma, V.; Cervantes, P.; Parikh, S.V.; Yatham, L.N. Do symptoms of bipolar disorder exhibit seasonal variation? A multisite prospective investigation. Bipolar Disord. 2011, 13, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Moncalvo, M.; Solia, F.; Maina, G. Involuntary admissions in Italy: The impact of seasonality. Int. J. Psychiatry Clin. Pract. 2016, 20, 232–238. [Google Scholar] [CrossRef]
- Bakstein, E.; Mladá, K.; Fárková, E.; Kolenič, M.; Španiel, F.; Manková, D.; Hajek, T. Cross-sectional and within-subject seasonality and regularity of hospitalizations: A population study in mood disorders and schizophrenia. Bipolar Disord. 2020, 22, 508–516. [Google Scholar] [CrossRef]
- Angermeyer, C. Schizophrenia and violence. Acta Psychiatr. Scand. 2000, 102, 63–67. [Google Scholar] [CrossRef]
- Fazel, S.; Gulati, G.; Linsell, L.; Geddes, J.R.; Grann, M. Schizophrenia and violence: Systematic review and meta-analysis. PLoS Med. 2009, 6, e1000120. [Google Scholar] [CrossRef] [PubMed]
- Volavka, J. Violence in schizophrenia and bipolar disorder. Psychiatr. Danub. 2013, 25, 24–33. [Google Scholar] [PubMed]
- Walsh, E.; Buchanan, A.; Fahy, T. Violence and schizophrenia: Examining the evidence. Br. J. Psychiatry 2002, 180, 490–495. [Google Scholar] [CrossRef]
- Amr, M.; Volpe, F.M. Seasonal influences on admissions for mood disorders and schizophrenia in a teaching psychiatric hospital in Egypt. J. Affect. Disord. 2012, 137, 56–60. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Lok, K.I.; Zhang, Q.; Hong, L.; Ungvari, G.S.; Cheung, T.; Bressington, D.T.; Xiang, Y.T. Voluntary admissions for patients with schizophrenia: A systematic review and meta-analysis. Asian J. Psychiatr. 2020, 48, 101902. [Google Scholar] [CrossRef]
- Saya, A.; Brugnoli, C.; Piazzi, G.; Liberato, D.; Di Ciaccia, G.; Niolu, C.; Siracusano, A. Criteria, procedures, and future prospects of involuntary treatment in psychiatry around the world: A narrative review. Front. Psychiatry 2019, 10, 271. [Google Scholar] [CrossRef]
- Wang, J.P.; Chiu, C.C.; Yang, T.H.; Liu, T.H.; Wu, C.Y.; Chou, P. The low proportion and associated factors of involuntary admission in the psychiatric emergency service in Taiwan. PLoS ONE 2015, 10, e0129204. [Google Scholar] [CrossRef]
- Flammer, E.; Steinert, T.; Eisele, F.; Bergk, J.; Uhlmann, C. Who is subjected to coercive measures as a psychiatric inpatient? A multi-level analysis. Clin. Pract. Epidemiology Ment. Health 2013, 9, 110. [Google Scholar] [CrossRef]
- De Berardis, D.; Fornaro, M.; Orsolini, L.; Iasevoli, F.; Tomasetti, C.; De Bartolomeis, A.; Serroni, N.; Valchera, A.; Carano, A.; Vellante, F.; et al. The Role of Inhaled Loxapine in the Treatment of Acute Agitation in Patients with Psychiatric Disorders: A Clinical Review. Int. J. Mol. Sci. 2017, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Fugger, G.; Gleiss, A.; Baldinger, P.; Strnad, A.; Kasper, S.; Frey, R. Psychiatric patients’ perception of physical restraint. Acta Psychiatr. Scand. 2016, 133, 221–231. [Google Scholar] [CrossRef]
- Fujishiro, K.; Gee, G.C.; de Castro, A.B. Associations of workplace aggression with work-related well-being among nurses in the Philippines. Am. J. Public Health 2011, 101, 861–867. [Google Scholar] [CrossRef]
- Krieger, E.; Moritz, S.; Lincoln, T.M.; Fischer, R.; Nagel, M. Coercion in psychiatry: A cross-sectional study on staff views and emotions. J. Psychiatr. Ment. Health Nurs. 2021, 28, 149–162. [Google Scholar] [CrossRef]
- Kaplan, Z.; Schild, K.; Levine, J. Violence in hospitalized psychiatric patients: Diurnal and seasonal patterns. Psychiatry Res. 1996, 60, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Aguglia, A.; Borsotti, A.; Cuniberti, F.; Serafini, G.; Amore, M.; Maina, G. The influence of sunlight exposure on hospitalization in emergency psychiatry. Chronobiol. Int. 2017, 34, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Borsotti, A.; Maina, G. Bipolar disorders: Is there an influence of seasonality or photoperiod? Braz. J. Psychiatry 2018, 40, 6–11. [Google Scholar] [CrossRef]
- Cassidy, F.; Carroll, B.J. Seasonal variation of mixed and pure episodes of bipolar disorder. J. Affect. Disord. 2002, 68, 25–31. [Google Scholar] [CrossRef]
- Clarke, M.; Moran, P.; Keogh, F.; Morris, M.; Kinsella, A.; Walsh, D.; O’Callaghan, E. Seasonal influences on admissions in schizophrenia and affective disorder in Ireland. Schizophr. Res. 1998, 34, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Moran, P.; Keogh, F.; Morris, M.; Kinsella, A.; Larkin, C.; O’Callaghan, E. Seasonal influences on admissions for affective disorder and schizophrenia in Ireland: A comparison of first and readmissions. Eur. Psychiatry 1999, 14, 251–255. [Google Scholar] [CrossRef]
- Daniels, B.A.; Kirkby, K.C.; Mitchell, P.; Hay, D.; Mowry, B. Seasonal variation in hospital admission for bipolar disorder, depression and schizophrenia in Tasmania. Acta Psychiatr. Scand. 2000, 102, 38–43. [Google Scholar] [CrossRef]
- Dominiak, M.; Swiecicki, L.; Rybakowski, J. Psychiatric hospitalizations for affective disorders in Warsaw, Poland: Effect of season and intensity of sunlight. Psychiatry Res. 2015, 229, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kaliaperumal, V.G.; Chatterji, S.; Rao, S.; Murthy, R.S. Climate and admissions for mania in the tropics. J. Affect. Disord. 1992, 26, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Hornsby, H.; Hay, D. Seasonality of mania: A Tasmanian study. Aust. N. Z. J. Psychiatry 1995, 29, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Kerr-Correa, F.; Souza, L.B.; Calil, H.M. Affective disorders, hospital admissions, and seasonal variation of mania in a subtropical area, southern hemisphere. Psychopathology 1998, 31, 265–269. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, L.; Joe, S.H.; Suh, K.Y. Effects of season and climate on the first manic episode of bipolar affective disorder in Korea. Psychiatry Res. 2002, 113, 151–159. [Google Scholar] [CrossRef]
- Medici, C.R.; Vestergaard, C.H.; Hadzi-Pavlovic, D.; Munk-Jørgensen, P.; Parker, G. Seasonal variations in hospital admissions for mania: Examining for associations with weather variables over time. J. Affect. Disord. 2016, 205, 81–86. [Google Scholar] [CrossRef]
- Modai, I.; Kikinzon, L.; Valevski, A. Environmental factors and admission rates in patients with major psychiatric disorders. Chronobiol. Int. 1994, 11, 196–199. [Google Scholar] [CrossRef]
- Morken, G.; Lilleeng, S.; Linaker, O.M. Seasonal variation in suicides and in admissions to hospital for mania and depression. J. Affect. Disord. 2002, 69, 39–45. [Google Scholar] [CrossRef]
- Parker, G.; Hadzi-Pavlovic, D.; Bayes, A.; Graham, R. Relationship between photoperiod and hospital admissions for mania in New South Wales, Australia. J. Affect. Disord. 2018, 226, 72–76. [Google Scholar] [CrossRef]
- Parker, G.B.; Graham, R.K. Seasonal variations in rates of hospitalisation for mania and hypomania in psychiatric hospitals in NSW. J. Affect. Disord. 2016, 191, 289–291. [Google Scholar] [CrossRef]
- Parker, G.B.; Hadzi-Pavlovic, D.; Graham, R.K. Examining for any impact of climate change on the association between seasonality and hospitalization for mania. J. Affect. Disord. 2017, 208, 431–435. [Google Scholar] [CrossRef]
- Rajkumar, R.P.; Sarkar, S. Seasonality of admissions for mania: Results from a general hospital psychiatric unit in Pondicherry, India. Prim. Care Companion CNS Disord. 2015, 17, 26834. [Google Scholar] [CrossRef][Green Version]
- Takei, N.; O’Callaghan, E.; Sham, P.; Glover, G.; Tamura, A.; Murray, R. Seasonality of admissions in the psychoses: Effect of diagnosis, sex, and age at onset. Br. J. Psychiatry 1992, 161, 506–511. [Google Scholar] [CrossRef]
- Volpe, F.M.; da Silva, E.M.; dos Santos, T.N.; de Freitas, D.E.G. Further evidence of seasonality of mania in the tropics. J. Affect. Disord. 2010, 124, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Volpe, F.M.; Del Porto, J.A. Seasonality of admissions for mania in a psychiatric hospital of Belo Horizonte, Brazil. J. Affect. Disord. 2006, 94, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.K.; Sharma, V.; Kueneman, K. Seasonality of manic depressive illness in Canada. J. Affect. Disord. 1999, 55, 99–105. [Google Scholar] [CrossRef]
- Yang, A.C.; Yang, C.H.; Hong, C.J.; Liou, Y.J.; Shia, B.C.; Peng, C.K.; Huang, N.E.; Tsai, S.J. Effects of Age, Sex, Index Admission, and Predominant Polarity on the Seasonality of Acute Admissions for Bipolar Disorder: A Population-Based Study. Chronobiol. Int. 2013, 30, 478–485. [Google Scholar] [CrossRef]
- Davies, G.; Ahmad, F.; Chant, D.; Welham, J.; McGrath, J. Seasonality of first admissions for schizophrenia in the Southern Hemisphere. Schizophr. Res. 2000, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hinterbuchinger, B.; König, D.; Gmeiner, A.; Listabarth, S.; Fellinger, M.; Thenius, C.; Pruckner, N. Seasonality in schizophrenia-An analysis of a nationwide registry with 110,735 hospital admissions. Eur. Psychiatry 2020, 63, e55. [Google Scholar] [CrossRef]
- Shiloh, R.; Shapira, A.; Potchter, O.; Hermesh, H.; Popper, M.; Weizman, A. Effects of climate on admission rates of schizophrenia patients to psychiatric hospitals. Eur. Psychiatry 2005, 20, 61–64. [Google Scholar] [CrossRef]
- Takei, N.; Murray, R.M. Gender difference of schizophrenia in seasonal admissions in Scotland. Br. J. Psychiatry 1993, 162, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.H.; Lee, H.C.; Liu, T.C.; Chen, C.S.; Lin, H.C. Seasonal variation in schizophrenia admissions in a Chinese population. Schizophr. Res. 2006, 86, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.W. Environmental connections: A deeper look into mental illness. Environ. Health Perspect. 2007, 115, 404–410. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Malig, B.J.; Basu, R. The association between ozone and fine particles and mental health-related emergency department visits in California, 2005–2013. PLoS ONE 2021, 16, e0249675. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Susser, E.S. Serologic evidence of prenatal influenza in the etiology of Schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol Res. Health 2001, 25, 85. [Google Scholar]
- Kishi, T.; Kitajima, T.; Ikeda, M.; Yamanouchi, Y.; Kinoshita, Y.; Kawashima, K.; Okochi, T.; Okumura, T.; Tsunoka, T.; Inada, T.; et al. Association study of clock gene (CLOCK) and schizophrenia and mood disorders in the Japanese population. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 293–297. [Google Scholar] [CrossRef]
- Garbazza, C.; Benedetti, F. Genetic factors affecting seasonality, mood, and the circadian clock. Front. Endocrinol. 2018, 9, 481. [Google Scholar] [CrossRef]
- Byrne, E.M.; Heath, A.C.; Madden, P.A.; Pergadia, M.L.; Hickie, I.B.; Montgomery, G.W.; Martin, N.G.; Wray, N.R. Testing the role of circadian genes in conferring risk for psychiatric disorders. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 254–260. [Google Scholar] [CrossRef]
- Lamont, E.W.; Legault-Coutu, D.; Cermakian, N.; Boivin, D.B. The role of circadian clock genes in mental disorders. Dialogues Clin. Neurosci. 2022, 9, 333–342. [Google Scholar] [CrossRef]
- Charrier, A.; Olliac, B.; Roubertoux, P.; Tordjman, S. Clock genes and altered sleep–wake rhythms: Their role in the development of psychiatric disorders. Int. J. Mol. Sci. 2017, 18, 938. [Google Scholar] [CrossRef]
- Gupta, T.; Sahni, D.; Gupta, S.K. An Investigation into the Role of Melatonin in Papez Circuit and Hypothalamic–pituitary Axis. J. Neurol. Surg. Part B Skull Base 2018, 79, P007. [Google Scholar] [CrossRef]
- Maruani, J.; Anderson, G.; Etain, B.; Lejoyeux, M.; Bellivier, F.; Geoffroy, P.A. The neurobiology of adaptation to seasons: Relevance and correlations in bipolar disorders. Chronobiol. Int. 2018, 35, 1335–1353. [Google Scholar] [CrossRef]
- Quera Salva, M.A.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian rhythms, melatonin and depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef]
- Wang, B.; Chen, D. Evidence for seasonal mania: A review. J. Psychiatr. Pract. 2013, 19, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Altunsoy, N.; Yüksel, R.N.; Cingi Yirun, M.; Kılıçarslan, A.; Aydemir, C. Exploring the relationship between vitamin D and mania: Correlations between serum vitamin D levels and disease activity. Nord. J. Psychiatry 2018, 72, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Brewerton, T.D.; Putnam, K.T.; Lewine, R.R.; Risch, S.C. Seasonality of cerebrospinal fluid monoamine metabolite concentrations and their associations with meteorological variables in humans. J. Psychiatr. Res. 2018, 99, 76–82. [Google Scholar] [CrossRef]
- Ljubičić, Đ.; Stipčević, T.; Pivac, N.; Jakovljević, M.; Mück-Šeler, D. The influence of daylight exposure on platelet 5-HT levels in patients with major depression and schizophrenia. J. Photochem. Photobiol. B Biol. 2007, 89, 63–69. [Google Scholar] [CrossRef]
- Sarrias, M.J.; Artigas, F.; Martínez, E.; Gelpí, E. Seasonal changes of plasma serotonin and related parameters: Correlation with environmental measures. Biol. Psychiatry 1989, 26, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Reid, C.; Kaye, D.M.; Jennings, G.L.; Esler, M.D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002, 360, 1840–1842. [Google Scholar] [CrossRef] [PubMed]
- Pail, G.; Huf, W.; Pjrek, E.; Winkler, D.; Willeit, M.; Praschak-Rieder, N.; Kasper, S. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology 2011, 64, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Flandreau, E.I.; Ressler, K.J.; Owens, M.J.; Nemeroff, C.B. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 2012, 37, 27–38. [Google Scholar] [CrossRef]
- Arnett, M.G.; Muglia, L.M.; Laryea, G.; Muglia, L.J. Genetic approaches to hypothalamic-pituitary-adrenal axis regulation. Neuropsychopharmacology 2016, 41, 245–260. [Google Scholar] [CrossRef]
- Dalla, C.; Antoniou, K.; Drossopoulou, G.; Xagoraris, M.; Kokras, N.; Sfikakis, A.; Papadopoulou-Daifoti, Z. Chronic mild stress impact: Are females more vulnerable? Neuroscience 2005, 135, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Rao, R.T.; Hartmanshenn, C.; Androulakis, I.P. Modeling the influence of seasonal differences in the HPA axis on synchronization of the circadian clock and cell cycle. Endocrinology 2018, 159, 1808–1826. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, J.; Doran, J.; Manicavasagar, V.; Parker, G. The precipitants of manic/hypomanic episodes in the context of bipolar disorder: A review. J. Affect. Disord. 2011, 133, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, J.; Whitton, A.; Parker, G.; Doran, J.; Manicavasagar, V.; Delmas, K. Triggers of mania and depression in young adults with bipolar disorder. J. Affect. Disord. 2012, 143, 196–202. [Google Scholar] [CrossRef]
- Fisekovic, S.; Licanin, I.; Cesir, A. Prevalence of neurotic, somatoform and stress induced disorders in relation to the seasons and climatic factors during the 2010/2011. Mater. Sociomed. 2012, 24, 190. [Google Scholar] [CrossRef]
- D’Mello, D.A.; McNeil, J.A. Seasons and bipolar disorder. Ann. Clin. Psychiatry 1995, 7, 11–18. [Google Scholar] [CrossRef]
- Peluola, A.; Mela, M.; Adelugba, O.O. A review of violent incidents in a multilevel secure forensic psychiatric hospital: Is there a seasonal variation? Med. Sci. Law 2013, 53, 72–79. [Google Scholar] [CrossRef]
- Volpe, F.M.; Tavares, A.; del Porto, J.A. Seasonality of three dimensions of mania: Psychosis, aggression and suicidality. J. Affect. Disord. 2008, 108, 95–100. [Google Scholar] [CrossRef]
- Paavola, P.; Tiihonen, J. Seasonal variation of seclusion incidents from violent and suicidal acts in forensic psychiatric patients. Int. J. Law Psychiatry 2010, 33, 27–34. [Google Scholar] [CrossRef]
- Roitman, G.; Orev, E.; Schreiber, G. Annual rhythms of violence in hospitalized affective patients: Correlation with changes in the duration of the daily photoperiod. Acta Psychiatr. Scand. 1990, 82, 73–76. [Google Scholar] [CrossRef]
- Reitan, S.K.; Helvik, A.S.; Iversen, V. Use of mechanical and pharmacological restraint over an eight-year period and its relation to clinical factors. Nord. J. Psychiatry 2018, 72, 24–30. [Google Scholar] [CrossRef]
- Wynn, R. Polar day and polar night: Month of year and time of day and the use of physical and pharmacological restraint in a north Norwegian university psychiatric hospital. Arct. Med. Res. 1996, 55, 174–181. [Google Scholar]
- Kuivalainen, S.; Vehviläinen-Julkunen, K.; Louheranta, O.; Putkonen, A.; Repo-Tiihonen, E.; Tiihonen, J. Seasonal variation of hospital violence, seclusion and restraint in a forensic psychiatric hospital. Int. J. Law Psychiatry 2017, 52, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Fan, H.; Liu, Y.; Ding, G. Influence of heat waves on daily hospital visits for mental illness in Jinan, China—A case-crossover study. Int. J. Environ. Res. Public Health 2019, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Z.; Ma, G.; Cheng, J.; Xu, H.; Qin, W.; Su, H. Effects of extreme precipitation on hospitalization risk and disease burden of schizophrenia in urban and rural Lu’an, China, from 2010 to 2019. Environ. Sci. Pollut. Res. 2022, 29, 19176–19184. [Google Scholar] [CrossRef] [PubMed]
- Stott, P. How climate change affects extreme weather events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Aguglia, A.; Serafini, G.; Escelsior, A.; Amore, M.; Maina, G. What is the role of meteorological variables on involuntary admission in psychiatric ward? An Italian cross-sectional study. Environ. Res. 2020, 180, 108800. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Gasparrini, A.; Armstrong, B.; Honda, Y.; Chung, Y.; Hashizume, M. Suicide and ambient temperature: A multi-country multi-city study. Environ. Health Perspect. 2019, 127, 117007. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Halonen, P.; Tiihonen, L.; Kautiainen, H.; Storvik, M.; Callaway, J. The association of ambient temperature and violent crime. Sci. Rep. 2017, 7, 6543. [Google Scholar] [CrossRef] [PubMed]
- Simister, J.; Cooper, C. Thermal stress in the USA: Effects on violence and on employee behaviour. Stress Health 2005, 21, 3–15. [Google Scholar] [CrossRef]
- Cianconi, P.; Betrò, S.; Janiri, L. The impact of climate change on mental health: A systematic descriptive review. Front. Psychiatry 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
| Authors/Year of Publication | Study Type/Source, Country | Period of Study/Years of Survey | Sample Size/Age | Diagnostic Criteria | Admissions Characteristics | Main Results |
|---|---|---|---|---|---|---|
| [28] Aguglia et al. (2016) | Cross-sectional (retrospective)/Turin, Italy | 2013–2015 (2) | 112/ mean age (±SD) 43.2 (±13.7) | DSM 5 | Sample of affective disorders and schizophrenia involuntary admissions. | Involuntary affective and schizophrenia disorders, significant peak in spring and summer. |
| [45] Aguglia et al. (2017) | Cross-sectional (retrospective)/Turin, Italy | 2013–2015 (2) | 730/ mean age (±SD) 43.4 (±13.9) | DSM 5 | Sample of affective and schizophrenia admissions. | Bipolar disorder, slightly higher prevalence in spring, manic episodes in spring/summer, unipolar depression and schizophrenia in autumn/winter. |
| [46] Aguglia et al. (2018) | Cross-sectional (retrospective)/Turin, Italy | 2013–2015 (2) | 730/ mean age (±SD) 43.4 (±13.9) | DSM 5 | Sample of affective admissions. | Manic episodes, significantly increased in spring and summer. Stepwise logistic regression analysis showed manic episode no longer predicted spring/summer pattern. |
| [30] Amr and Volpe (2012) | Cross-sectional (retrospective)/Mansoura, Egypt | 2003–2007 (5) | 3346/ mean age (±SD) 27.3 (±5.2) | DSM IV | All affective and schizophrenia admissions. | Manic and mixed episodes, significantly increased in summer (June), unipolar and bipolar depression in winter (December). Schizophrenia, no seasonal variation. |
| [29] Bakstein et al. (2020) | Cross-sectional (retrospective)/Czech Republic | 1994–2013 (20) | 231,573/ Not specified | ICD-10 | All affective and schizophrenia admissions. | Mania, significantly increased in summer (August), bipolar and unipolar depression in spring (April), schizophrenia in summer (June). Unipolar depression, significantly lower rates in summer (August). |
| [47] Cassidy et al. (2002) | Cross-sectional (retrospective)/ North Carolina, United States | 1993–1996 (3) | 304/ 18–82 | DSM-III-R | First mixed or manic bipolar admissions. | First manic episodes, peak in early spring (March) and nadir in late fall (November). First mixed episodes, peak in late summer (August) and nadir in late fall (November). |
| [48] Clarke et al. (1998) | Cross-sectional (retrospective)/Ireland | 1989–1994 (6) | 13,240/ Not specified | ICD-9 & ICD-10 | First schizophrenia and affective admissions. | First bipolar and unipolar depression, significant peak in summer (August), schizophrenia also in summer (July). |
| [49] Clarke et al. (1999) | Cross-sectional (retrospective)/Ireland | 1989–1994 (6) | 65,738/ Not specified | ICD-9 & ICD-10 | All schizophrenia and affective disorders admissions. | First manic episodes and depressive disorder, significant peak in summer (August), schizophrenia also in summer (July). Bipolar disorder readmissions, significant peak in summer (July). |
| [50] Daniels et al. (2000) | Cross-sectional (retrospective)/Tasmania, Australia | 1983–1989 (7) | 8464/ Not specified | ICD-9 | All schizophrenia and affective disorders admissions. | Schizoaffective disorder, significant winter peak for males, a similar but not significant peak for total sample and females. Bipolar disorder, not significant peak in late spring and early summer. Bipolar depression, significant low trend in autumn, no statistical significance by gender. Mania, depression and schizophrenia, no significant seasonal variation, either the entire sample or by gender. |
| [51] Dominiak et al. (2015) | Cross-sectional (retrospective)/Warsaw, Poland | 2002–2010 (9) | 2837/ >18 | ICD-10 | Sample of affective disorders admissions. | Mania, significantly increased in winter (January), spring and summer (May and August). Significantly lowest number in autumn (November). For females, significant variation in winter (January), for males in summer (August). Mixed episodes, extra admissions in winter (December to February) and spring/summer (May to June), significant lowest rate in autumn (November). For males, peaks in winter (February), spring/summer and a small extra peak in summer (August), significantly lowest rates in spring (April) and summer (July). Bipolar depression, significant highest in spring (April), significant lowest in summer (August). In males aged 36–65 years, significant highest in winter (February) and autumn (November), significant lowest in spring/summer (from May to August) and in winter (December). Recurrent depression peaks in spring (March) and autumn (November), significant lowest rates in winter (December). Single depressive episode, significant highest number in spring (May), lowest admissions rate late autumn (November). |
| [52] Jain et al. (1992) | Cross-sectional (retrospective)/Bangalore, India | 1980–1988 (9) | 270/ Not specified | ICD-9 | All mania admissions. | Mania, no seasonal variation. |
| [53] Jones et al. (1995) | Cross-sectional (retrospective)/Tasmania, Australia | 1983–1989 (7) | 1280/ Not specified | ICD-9 | All mania admissions. | Mania, spring/summer total admissions peaks. Mania, spring readmissions peak, no seasonal pattern for first admissions. Mania, spring peak in female readmissions. Mania, spring peak in patients over 50 years old, no seasonal pattern for under 30 and 30–49 age groups. |
| [54] Kerr-Correa et al. (1998) | Cross-sectional (retrospective)/Botucatu, Brazil | 1982–1991 (10) | 157/ Not specified | DSM-III-R & ICD-9 | All bipolar disorder admissions. | Mania, no significant seasonal variation for total admissions. Mania, significant spring/summer peak in women, no significant difference in men. Bipolar and unipolar depression, no significant seasonal pattern. |
| [55] Lee et al. (2002) | Cross-sectional (retrospective)/Seoul, Korea | 1996–1999 (4) | 152/ 18–33 | DSM-III-R | First manic admissions. | Mania, peaks in spring (March) and autumn (October), lowest in winter (January). |
| [56] Medici et al. (2016) | Cross-sectional (retrospective)/Denmark | 1995–2012 (18) | 24,313/ ≥15 | ICD-8 & ICD-10 | All mania admissions. | Mania, summer (August) peak. |
| [57] Modai et al. (1994) | Cross-sectional (retrospective)/Petah Tikva, Israel | 1988–1990 (3) | 63/ mean age (±SD) 59.1 (±15.18) | DSM III | All bipolar depression admissions. | Bipolar depression, winter increase. |
| [58] Morken et al. (2002) | Cross-sectional (retrospective)/Norway | 1992–1996 (5) | 4341/ ≥18 | ICD-9 & ICD-10 | All affective disorder admissions | Mania, significant spring peak among men, but not among total sample or women. Bipolar depression, significant spring peak for total sample (March–April) and females (April) with a nadir in autumn (November), no seasonal pattern for males. Unipolar depression, significant spring (April) peak for total sample and males, a significant autumn (November) peak and a non-significant spring (April) peak for females. |
| [59] Parker et al. (2018) | Cross-sectional (retrospective)/New South Wales, Australia | 2000–2014 (15) | 21,882/ Not specified | ICD-10 | All mania admissions. | Mania, peak in spring, abrupt change in winter (June to August in NSW). |
| [60] Parker & Graham (2016) | Cross-sectional (retrospective)/New South Wales, Australia | 1999–2014 (14) | 27,255/ Not specified | ICD-10 | All mania and hypomania admissions. | Mania and hypomania, similar pattern, lowest in autumn, increasing in winter, highest in spring. |
| [61] Parker et al. (2017) | Cross-sectional (retrospective), New South Wales, Australia | 2000–2014 (14) | 21,882/ Not specified | ICD-10 | All mania admissions. | Mania, peak in spring (October and November NSW). |
| [62] Rajkumar & Sarkar (2015) | Cross-sectional (retrospective)/Pondicherry, India | 2010–2013 (4) | 357/ Adults | ICD-10 | All mania admissions. | Highly significant 3-month peak (November to January, most significant 5-month peak (November to March). Males, significant winter peak (December to January), no peak in females. Patients over the age of 25, significant 2-month peak in December and January, a 3-month peak from November to January. Patients below the age of 25, some evidence for a 5-month peak (November to March), and a 6-month peak (November and April). |
| [63] Takei et al. (1992) | Cross-sectional (retrospective)/England and Wales | 1976–1986 (11) | 38,615/ Not specified | ICD-8 & ICD-9 | First schizophrenia and affective disorders admissions. | Mania, significant summer peak for total sample (August), males (August) and females (July). Schizophrenia, total and females significant peak in summer (July). Four of the six schizophrenia subtypes, significant summer cyclical trend in females, none in males. Schizoaffective disorder, total sample and both sexes, no significant seasonal variation. |
| [64] Volpe et al. (2010) | Cross-sectional (retrospective)/Belo Horizonte, Brazil | 2000–2007 (8) | 5172/mean age (±SD) 41.3 (±12.5) | ICD-10 | All manic admissions | Mania, significant peak in late winter, minimum in late summer. |
| [65] Volpe & Del Porto (2006) | Cross-sectional (retrospective)/Belo Horizonte, Brazil | 1996–2000 (5) | 425/ mean age (±SD) 42 (±13) | ICD-10 | All manic admissions. | Mania, significant peak in late winter, minimum in late summer. |
| [66] Whitney et al. (1999) | Cross-sectional (retrospective)/Ontario, Canada | 1920–1995 (75) | 5317/ Not specified | Case conference (1920–1960) ICD-9 (1970–1990) | All bipolar disorder admissions. | Bipolar disorder, significant peak in summer. Mania, total and both sexes preponderance in autumn. Bipolar depression, preponderance in spring and summer, both sexes in autumn. Mixed total and females episodes statistically higher in summer (June). |
| [67] Yang et al. (2013) | Cross-sectional (retrospective)/Taiwan | 2002–2007 (6) | 9619/ 18–55 | ICD-9 | Sample of bipolar disorder admissions. | Bipolar disorder, total and both sexes, significant spring (May) peak. Manic episodes, total and both sexes, significant spring peak. Bipolar depression, significant autumn (November) peak for females, not significant peak for males (September) Mixed episodes, significant spring peak (May) for females and significant summer (June) peak for males. Young adult (18–35), significant summer (June) peak for bipolar disorder, manic and mixed episodes, significant autumn (October) peak for bipolar depression. Middle adult (35–55), significant spring peak for bipolar disorder (April), manic (March) and mixed (May) episodes, not significant winter (December) peak for bipolar depression. |
| [68] Davies et al. (2000) | Cross-sectional register-based/Queensland, Australia | 1973–1991 (19) | 7739/ Not specified | ICD-8 & ICD-9 | First schizophrenia admissions. | Most admission during winter, both sexes peak in summer (August), seasonality more pronounced for males. |
| [69] Hinterbuchinger et al. (2020) | Cross-sectional register-based/Austria | 2003–2016 (14) | 110,735/ ≥15 | ICD-10 | Sample of schizophrenia admissions. | Significant variation peaks in midwinter (January) and summer (June) and a trough in early winter (December). Hospitalizations follow a seasonal pattern in both men and women |
| [70] Shiloh et al. (2005) | Cross-sectional register-based/Tel-Aviv, Israel | 1981–1991 (11) | 33,614/ >18 | ICD-9 | All schizophrenia, schizoaffective disorder admissions. | Schizophrenia significantly higher in summer. Schizoaffective significantly higher in autumn (October and November). |
| [71] Takei & Murray (1993) | Cross-sectional register-based/Scotland | 1961–1990 (30) | 14,964/ Not specified | ICD-7 & ICD-8 & ICD-9 | First schizophrenia admissions. | Schizophrenia total and females significant peak in spring (early May). |
| [72] Tian et al. (2006) | Cross-sectional register-based/Taiwan | 1997–2003 (7) | 759,611/ ≥18 | ICD-9 | All schizophrenia admissions. | Schizophrenia peak in spring (March). |
| Spring | Summer | Autumn | Winter | Spring/Summer | Spring/Autumn | Spring/Summer/Winter | Summer/Winter | Autumn/Winter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | PC | SA | PC | SA | PC | SA | PC | SA | PC | SA | PC | SA | PC | SA | PC | SA | PC | |
| Bipolar disorder | 1 [67] | 1 [45] | 4 [48,49,63,66] | 1 [50] | ||||||||||||||
| Manic episodes | 1 [67] | 5 [47,53,59,60,61] | 3 [29,49,63] | 1 [56] | 1 [66] | 3 [62,64,65] | 3 [45,46,53] | 1 [55] | 1 [51] | |||||||||
| Hypomanic episodes | 1 [60] | |||||||||||||||||
| Mixed episodes | 1 [51] | 1 [66] | 1 [47] | 1 [67] | ||||||||||||||
| Bipolar depression | 3 [29,51,58] | 1 [67] | 1 [57] | 1 [66] | ||||||||||||||
| Unipolar depression | 1 [58] | 2 [48,49] | 2 [29,51] | 1 [45] | ||||||||||||||
| Schizophrenia | 1 [71] | 1 [72] | 5 [29,48,49,63,70] | 1 [68] | 1 [69] | 1 [45] | ||||||||||||
| Schizoaffective | 1 [70] | 1 [50] | ||||||||||||||||
| Total | N = 7 | N = 9 | N = 15 | N = 2 | N = 2 | N = 1 | N = 6 | N = 1 | N = 5 | N = 2 | N = 1 | N = 1 | N = 1 | N = 2 | ||||
| Total sum | N = 16 | N = 17 | N = 3 | N = 6 | N = 6 | N = 3 | N = 1 | N = 1 | N = 2 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizavas, I.; Gournellis, R.; Douzenis, P.; Efstathiou, V.; Bali, P.; Lagouvardos, K.; Douzenis, A. A Systematic Review on the Impact of Seasonality on Severe Mental Illness Admissions: Does Seasonal Variation Affect Coercion? Healthcare 2023, 11, 2155. https://doi.org/10.3390/healthcare11152155
Rizavas I, Gournellis R, Douzenis P, Efstathiou V, Bali P, Lagouvardos K, Douzenis A. A Systematic Review on the Impact of Seasonality on Severe Mental Illness Admissions: Does Seasonal Variation Affect Coercion? Healthcare. 2023; 11(15):2155. https://doi.org/10.3390/healthcare11152155
Chicago/Turabian StyleRizavas, Ioannis, Rossetos Gournellis, Phoebe Douzenis, Vasiliki Efstathiou, Panagiota Bali, Kostas Lagouvardos, and Athanasios Douzenis. 2023. "A Systematic Review on the Impact of Seasonality on Severe Mental Illness Admissions: Does Seasonal Variation Affect Coercion?" Healthcare 11, no. 15: 2155. https://doi.org/10.3390/healthcare11152155
APA StyleRizavas, I., Gournellis, R., Douzenis, P., Efstathiou, V., Bali, P., Lagouvardos, K., & Douzenis, A. (2023). A Systematic Review on the Impact of Seasonality on Severe Mental Illness Admissions: Does Seasonal Variation Affect Coercion? Healthcare, 11(15), 2155. https://doi.org/10.3390/healthcare11152155







