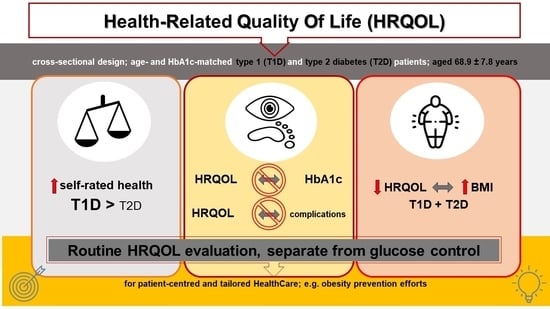
Health-Related Quality of Life Assessment in Older Patients with Type 1 and Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Medical Outcomes Short Form-36 (SF-36)
2.2. The EuroQol-5 Dimension
2.3. The EQ Visual Analogue Scale
2.4. The Hospital Anxiety and Depression Scale (HADS)
2.5. The Problem Areas in Diabetes (PAID) Scale
3. Statistical Analysis
4. Results
4.1. Baseline Patient Characteristics
4.2. HRQOL Outcome Measures
4.2.1. Medical Outcomes Short Form-36 (SF-36)—Health Dimensions
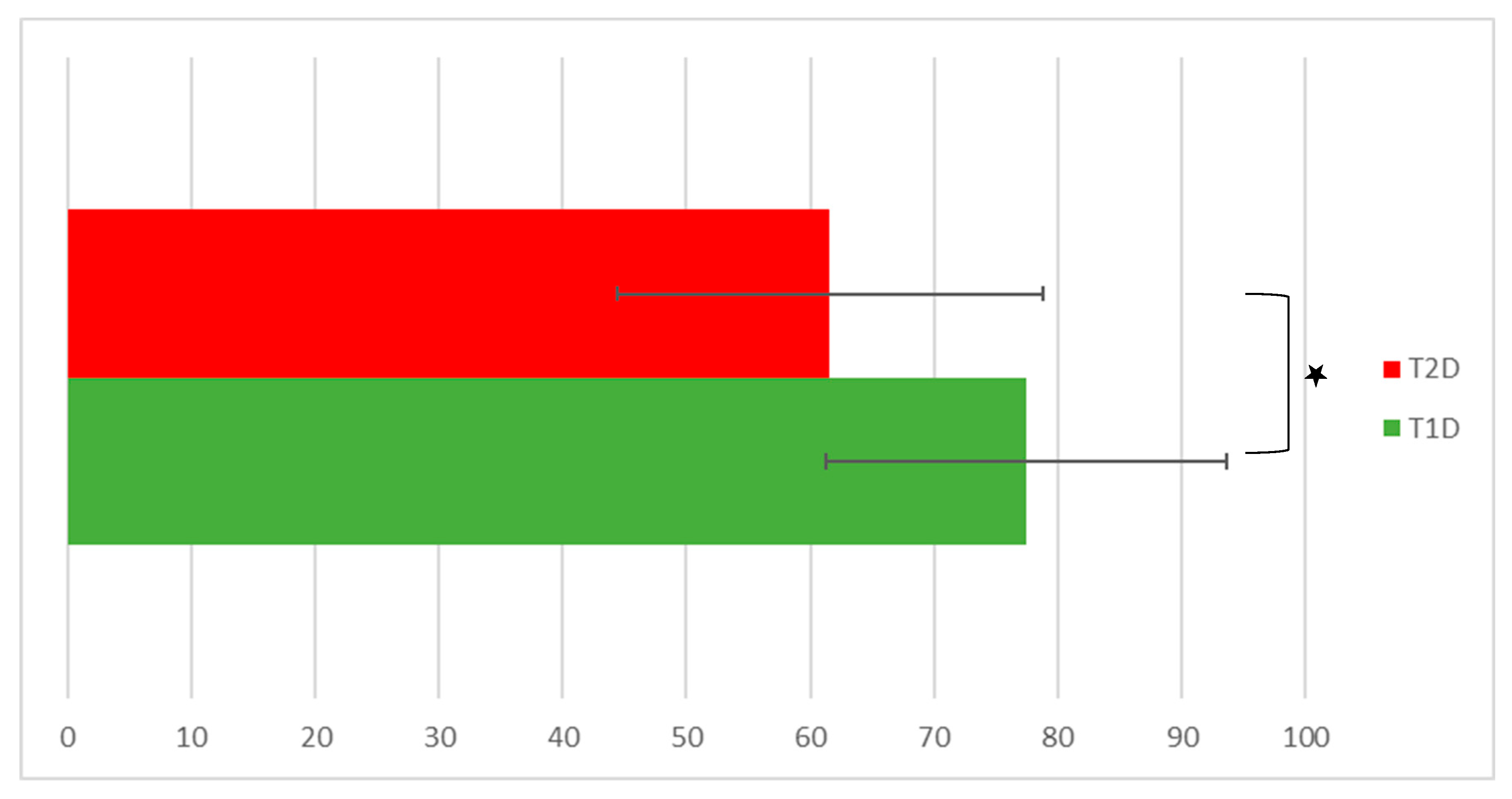
4.2.2. EQ-VAS State of Health
4.2.3. EQ-5D Dimensions
4.2.4. The Hospital Anxiety and Depression Scale (HADS) and the Problem Areas in Diabetes (PAID) Scale
4.2.5. Predictors (Diabetes Type, Presence of Diabetes Complications, Diabetes Duration, Basic Demographics, and BMI) Affecting Respondents’ Self-Rated Health Scores
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed. Brussels, Belgium. 2021. Available online: https://www.diabetesatlas.org/en/ (accessed on 3 July 2023).
- Ožji Nabor Kazalnikov za Spremljanje Obvladovanja Sladkorne Bolezni v Sloveniji. Nijz. Available online: https://nijz.si/publikacije/ozji-nabor-kazalnikov-za-spremljanje-obvladovanja-sladkorne-bolezni-v-sloveniji/ (accessed on 19 July 2023).
- Tang, T.S.; Yusuf, F.L.A.; Polonsky, W.H.; Fisher, L. Assessing Quality of Life in Diabetes: II—Deconstructing Measures into a Simple Framework. Diabetes Res. Clin. Pract. 2017, 126, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Natoli, G.; Nobili, A.; Mannucci, P.M.; Perticone, F.; Arcoraci, V.; Argano, C. The “Diabetes Comorbidome”: A Different Way for Health Professionals to Approach the Comorbidity Burden of Diabetes. Healthcare 2022, 10, 1459. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.S.; Thompson, W.W.; Zack, M.M.; Arnold, S.E.; Barile, J.P. Associations between Health-Related Quality of Life and Mortality in Older Adults. Prev. Sci. Off. J. Soc. Prev. Res. 2015, 16, 21–30. [Google Scholar] [CrossRef]
- Davis, W.K.; Hess, G.E.; Hiss, R.G. Psychosocial Correlates of Survival in Diabetes. Diabetes Care 1988, 11, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Dominick, K.L.; Ahern, F.M.; Gold, C.H.; Heller, D.A. Relationship of Health-Related Quality of Life to Health Care Utilization and Mortality among Older Adults. Aging Clin. Exp. Res. 2002, 14, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Garratt, A.M.; Schmidt, L.; Fitzpatrick, R. Patient-Assessed Health Outcome Measures for Diabetes: A Structured Review. Diabet. Med. 2002, 19, 1–11. [Google Scholar] [CrossRef]
- Kleefstra, N.; Landman, G.W.D.; Houweling, S.T.; Ubink-Veltmaat, L.J.; Logtenberg, S.J.J.; Meyboom-de Jong, B.; Coyne, J.C.; Groenier, K.H.; Bilo, H.J.G. Prediction of Mortality in Type 2 Diabetes From Health-Related Quality of Life (ZODIAC-4). Diabetes Care 2008, 31, 932–933. [Google Scholar] [CrossRef][Green Version]
- Li, C.-L.; Chang, H.-Y.; Hsu, C.-C.; Lu, J.R.; Fang, H.-L. Joint Predictability of Health Related Quality of Life and Leisure Time Physical Activity on Mortality Risk in People with Diabetes. BMC Public Health 2013, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- McEwen, L.N.; Kim, C.; Haan, M.N.; Ghosh, D.; Lantz, P.M.; Thompson, T.J.; Herman, W.H. Are Health-Related Quality-of-Life and Self-Rated Health Associated with Mortality? Insights from Translating Research Into Action for Diabetes (TRIAD). Prim. Care Diabetes 2009, 3, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Ferrer, M.; Gandek, B.; Ware, J.E.; Aaronson, N.K.; Mosconi, P.; Rasmussen, N.K.; Bullinger, M.; Fukuhara, S.; Kaasa, S.; et al. Health-Related Quality of Life Associated with Chronic Conditions in Eight Countries: Results from the International Quality of Life Assessment (IQOLA) Project. Qual. Life Res. 2004, 13, 283–298. [Google Scholar] [CrossRef]
- Testa, M.A.; Simonson, D.C. Assessment of Quality-of-Life Outcomes. N. Engl. J. Med. 1996, 334, 835–840. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Measuring Healthy Days: Population Assessment of Health-Related Quality of Life. 2001. Available online: http://www.cdc.gov/hrqol/pdfs/mhd.pdf (accessed on 20 July 2023).
- Grandy, S.; Fox, K.M. EQ-5D Visual Analog Scale and Utility Index Values in Individuals with Diabetes and at Risk for Diabetes: Findings from the Study to Help Improve Early Evaluation and Management of Risk Factors Leading to Diabetes (SHIELD). Health Qual. Life Outcomes 2008, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- The EuroQol Group. EuroQol—A New Facility for the Measurement of Health-Related Quality of Life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and Preliminary Testing of the New Five-Level Version of EQ-5D (EQ-5D-5L). Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr. SF-36 Health Survey. In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 1227–1246. ISBN 978-0-8058-2761-3. [Google Scholar]
- Solli, O.; Stavem, K.; Kristiansen, I. Health-Related Quality of Life in Diabetes: The Associations of Complications with EQ-5D Scores. Health Qual. Life Outcomes 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Anderson, B.J.; Lohrer, P.A.; Welch, G.; Jacobson, A.M.; Aponte, J.E.; Schwartz, C.E. Assessment of Diabetes-Related Distress. Diabetes Care 1995, 18, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. An Updated Literature Review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Feng, X.; Astell-Burt, T. Impact of a Type 2 Diabetes Diagnosis on Mental Health, Quality of Life, and Social Contacts: A Longitudinal Study. BMJ Open Diabetes Res. Care 2017, 5, e000198. [Google Scholar] [CrossRef] [PubMed]
- Golicki, D.; Dudzińska, M.; Zwolak, A.; Tarach, J.S. Quality of Life in Patients with Type 2 Diabetes in Poland—Comparison with the General Population Using the EQ-5D Questionnaire. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2015, 24, 139–146. [Google Scholar] [CrossRef]
- Hart, H.E.; Bilo, H.J.G.; Redekop, W.K.; Stolk, R.P.; Assink, J.H.; Meyboom-de Jong, B. Quality of Life of Patients with Type I Diabetes Mellitus. Qual. Life Res. 2003, 12, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, K.; Aarnio, E.; Lavikainen, P.; Jauhonen, H.-M.; Enlund, H.; Martikainen, J. Impact of Type 2 Diabetes Treated with Non-Insulin Medication and Number of Diabetes-Coexisting Diseases on EQ-5D-5 L Index Scores in the Finnish Population. Health Qual. Life Outcomes 2019, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, N.; Chen, Y.; Nie, X.; Li, Q.; Han, B.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; et al. Health-Related Quality of Life in Type-2 Diabetes Patients: A Cross-Sectional Study in East China. BMC Endocr. Disord. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Nieuwesteeg, A.; Pouwer, F.; van der Kamp, R.; van Bakel, H.; Aanstoot, H.-J.; Hartman, E. Quality of Life of Children with Type 1 Diabetes: A Systematic Review. Curr. Diabetes Rev. 2012, 8, 434–443. [Google Scholar] [CrossRef]
- Alsayed Hassan, D.; Helaluddin, F.; Chahestani, O.H.; Mohamed, O.; Islam, N. Diabetes Self-Management and Health-Related Quality of Life among Primary Care Patients with Diabetes in Qatar: A Cross-Sectional Study. Healthcare 2022, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Martel, D.; Velasco, R.; Sánchez-Hernández, R.M.; Carrillo, A.; Nóvoa, F.J.; Wägner, A.M. Quality of Life and Type 1 Diabetes: A Study Assessing Patients’ Perceptions and Self-Management Needs. Patient Prefer. Adherence 2015, 9, 1315–1323. [Google Scholar] [CrossRef]
- Coffey, J.T.; Brandle, M.; Zhou, H.; Marriott, D.; Burke, R.; Tabaei, B.P.; Engelgau, M.M.; Kaplan, R.M.; Herman, W.H. Valuing Health-Related Quality of Life in Diabetes. Diabetes Care 2002, 25, 2238–2243. [Google Scholar] [CrossRef]
- Imayama, I.; Plotnikoff, R.C.; Courneya, K.S.; Johnson, J.A. Determinants of Quality of Life in Adults with Type 1 and Type 2 Diabetes. Health Qual. Life Outcomes 2011, 9, 115. [Google Scholar] [CrossRef]
- Nutakor, J.A.; Zhou, L.; Larnyo, E.; Addai-Danso, S.; Tripura, D. Socioeconomic Status and Quality of Life: An Assessment of the Mediating Effect of Social Capital. Healthcare 2023, 11, 749. [Google Scholar] [CrossRef]
- Rodríguez-Almagro, J.; García-Manzanares, Á.; Lucendo, A.J.; Hernández-Martínez, A. Health-Related Quality of Life in Diabetes Mellitus and Its Social, Demographic and Clinical Determinants: A Nationwide Cross-Sectional Survey. J. Clin. Nurs. 2018, 27, 4212–4223. [Google Scholar] [CrossRef]
- Rubin, R.R.; Wadden, T.A.; Bahnson, J.L.; Blackburn, G.L.; Brancati, F.L.; Bray, G.A.; Coday, M.; Crow, S.J.; Curtis, J.M.; Dutton, G.; et al. Impact of Intensive Lifestyle Intervention on Depression and Health-Related Quality of Life in Type 2 Diabetes: The Look AHEAD Trial. Diabetes Care 2014, 37, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, M.; Cvetanović, G.; Anđelković Apostolović, M.; Stojanović, D.; Rančić, N. Impact of Socio-Demographic Characteristics and Long-Term Complications on Quality of Life in Patients with Diabetes Mellitus. Cent. Eur. J. Public Health 2018, 26, 104–110. [Google Scholar] [CrossRef]
- Currie, C.J.; Poole, C.D.; Woehl, A.; Morgan, C.L.; Cawley, S.; Rousculp, M.D.; Covington, M.T.; Peters, J.R. The Health-Related Utility and Health-Related Quality of Life of Hospital-Treated Subjects with Type 1 or Type 2 Diabetes with Particular Reference to Differing Severity of Peripheral Neuropathy. Diabetologia 2006, 49, 2272–2280. [Google Scholar] [CrossRef]
- Hayes, A.; Arima, H.; Woodward, M.; Chalmers, J.; Poulter, N.; Hamet, P.; Clarke, P. Changes in Quality of Life Associated with Complications of Diabetes: Results from the Advance Study. Value Health 2016, 19, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.; McGill, S.; Kind, P.; Bottomley, J.; Gillam, S.; Murphy, M. Health-Related Quality of Life in Type 2 Diabetes (TARDIS-2). Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2000, 3 (Suppl. 1), 47–51. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.M.; Braffett, B.H.; Cleary, P.A.; Gubitosi-Klug, R.A.; Larkin, M.E. DCCT/EDIC Research Group The Long-Term Effects of Type 1 Diabetes Treatment and Complications on Health-Related Quality of Life: A 23-Year Follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications Cohort. Diabetes Care 2013, 36, 3131–3138. [Google Scholar] [CrossRef]
- Koopmanschap, M. CODE-2 Advisory Board Coping with Type II Diabetes: The Patient’s Perspective. Diabetologia 2002, 45, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Sawyer, W.; Hopkinson, P. Impact of Long-Term Complications on Quality of Life in Patients with Type 2 Diabetes Not Using Insulin. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Venkataraman, K.; Wee, H.L.; Leow, M.K.S.; Tai, E.S.; Lee, J.; Lim, S.C.; Tavintharan, S.; Wong, T.Y.; Ma, S.; Heng, D.; et al. Associations between Complications and Health-Related Quality of Life in Individuals with Diabetes. Clin. Endocrinol. 2013, 78, 865–873. [Google Scholar] [CrossRef]
- Wexler, D.J.; Grant, R.W.; Wittenberg, E.; Bosch, J.L.; Cagliero, E.; Delahanty, L.; Blais, M.A.; Meigs, J.B. Correlates of Health-Related Quality of Life in Type 2 Diabetes. Diabetologia 2006, 49, 1489–1497. [Google Scholar] [CrossRef]
- Al-Taie, N.; Maftei, D.; Kautzky-Willer, A.; Krebs, M.; Stingl, H. Assessing the Quality of Life among Patients with Diabetes in Austria and the Correlation between Glycemic Control and the Quality of Life. Prim. Care Diabetes 2020, 14, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Laffel, L.M.; Domenger, C.; Danne, T.; Phillip, M.; Mazza, C.; Hanas, R.; Waldron, S.; Beck, R.W.; Calvi-Gries, F.; et al. Factors Associated With Diabetes-Specific Health-Related Quality of Life in Youth With Type 1 Diabetes: The Global TEENs Study. Diabetes Care 2017, 40, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.-K.; Karjalainen, M.; Tiihonen, M.; Kautiainen, H.; Saltevo, J.; Haanpää, M.; Mäntyselkä, P. Glycemic Control and Health-Related Quality of Life among Older Home-Dwelling Primary Care Patients with Diabetes. Prim. Care Diabetes 2017, 11, 577–582. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Griffin, S.J.; Davies, M.J.; Lauritzen, T.; Khunti, K.; Rutten, G.E.H.M.; Simmons, R.K. Diabetes-Specific Quality of Life but Not Health Status Is Independently Associated with Glycaemic Control among Patients with Type 2 Diabetes: A Cross-Sectional Analysis of the ADDITION-Europe Trial Cohort. Diabetes Res. Clin. Pract. 2014, 104, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Svedbo Engström, M.; Leksell, J.; Johansson, U.-B.; Borg, S.; Palaszewski, B.; Franzén, S.; Gudbjörnsdottir, S.; Eeg-Olofsson, K. Health-Related Quality of Life and Glycaemic Control among Adults with Type 1 and Type 2 Diabetes—A Nationwide Cross-Sectional Study. Health Qual. Life Outcomes 2019, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Kleefstra, N.; Ubink-Veltmaat, L.J.; Houweling, S.T.; Groenier, K.H.; Meyboom-de Jong, B.; Bilo, H.J.G. Cross-Sectional Relationship between Glycaemic Control, Hyperglycaemic Symptoms and Quality of Life in Type 2 Diabetes (ZODIAC-2). Neth. J. Med. 2005, 63, 215–221. [Google Scholar] [PubMed]
- Redekop, W.K.; Koopmanschap, M.A.; Stolk, R.P.; Rutten, G.E.H.M.; Wolffenbuttel, B.H.R.; Niessen, L.W. Health-Related Quality of Life and Treatment Satisfaction in Dutch Patients with Type 2 Diabetes. Diabetes Care 2002, 25, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Wexler, D.J.; Porneala, B.; Chang, Y.; Huang, E.S.; Huffman, J.C.; Grant, R.W. Diabetes Differentially Affects Depression and Self-Rated Health by Age in the U.S. Diabetes Care 2012, 35, 1575–1577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shallcross, A.J.; Ford, B.Q.; Floerke, V.A.; Mauss, I.B. Getting Better with Age: The Relationship between Age, Acceptance, and Negative Affect. J. Pers. Soc. Psychol. 2013, 104, 734–749. [Google Scholar] [CrossRef]
- Grandy, S.; Fox, K.M. SHIELD Study Group Change in Health Status (EQ-5D) over 5 Years among Individuals with and without Type 2 Diabetes Mellitus in the SHIELD Longitudinal Study. Health Qual. Life Outcomes 2012, 10, 99. [Google Scholar] [CrossRef]
- Jing, X.; Chen, J.; Dong, Y.; Han, D.; Zhao, H.; Wang, X.; Gao, F.; Li, C.; Cui, Z.; Liu, Y.; et al. Related Factors of Quality of Life of Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Health Qual. Life Outcomes 2018, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Sach, T.H.; Barton, G.R.; Doherty, M.; Muir, K.R.; Jenkinson, C.; Avery, A.J. The Relationship between Body Mass Index and Health-Related Quality of Life: Comparing the EQ-5D, EuroQol VAS and SF-6D. Int. J. Obes. 2007, 31, 189–196. [Google Scholar] [CrossRef]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 Diabetes and Quality of Life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wermeling, P.R.; Gorter, K.J.; van Stel, H.F.; Rutten, G.E.H.M. Both Cardiovascular and Non-Cardiovascular Comorbidity Are Related to Health Status in Well-Controlled Type 2 Diabetes Patients: A Cross-Sectional Analysis. Cardiovasc. Diabetol. 2012, 11, 121. [Google Scholar] [CrossRef]
- Kalda, R.; Rätsep, A.; Lember, M. Predictors of Quality of Life of Patients with Type 2 Diabetes. Patient Prefer. Adherence 2008, 2, 21–26. [Google Scholar]
- Eckert, K. Impact of Physical Activity and Bodyweight on Health-Related Quality of Life in People with Type 2 Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 303–311. [Google Scholar] [CrossRef]
- Porter, T.; Ong, B.N.; Sanders, T. Living with Multimorbidity? The Lived Experience of Multiple Chronic Conditions in Later Life. Health 2020, 24, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Brito-Brito, P.-R.; Rodríguez-Álvaro, M.; Fernández-Gutiérrez, D.-Á.; Martínez-Alberto, C.-E.; Cabeza-Mora, A.; García-Hernández, A.-M. Nursing Diagnoses, Planned Outcomes and Associated Interventions with Highly Complex Chronic Patients in Primary Care Settings: An Observational Study. Healthcare 2022, 10, 2512. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H. Development and Psychometric Validation of the Diabetes Therapy-Related QOL (DTR-QOL) Questionnaire. J. Med. Econ. 2012, 15, 556–563. [Google Scholar] [CrossRef]
- Kutubaeva, R.Z. Analysis of Life Satisfaction of the Elderly Population on the Example of Sweden, Austria and Germany. Popul. Econ. 2019, 3, 102–116. [Google Scholar] [CrossRef][Green Version]
- American Diabetes Association. 12 Older Adults: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S168–S179. [Google Scholar] [CrossRef] [PubMed]
- Young-Hyman, D.; de Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial Care for People With Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef] [PubMed]
- Volčanšek, Š.; Lunder, M.; Janež, A. Acceptability of Continuous Glucose Monitoring in Elderly Diabetes Patients Using Multiple Daily Insulin Injections. Diabetes Technol. Ther. 2019, 21, 566–574. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S. [Google Scholar]
- Garratt, A.M.; Ruta, D.A.; Abdalla, M.I.; Buckingham, J.K.; Russell, I.T. The SF36 Health Survey Questionnaire: An Outcome Measure Suitable for Routine Use within the NHS? BMJ 1993, 306, 1440–1444. [Google Scholar] [CrossRef]
- Feng, Y.; Parkin, D.; Devlin, N.J. Assessing the Performance of the EQ-VAS in the NHS PROMs Programme. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2014, 23, 977–989. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Brod, M.; Skovlund, S.E.; Wittrup-Jensen, K.U. Measuring the Impact of Diabetes Through Patient Report of Treatment Satisfaction, Productivity and Symptom Experience. Qual. Life Res. 2006, 15, 481–491. [Google Scholar] [CrossRef]
- Brod, M.; Hammer, M.; Christensen, T.; Lessard, S.; Bushnell, D.M. Understanding and Assessing the Impact of Treatment in Diabetes: The Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device). Health Qual. Life Outcomes 2009, 7, 83. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Ishii, H.; Takamura, H.; Nishioka, Y.; Langer, J.; Watanabe, M.; Kim, H.R.; Crawford, B. Quality of Life and Utility Values for Cost-Effectiveness Modeling in Japanese Patients with Type 2 Diabetes. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 2931–2943. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Ruggiero, L.; Eakin, E.G.; Dryfoos, J.; Chobanian, L. Quality of Life and Associated Characteristics in a Large National Sample of Adults with Diabetes. Diabetes Care 1997, 20, 562–567. [Google Scholar] [CrossRef]
- Abegaz, T.M.; Ali, A.A. Health-Related Quality of Life and Healthcare Events in Patients with Monotherapy of Anti-Diabetes Medications. Healthcare 2023, 11, 541. [Google Scholar] [CrossRef]
- Khunkaew, S.; Fernandez, R.; Sim, J. Demographic and Clinical Predictors of Health-Related Quality of Life among People with Type 2 Diabetes Mellitus Living in Northern Thailand: A Cross-Sectional Study. Health Qual. Life Outcomes 2019, 17, 177. [Google Scholar] [CrossRef]
- Rubin, R.R. Diabetes and Quality of Life. Diabetes Spectr. 2000, 13, 21. [Google Scholar] [CrossRef]
- Jacobson, A.M.; de Groot, M.; Samson, J.A. The Evaluation of Two Measures of Quality of Life in Patients with Type I and Type II Diabetes. Diabetes Care 1994, 17, 267–274. [Google Scholar] [CrossRef]
- Selenius, J.S.; Wasenius, N.S.; Kautiainen, H.; Salonen, M.; von Bonsdorff, M.; Eriksson, J.G. Impaired Glucose Regulation, Depressive Symptoms, and Health-Related Quality of Life. BMJ Open Diabetes Res. Care 2020, 8, e001568. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.F. The Hospital Anxiety and Depression Scale. Occup. Med. 2014, 64, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-W.; Lovblom, L.E.; Cardinez, M.; Weisman, A.; Farooqi, M.A.; Halpern, E.M.; Boulet, G.; Eldelekli, D.; Lovshin, J.A.; Lytvyn, Y.; et al. Neuropathy and Presence of Emotional Distress and Depression in Longstanding Diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. J. Diabetes Complicat. 2017, 31, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, L.; Long, G.H.; Griffin, S.J.; Simmons, R.K. Are Changes in Glycaemic Control Associated with Diabetes-Specific Quality of Life and Health Status in Screen-Detected Type 2 Diabetes Patients? Four-Year Follow up of the ADDITION-Cambridge Cohort. Diabetes Metab. Res. Rev. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.; Gray, A.; Holman, R. Estimating Utility Values for Health States of Type 2 Diabetic Patients Using the EQ-5D (UKPDS 62). Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2002, 22, 340–349. [Google Scholar] [CrossRef]
- Alva, M.; Gray, A.; Mihaylova, B.; Clarke, P. The Effect of Diabetes Complications on Health-Related Quality of Life: The Importance of Longitudinal Data to Address Patient Heterogeneity. Health Econ. 2014, 23, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cavrini, G.; Broccoli, S.; Puccini, A.; Zoli, M. EQ-5D as a Predictor of Mortality and Hospitalization in Elderly People. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2012, 21, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Kamp, A.; Askegaard, S. Putting Patients into the Centre: Patient Empowerment in Everyday Health Practices. Health 2020, 24, 625–645. [Google Scholar] [CrossRef]
- Khemakhem, R.; Dridi, Y.; Hamza, M.; Ben Hamouda, A.; Khlayfia, Z.; Ouerda, H.; Halioui, S.; Siala, N.; Belhadj, A.; Maherzi, A. Living with Type 1 Diabetes Mellitus: How Does the Condition Affect Children’s and Adolescents’ Quality of Life? Arch. Pédiatrie 2020, 27, 24–28. [Google Scholar] [CrossRef]


| T1D (n = 25) | T2D (n = 31) | p-Value | |
|---|---|---|---|
| Age (years) | 64.79 ± 5.89 | 72.26 ± 7.91 | 0.062 |
| Female gender (%) | 46 | 44 | 0.45 |
| HbA1c (%; mmol/mol) | 7.60 ± 1.37; 59.6 ± 14.0 | 8.12 ± 1.66; 65.2 ± 17.2 | 0.097 |
| BMI (kg/m2) | 27.68 ± 6.26 | 31.49 ± 7.49 | 0.047 * |
| Diabetes duration (years) | 37.62 ± 15.7 | 20.45 ± 11.21 | 0.0001 * |
| Arterial hypertension prevalence (%) | 80 | 88.5 | 0.38 |
| Presence of microvascular complications (%) | 92 | 76 | 0.14 |
| Presence of macrovascular complications (%) | 20.8 | 37.5 | 0.21 |
| SF-36 Health Dimensions | T1D | T2D | p-Value |
|---|---|---|---|
| SF-PF | 79.2 ± 20.3 | 67.6 ± 27.4 | 0.2 |
| SF-LPH | 75.0 ± 28.8 | 71.25 ± 29.5 | 0.6 |
| SF-LEH | 74.6 ± 25.6 | 83.33 ± 26.0 | 0.3 |
| SF-V | 62.5 ± 18.9 | 57.9 ± 19.5 | 0.5 |
| SF-MH | 76.0 ± 15.8 | 70.6 ± 16.9 | 0.5 |
| SF-SF | 78.4 ± 21.5 | 80.5 ± 16.0 | 0.6 |
| SF-BP | 63.1 ± 26.8 | 68.1 ± 27.4 | 0.6 |
| SF-GH | 58.6 ± 20.8 | 46.8 ± 20.1 | 0.048 * |
| Predictor Outcome | Sex | Age | Duration | Diabetes Type | HbA1c | MicroV | MacroV | BMI |
|---|---|---|---|---|---|---|---|---|
| SF-PF | NS | NS | NS | NS | NS | NS | NS | –0.465 (0.002) |
| SF-BP | NS | NS | NS | NS | NS | NS | NS | −0.369 (0.043) |
| SF-V | NS | NS | NS | NS | NS | NS | NS | −0.417 (0.004) |
| SF-GH | NS | NS | NS | NS | NS | NS | NS | −0.346 (0.018) |
| EQ-5D VAS | NS | NS | NS | −0.451 (0.005) | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volčanšek, Š.; Lunder, M.; Janež, A. Health-Related Quality of Life Assessment in Older Patients with Type 1 and Type 2 Diabetes. Healthcare 2023, 11, 2154. https://doi.org/10.3390/healthcare11152154
Volčanšek Š, Lunder M, Janež A. Health-Related Quality of Life Assessment in Older Patients with Type 1 and Type 2 Diabetes. Healthcare. 2023; 11(15):2154. https://doi.org/10.3390/healthcare11152154
Chicago/Turabian StyleVolčanšek, Špela, Mojca Lunder, and Andrej Janež. 2023. "Health-Related Quality of Life Assessment in Older Patients with Type 1 and Type 2 Diabetes" Healthcare 11, no. 15: 2154. https://doi.org/10.3390/healthcare11152154
APA StyleVolčanšek, Š., Lunder, M., & Janež, A. (2023). Health-Related Quality of Life Assessment in Older Patients with Type 1 and Type 2 Diabetes. Healthcare, 11(15), 2154. https://doi.org/10.3390/healthcare11152154