Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
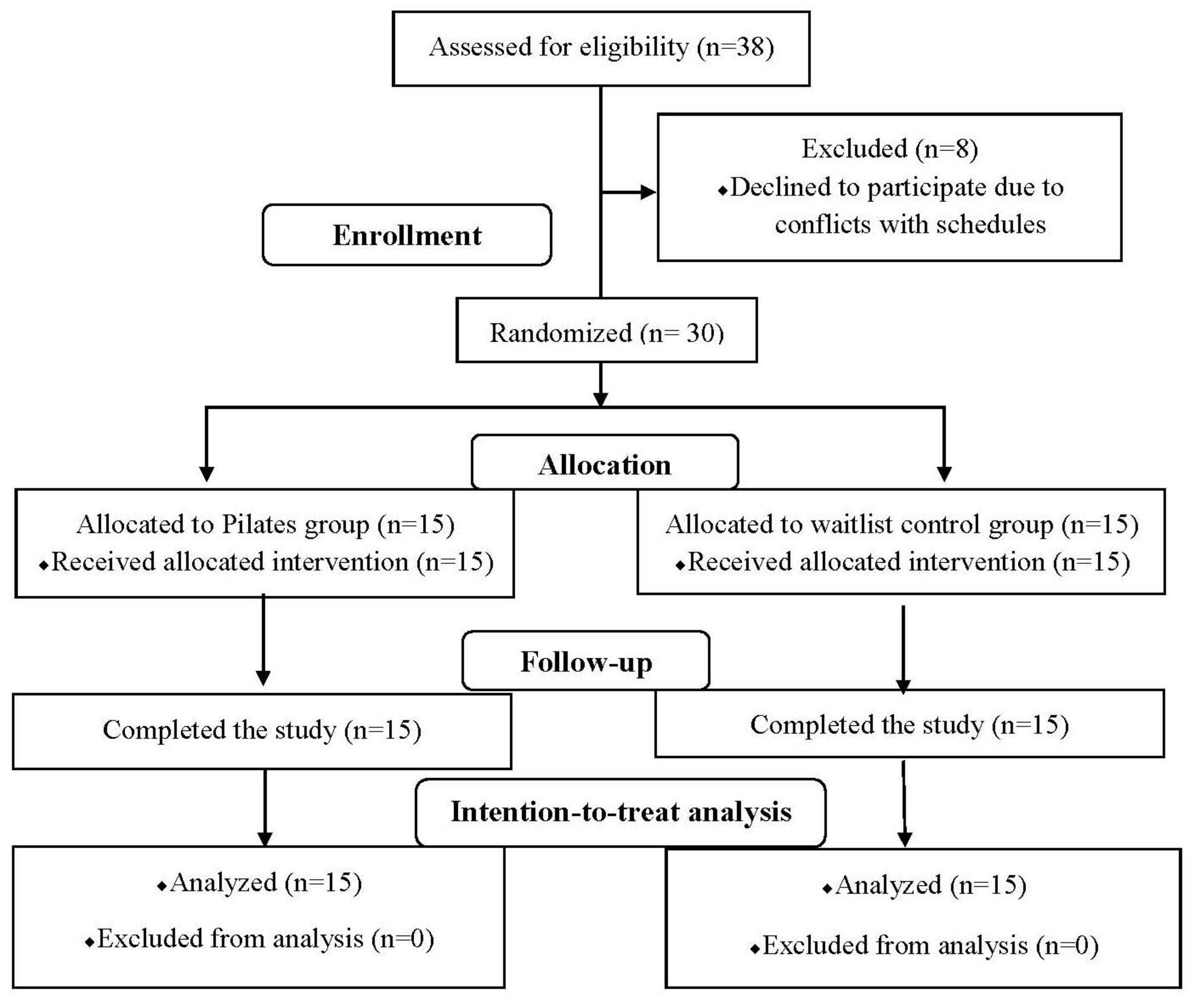
2.1. Study Design and Procedures
2.2. Ethical Considerations
2.3. Subjects
2.4. Pilates Protocol
2.5. General, Menstrual, and Lifestyle Characteristics
2.6. Primary Outcomes
2.6.1. Dysmenorrhea and Premenstrual Syndrome
2.6.2. Muscle Strength and Flexibility
2.7. Secondary Outcomes
2.7.1. Sleep Quality and Duration
2.7.2. Psychological Variables
2.8. Sample Size
2.9. Statistical Methods
3. Results
3.1. Subject Characteristics
| Variables | Categories | Pilates Group (n = 15) | Control Group (n = 15) | p-Value a |
|---|---|---|---|---|
| Age (years) | 33.9 ± 3.5 | 31.3 ± 4.5 | 0.096 | |
| Body mass index (kg/m2) | 21.4 ± 2.1 | 21.2 ± 1.9 | 0.818 | |
| Body fat (%) | 31.0 ± 4.7 | 29.8 ± 5.1 | 0.504 | |
| Menarcheal age (year) | 12.9 ± 1.1 | 12.9 ± 1.6 | 1.000 | |
| Period length (day) | 5.3 ± 1.3 | 5.1 ± 1.2 | 0.683 b | |
| Length of menstrual cycle (day) | 30.3 ± 5.1 | 29.9 ± 5.5 | 0.806 b | |
| Period regularity | Regular | 14 (93.3) | 7 (46.7) | 0.014 |
| Irregular | 1 (6.7) | 8 (53.3) | ||
| Volume of menstrual fluid | Light | 3 (20.0) | 0 (0.0) | 0.117 c |
| Moderate | 10 (66.7) | 10 (66.7) | ||
| Heavy | 2 (13.3) | 5 (33.3) | ||
| Family history of dysmenorrhea | No | 2 (13.3) | 5 (33.3) | 0.135 c |
| Yes | 8 (53.3) | 9 (60.0) | ||
| Not know | 5 (33.3) | 1 (6.7) | ||
| Marital status | Married | 2 (13.3) | 2 (13.3) | 1.000 |
| Unmarried | 13 (86.7) | 13 (86.7) | ||
| Smoking | No | 15 (100.0) | 14 (93.3) | 1.000 |
| Yes | 0 (0.0) | 1 (6.7) | ||
| Drinking | No | 4 (26.7) | 4 (26.7) | 1.000 |
| Yes | 11 (73.3) | 11 (73.3) | ||
| Moderate-intensity exercise (≥150 min/wk) | No | 14 (93.3) | 15 (100.0) | 1.000 |
| Yes | 1 (6.7) | 0 (0.0) | ||
| Sleep quality | Poor | 15 (100.0) | 12 (80.0) | 0.224 |
| Good | 0 (0.0) | 3 (20.0) | ||
| Use of pain relievers to manage dysmenorrhea | No | 4 (26.7) | 1 (6.7) | 0.330 |
| Yes | 11 (73.3) | 14 (93.3) | ||
3.2. Changes in Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Smith, C.A.; Steel, K.A.; Macmillan, F. The effectiveness of self-care and lifestyle interventions in primary dysmenorrhea: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, I.; Povoa, A.M. Primary Dysmenorrhea: Assessment and Treatment. Rev. Bras. Ginecol. Obstet. 2020, 42, 501–507. [Google Scholar] [CrossRef]
- Kim, M.J.; Baek, I.H.; Goo, B.O. The effect of lumbar-pelvic alignment and abdominal muscle thickness on primary dysmenorrhea. J. Phys. Ther. Sci. 2016, 28, 2988–2990. [Google Scholar] [CrossRef]
- Bajalan, Z.; Moafi, F.; MoradiBaglooei, M.; Alimoradi, Z. Mental health and primary dysmenorrhea: A systematic review. J. Psychosomatic. Obstet. Gynaecol. 2019, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.Y. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef]
- Kannan, P.; Cheung, K.K.; Lau, B.W. Does aerobic exercise induced-analgesia occur through hormone and inflammatory cytokine-mediated mechanisms in primary dysmenorrhea? Med. Hypotheses 2019, 123, 50–54. [Google Scholar] [CrossRef]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef]
- Sharghi, M.; Mansurkhani, S.M.; Larky, D.A.; Kooti, W.; Niksefat, M.; Firoozbakht, M.; Behzadifar, M.; Azami, M.; Servatyari, K.; Jouybari, L. An update and systematic review on the treatment of primary dysmenorrhea. JBRA Assist. Reprod. 2019, 23, 51–57. [Google Scholar] [CrossRef]
- Osayande, A.S.; Mehulic, S. Diagnosis and initial management of dysmenorrhea. Am. Fam. Physician 2014, 89, 341–346. [Google Scholar] [PubMed]
- Aboualsoltani, F.; Bastani, P.; Khodaie, L.; Fazljou, S.M.B. Non-Pharmacological Treatments of Primary Dysmenorrhea: A systematic Review. Arch. Pharma. Pract. 2020, 11 (Suppl. S1), 136–142. Available online: https://archivepp.com/article/non-pharmacological-treatments-of-primary-dysmenorrhea-a-systematic-review (accessed on 2 May 2023).
- Ortiz, M.I.; Cortes-Marquez, S.K.; Romero-Quezada, L.C.; Murguia-Canovas, G.; Jaramillo-Diaz, A.P. Effect of a physiotherapy program in women with primary dysmenorrhea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide survey reveals fitness trends for 2009. ACSM Health Fit. J. 2008, 12, 7–14. [Google Scholar] [CrossRef]
- Wells, C.; Kolt, G.S.; Bialocerkowski, A. Defining Pilates exercise: A systematic review. Complement. Ther. Med. 2012, 20, 253–262. [Google Scholar] [CrossRef]
- Cruz-Díaz, D.; Romeu, M.; Velasco-González, C.; Martínez-Amat, A.; Hita-Contreras, F. The effectiveness of 12 weeks of Pilates intervention on disability, pain and kinesiophobia in patients with chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2018, 32, 1249–1257. [Google Scholar] [CrossRef]
- de Araujo Cazotti, L.; Jones, A.; Roger-Silva, D.; Ribeiro, L.H.C.; Natour, J. Effectiveness of the Pilates Method in the Treatment of Chronic Mechanical Neck Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 1740–1746. [Google Scholar] [CrossRef]
- Gonzalez-Galvez, N.; Marcos-Pardo, P.J.; Carrasco-Poyatos, M. Functional improvements after a pilates program in adolescents with a history of back pain: A randomised controlled trial. Complement Ther. Clin. Pract. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Miyamoto, G.C.; Franco, K.F.M.; van Dongen, J.M.; Franco, Y.R.D.S.; de Oliveira, N.T.B.; Amaral, D.D.V.; Branco, A.N.C.; da Silva ML, M.L.; van Tulder, M.W.; Cabral, C.M.N. Different doses of Pilates-based exercise therapy for chronic low back pain: A randomised controlled trial with economic evaluation. Br. J. Sports Med. 2018, 52, 859–868. [Google Scholar] [CrossRef]
- Alikiani, Z.; Toloee, M.E.; Jafari, A.K. The effects of Pilates exercise and caraway supplementation on the levels of prostaglandin E2 and perception dysmenorrhea in adolescent girl’s non-athlete. Asian Exerc. Sport Sci. J. 2017, 1, 11. [Google Scholar] [CrossRef]
- Chang, E.A.; Koo, I.S.; Choi, J.H. The Effect of Pilates Stabilization Exercise and Kinesio taping on the Dysmenorrhea and Prostaglandin F2α of Female University Students. J. Int. Acad. Phys. Ther. Res. 2018, 9, 1558–1563. [Google Scholar] [CrossRef]
- Cruz-Ferreira, A.; Fernandes, J.; Kuo, Y.L.; Bernardo, L.M.; Fernandes, O.; Laranjo, L.; Silva, A. Does pilates-based exercise improve postural alignment in adult women? Women Health 2013, 53, 597–611. [Google Scholar] [CrossRef]
- Geremia, J.M.; Iskiewicz, M.M.; Marschner, R.A.; Lehnen, T.E.; Lehnen, A.M. Effect of a physical training program using the Pilates method on flexibility in elderly subjects. Age 2015, 37, 119. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Galvez, N.; Marcos-Pardo, P.J.; Trejo-Alfaro, H.; Vaquero-Cristobal, R. Effect of 9-month Pilates program on sagittal spinal curvatures and hamstring extensibility in adolescents: Randomised controlled trial. Sci. Rep. 2020, 10, 9977. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yim, J. Core Stability and Hip Exercises Improve Physical Function and Activity in Patients with Non-Specific Low Back Pain: A Randomized Controlled Trial. Tohoku J. Exp. Med. 2020, 251, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Kumar, S.; Nezamuddin, M.; Sharma, V.P. Efficacy of core muscle strengthening exercise in chronic low back pain patients. J. Back Musculoskelet. Rehabil. 2015, 28, 699–707. [Google Scholar] [CrossRef]
- Borjigen, A.; Huang, C.; Liu, M.; Lu, J.; Peng, H.; Sapkota, C.; Sheng, J. Status and Factors of Menstrual Knowledge, Attitudes, Behaviors and Their Correlation with Psychological Stress in Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2019, 32, 584–589. [Google Scholar] [CrossRef]
- Aibar-Almazan, A.; Hita-Contreras, F.; Cruz-Diaz, D.; de la Torre-Cruz, M.; Jimenez-Garcia, J.D.; Martinez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: A randomized controlled trial. Maturitas 2019, 124, 62–67. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef]
- Lademann, A.; Lademann, R. Pilates and Conditioning for Athletes: An Integrated Approach to Performance and Recovery, 1st ed.; Human Kinetics: Champaign, IL, USA, 2018. [Google Scholar]
- Raneri, S.M. Pilates Fisios—Matwork and Small Equipment; Piccin Nuova Libraria S.p.A.: Padova, Italy, 2016. [Google Scholar]
- Chen, C.X.; Kwekkeboom, K.L.; Ward, S.E. Self-report pain and symptom measures for primary dysmenorrhoea: A critical review. Eur. J. Pain 2015, 19, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J. Menstrual symptom questionnaire: Further psychometric evaluation. Behav. Res. Ther. 1977, 15, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.I.; Ryu, S.J.; Nam, E.Y.; Kim, J.H.; Kim, S.J.; Park, D.S. The comparative study on the effects of chuna and combined with acupuncture in patients with dysmenorrhea. JKMR 2014, 24, 157–164. (In Korean). Available online: https://kiss.kstudy.com/Detail/Ar?key=3254576 (accessed on 2 May 2023).
- Steiner, M.; Macdougall, M.; Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Womens Ment. Health 2003, 6, 203–209. [Google Scholar] [CrossRef]
- Choi, J.A. Study on the Seasonality and Premenstrual Symptoms in Bipolar I and Bipolar II Disorders. Ph.D. Dissertation, Sungkyunkwan University, Seoul, Republic of Korea, 2012. (In Korean). Available online: http://www.riss.kr/link?id=T12669973 (accessed on 2 May 2023).
- Chamali, R.; Emam, R.; Mahfoud, Z.R.; Al-Amin, H. Dimensional (premenstrual symptoms screening tool) vs categorical (mini diagnostic interview, module U) for assessment of premenstrual disorders. World J. Psychiatry 2022, 12, 603. [Google Scholar] [CrossRef]
- Choi, J.; Baek, J.H.; Noh, J.; Kim, J.S.; Choi, J.S.; Ha, K.; Kwon, J.S.; Hong, K.S.J.H.; Baek, J.; Noh, J.S.; et al. Hong, Association of seasonality and premenstrual symptoms in bipolar I and bipolar II disorders. J. Affect. Disord. 2011, 129, 313–316. [Google Scholar] [CrossRef]
- Buckinx, F.; Croisier, J.L.; Reginster, J.Y.; Dardenne, N.; Beaudart, C.; Slomian, J.; Leonard, S.; Bruyère, O. Reliability of Muscle Strength Measures Obtained with a Hand-Held Dynamometer in an Elderly Population. Clin. Physiol. Funct. Imaging 2017, 37, 332–340. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Adair, B.; Pua, Y.H.; Williams, G.P.; McGaw, R.; Clark, R.A. Assessment of Lower Limb Muscle Strength and Power Using Hand-Held and Fixed Dynamometry: A Reliability and Validity Study. PLoS ONE 2015, 10, e0140822. [Google Scholar] [CrossRef]
- Thorborg, K.; Petersen, J.; Magnusson, S.P.; Hölmich, P. Clinical assessment of hip strength using a handheld dynamometer is reliable. Scand. J. Med. Sci. Sports 2010, 20, 493–501. [Google Scholar] [CrossRef]
- Cuenca-Garcia, M.; Marin-Jimenez, N.; Perez-Bey, A.; Sánchez-Oliva, D.; Camiletti-Moiron, D.; Alvarez-Gallardo, I.C.; Ortega, F.B.; Castro-Piñero, J. Reliability of Field-Based Fitness Tests in Adults: A Systematic Review. Sports Med. 2022, 52, 1961–1979. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Mon, T.H.; Berman, S.R.; Kupfe, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, Y.J.; Lee, J.E.; Kim, Y.T.; Hong, S.C.; Choi, Y.J.; Song, M.K.; Kim, H.Y. Factors associated with poor sleep quality in the Korean general population: Providing information from the Korean version of the Pittsburgh Sleep Quality Index. J. Affect. Disord. 2020, 271, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath 2012, 16, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Park, J.N.; Seo, Y.S. Validation of the Perceived Stress Scale on samples of Korean university students. Korean J. Psychol. Gen. 2010, 29, 611–629. (In Korean) [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Geisser, M.E.; Roth, R.S.; Robinson, M.E. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: A comparative analysis. Clin. J. Pain. 1997, 13, 163–170. [Google Scholar] [CrossRef]
- Heo, E.H.; Choi, K.S.; Yu, J.C.; Nam, J.A. Validation of the Center for Epidemiological Studies Depression Scale among Korean Adolescents. Psychiatry Investig. 2018, 15, 124–132. [Google Scholar] [CrossRef]
- Hahn, D.W.; Lee, C.H.; Chon, K.K. Korean adaptation of Spielberger’s STAI (K-STAI). Korean J. Health Psychol. Health 1996, 1, 1–14. (In Korean) [Google Scholar]
- Julian, L.J. Measures of anxiety: State-trait anxiety inventory (STAI), beck anxiety inventory (BAI), and hospital anxiety and depression scale-anxiety (HADS-A). Arthritis Care Res. 2011, 63, S467–S472. [Google Scholar] [CrossRef]
- Wickström, K.; Edelstam, G. Minimal clinically important difference for pain on the VAS scale and the relation to quality of life in women with endometriosis. Sex Reprod. Healthc. 2017, 13, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Beit Yosef, A.; Jacobs, J.M.; Shenkar, S.; Shames, J.; Schwartz, I.; Doryon, Y.; Naveh, Y.; Khalailh, F.; Berrous, S.; Gilboa, Y. Activity Performance, Participation, and Quality of Life Among Adults in the Chronic Stage After Acquired Brain Injury-The Feasibility of an Occupation-Based Telerehabilitation Intervention. Front. Neurol. 2019, 10, 1247. [Google Scholar] [CrossRef]
- Cook, C.E. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J. Man. Manip. Ther. 2008, 16, 82E–83E. [Google Scholar] [CrossRef] [PubMed]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Wojcik, M.; Plagens-Rotman, K.; Merks, P.; Mizgier, M.; Kedzia, W.; Jarzabek-Bielecka, G. Visceral therapy in disorders of the female reproductive organs. Ginekol. Pol. 2022, 93, 511–518. [Google Scholar] [CrossRef]
- Di Lorenzo, C.E. Pilates: What is it? Should it be used in rehabilitation? Sports Health 2011, 3, 352–361. [Google Scholar] [CrossRef]
- Jose, A.; Nayak, S.; Rajesh, A.; Kamath, N.; Nalini, M. Impact of relaxation therapy on premenstrual symptoms: A systematic review. J. Educ. Health Promot. 2022, 11, 401. [Google Scholar] [CrossRef]
- Ravichandran, H.; Janakiraman, B. Effect of Aerobic Exercises in Improving Premenstrual Symptoms Among Healthy Women: A Systematic Review of Randomized Controlled Trials. Int. J. Womens Health 2022, 14, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Desai, D. Effectiveness of Pilates and Self-Stretching Exercise on Pain and Quality of Life in Primary Dysmenorrhea-A Comparative Study. Indian J. Physiother. Occup. 2021, 15, 129–138. [Google Scholar] [CrossRef]
- Paithankar, S.M.; Hande, D. Effectiveness of pilates over conventional physiotherapeutic treatment in females with primary dysmmenorrhea. IOSR-JDMS 2016, 15, 156–163. [Google Scholar]
- Çitil, E.T.; Kaya, N. Effect of pilates exercises on premenstrual syndrome symptoms: A quasi-experimental study. Complement. Ther. Med. 2021, 57, 102623. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Y.; Qiu, H.; Xu, D.; Zhu, J.; Liu, J.; Li, H. Prevalence and Risk Factors of Primary Dysmenorrhea in Students: A Meta-Analysis. Value Health 2022, 25, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, X.; Shen, Z.; Chen, G.; Chen, W.; He, T.; Xu, X. Effect of Pilates on Sleep Quality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2020, 11, 158. [Google Scholar] [CrossRef]

| Variables | Group | Baseline | Week 12 | p-Value a | Effect Size d | Week 12—Baseline | p-Value c | Effect Size d |
|---|---|---|---|---|---|---|---|---|
| VAS (cm) | Pilates group | 7.6 ± 1.2 | 2.4 ± 1.5 | 0.000 | 3.64 | −5.2 ± 1.4 | 0.000 | −3.30 |
| Control group | 6.4 ± 1.4 | 6.7 ± 1.2 | 0.468 b | 0.19 e | 0.3 ± 1.9 | |||
| CMSS severity | Pilates group | 30.1 ± 13.5 | 14.1 ± 9.7 | 0.000 | 1.40 | −15.9 ± 11.4 | 0.000 | 1.75 |
| Control group | 21.9 ± 9.3 | 21.7 ± 7.0 | 0.806 b | −0.06 e | 0.2 ± 1.9 | |||
| CMSS frequency | Pilates group | 34.0 ± 12.7 | 17.9 ± 12.5 | 0.002b | −0.80 e | −16.1 ± 14.0 | 0.000 | −1.60 |
| Control group | 24.3 ± 7.7 | 25.8 ± 7.4 | 0.411 | −0.22 | 1.5 ± 6.7 | |||
| PSST symptoms | Pilates group | 26.8 ± 9.7 | 14.7 ± 9.2 | 0.000 | 1.38 | −12.1 ± 8.7 | 0.000 | −1.92 |
| Control group | 17.2 ± 9.5 | 20.5 ± 9.1 | 0.096 | −0.46 | 3.3 ± 7.2 | |||
| PSST functional impairment | Pilates group | 9.1 ± 3.4 | 4.5 ± 3.7 | 0.001 b | −0.89 e | −4.6 ± 2.9 | 0.000 | −1.83 |
| Control group | 5.4 ± 3.2 | 6.4 ± 4.0 | 0.241 | −0.32 | 1.0 ± 3.2 | |||
| Back flexibility (cm) | Pilates group | 29.4 ± 6.8 | 33.5 ± 4.7 | 0.006b | 0.71 f | 4.1 ± 5.4 | 0.000 d | −0.70 f |
| Control group | 31.8 ± 8.6 | 28.4 ± 8.2 | 0.010 | 0.77 | −3.4 ± 4.5 | |||
| Muscle strength | ||||||||
| Left hip flexors (kg) | Pilates group | 8.4 ± 0.9 | 11.8 ± 1.5 | 0.000 | −1.81 | 3.5 ± 1.9 | 0.000 | 2.28 |
| Control group | 8.2 ± 1.9 | 8.1 ± 1.3 | 0.764 | 0.08 | −0.1 ± 1.1 | |||
| Right hip flexors (kg) | Pilates group | 8.9 ± 1.7 | 11.7 ± 1.6 | 0.001 | −1.13 | 2.8 ± 2.5 | 0.000 | 1.82 |
| Control group | 8.3 ± 0.8 | 7.7 ± 0.9 | 0.029 | 0.63 | −0.6 ± 1.0 | |||
| Left hip Extensors (kg) | Pilates group | 10.3 ± 1.5 | 11.5 ± 1.4 | 0.002 | −1.00 | 1.2 ± 1.2 | 0.007 | 1.06 |
| Control group | 10.9 ± 2.0 | 10.7 ± 1.8 | 0.562 | 0.15 | −0.2 ± 1.5 | |||
| Right hip Extensors (kg) | Pilates group | 10.3 ± 1.7 | 12.0 ± 1.5 | 0.000 | −1.46 | 1.6 ± 1.1 | 0.000 | 1.54 |
| Control group | 11.2 ± 2.0 | 10.5 ± 1.9 | 0.168 | 0.38 | −0.7 ± 1.8 | |||
| Left hip abductors (kg) | Pilates group | 9.8 ± 1.4 | 12.3 ± 2.0 | 0.002 b | 0.80 f | 2.4 ± 2.1 | 0.000 | 0.98 |
| Control group | 9.7 ± 1.6 | 10.0 ± 1.9 | 0.619 | −0.13 | 0.3 ± 2.2 | |||
| Right hip abductors (kg) | Pilates group | 8.3 ± 1.6 | 12.4 ± 2.1 | 0.000 | −1.46 | 4.1 ± 2.8 | 0.004 d | −0.51 f |
| Control group | 9.5 ± 1.9 | 9.6 ± 1.7 | 0.844 | −0.05 | 0.1 ± 2.1 |
| Variables | Group | Baseline | Week 12 | p-Value a | Effect Size e | Week 12—Baseline | p-Value c | Effect Size e |
|---|---|---|---|---|---|---|---|---|
| Body mass index (kg/m2) | Pilates group | 21.4 ± 2.1 | 21.3 ± 1.7 | 0.531 | 0.17 | −0.1 ± 0.6 | 0.129 | −0.57 |
| Control group | 21.2 ± 1.9 | 21.5 ± 2.0 | 0.148 | −0.40 | 0.2 ± 0.6 | |||
| Body fat (%) | Pilates group | 31.0 ± 4.7 | 29.9 ± 5.1 | 0.018 | 0.69 | −1.1 ± 1.6 | 0.126 d | 0.28 f |
| Control group | 29.8 ± 5.1 | 30.4 ± 5.7 | 0.347 | −0.25 | 0.6 ± 2.5 | |||
| Total score of sleep quality | Pilates group | 8.0 ± 2.0 | 6.0 ± 1.1 | 0.006 b | −0.71 f | −2.0 ± 1.9 | 0.015 d | 0.46 f |
| Control group | 8.8 ± 3.5 | 8.6 ± 3.0 | 0.317 b | −0.26 f | −0.2 ± 0.8 | |||
| Sleep duration (hour) | Pilates group | 6.3 ± 0.8 | 6.4 ± 1.1 | 0.675 b | 0.11 f | 0.1 ± 0.8 | 0.067 d | −0.34 f |
| Control group | 5.8 ± 1.1 | 4.9 ± 1.5 | 0.040b | −0.52 f | −0.9 ± 1.5 | |||
| Perceived stress | Pilates group | 21.6 ± 3.5 | 20.0 ± 3.8 | 0.036 | 0.60 | −1.6 ± 2.7 | 0.683 d | 0.12 f |
| Control group | 20.7 ± 3.8 | 19.4 ± 4.6 | 0.195 | 0.35 | −1.3 ± 3.8 | |||
| Depression | Pilates group | 15.7 ± 8.9 | 12.0 ± 4.8 | 0.081 | 0.42 | −3.7 ± 8.8 | 0.653 d | 0.08 f |
| Control group | 21.1 ± 8.1 | 19.3 ± 10.6 | 0.267 | 0.30 | −1.8 ± 6.0 | |||
| State anxiety | Pilates group | 43.8 ± 9.1 | 40.1 ± 7.6 | 0.110 | 0.44 | −3.7 ± 8.3 | 0.461 d | 0.14 f |
| Control group | 43.8 ± 8.0 | 43.1 ± 11.0 | 0.729 | 0.09 | −0.7 ± 8.0 | |||
| Trait anxiety | Pilates group | 41.2 ± 6.7 | 39.6 ± 6.2 | 0.281 | 0.29 | −1.6 ± 5.5 | 0.345 d | 0.18 f |
| Control group | 45.5 ± 5.8 | 46.1 ± 7.7 | 0.864 b | −0.10 | 0.6 ± 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.-H.; Kim, J. Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study. Healthcare 2023, 11, 2076. https://doi.org/10.3390/healthcare11142076
Song B-H, Kim J. Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study. Healthcare. 2023; 11(14):2076. https://doi.org/10.3390/healthcare11142076
Chicago/Turabian StyleSong, Bo-Hwa, and Jaehee Kim. 2023. "Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study" Healthcare 11, no. 14: 2076. https://doi.org/10.3390/healthcare11142076
APA StyleSong, B.-H., & Kim, J. (2023). Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study. Healthcare, 11(14), 2076. https://doi.org/10.3390/healthcare11142076






