Efficacy and Safety of Woohwangchungsimwon Combined with Donepezil in Behavioral and Psychological Symptoms of Dementia in Patients with Probable Alzheimer’s Disease: Study Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
2. Experimental Design
2.1. Objective
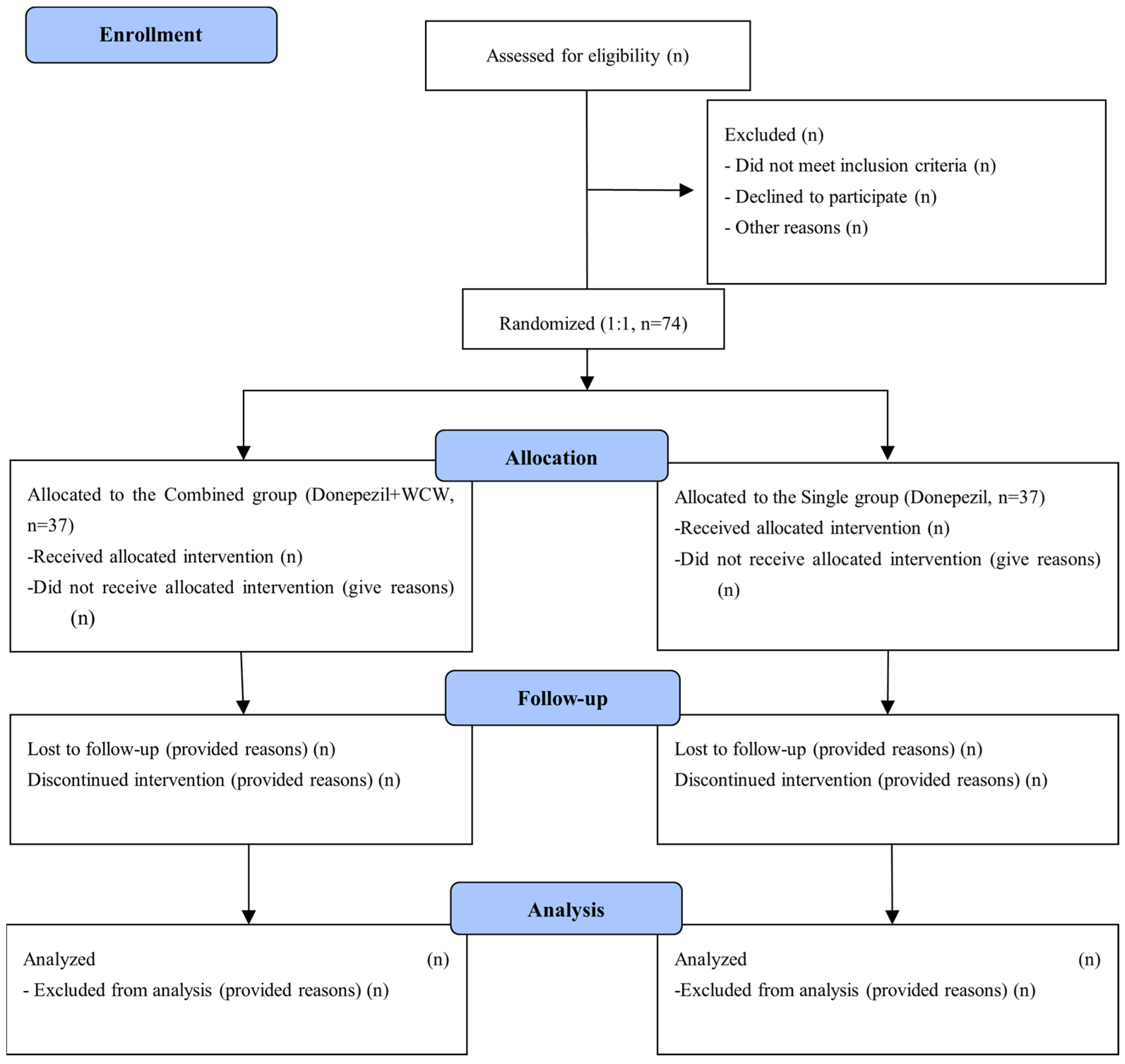
2.2. Study Design and Recruitment
2.3. Eligibility Criteria
2.4. Randomization
2.5. Blinding
3. Detailed Procedure
3.1. Intervention
3.2. Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.3. Additional Analysis
3.3.1. Functional Magnetic Resonance Imaging
3.3.2. Economic Evaluation
3.4. Statistical Methods
3.4.1. Sample Size Calculation
3.4.2. Statistical Analysis
3.5. Safety Evaluation
3.6. Quality Assurance
3.7. Data Management and Monitoring
3.8. Criteria for Clinical Trial Termination
- (1)
- An unforeseen obvious or unacceptable risk to the participant.
- (2)
- Moderate or severe adverse reactions related to the test drugs occur in >25% of the participants.
- (3)
- The principal investigator decides to suspend or discontinue the trial.
3.9. Ethical Statement
4. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Trial Status
References
- Prince, M.J.; Wimo, A.; Guerchet, M.M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analyses of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Grand, J.H.; Caspar, S.; MacDonald, S.W. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [PubMed]
- Walaszek, A.; Schroeder, M.; Albrecht, T.; Le Caire, T.; Carlsson, C.M. Using academic detailing to enhance the knowledge, skills and attitudes of clinicians caring for patients with behavioral and psychological symptoms of dementia. Alzheimers Dement. 2021, 17, e051961. [Google Scholar] [CrossRef]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Zahodne, L.B.; Ornstein, K.; Cosentino, S.; Devanand, D.P.; Stern, Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am. J. Geriatr. Psychiatry 2015, 23, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bessey, L.J.; Walaszek, A. Management of behavioral and psychological symptoms of dementia. Curr. Psychiatry Rep. 2019, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- May, B.H.; Lu, C.; Lu, Y.; Zhang, A.L.; Xue, C.C. Chinese herbs for memory disorders: A review and systematic analysis of classical herbal literature. J. Acupunct. Meridian Stud. 2013, 6, 2–11. [Google Scholar] [CrossRef]
- Yang, W.T.; Zheng, X.W.; Chen, S.; Shan, C.S.; Xu, Q.Q.; Zhu, J.Z.; Bao, X.Y.; Lin, Y.; Zheng, G.Q.; Wang, Y. Chinese herbal medicine for Alzheimer’s disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 2017, 141, 143–155. [Google Scholar] [CrossRef]
- Hyde, A.J.; May, B.H.; Dong, L.; Feng, M.; Liu, S.; Guo, X.; Zhang, A.L.; Lu, C.; Xue, C.C. Herbal medicine for management of the behavioural and psychological symptoms of dementia (BPSD): A systematic review and meta-analysis. J. Psychopharmacol. 2017, 31, 169–183. [Google Scholar] [CrossRef]
- Financial Supervisory Service. Data Analysis, Retrieval and Transfer System. Available online: http://dart.fss.or.kr (accessed on 5 May 2021).
- May, B.H.; Feng, M.; Zhou, I.W.; Chang, S.Y.; Lu, S.C.; Zhang, A.L.; Guo, X.F.; Lu, C.J.; Xue, C.C. Memory impairment, dementia, and Alzheimer’s disease in classical and contemporary traditional Chinese medicine. J. Altern. Complement. Med. 2016, 22, 695–705. [Google Scholar] [CrossRef]
- Oh, Y.T.; Oh, H.M.; Kim, S.W.; Kim, W.Y.; Son, C.G.; Cho, J.H. A survey on ancient literature records on Woohwangchungsim-won and its potential clinical application. J. Haehwa Med. 2017, 26, 1–10, (In Korean, English abstract). [Google Scholar]
- Choi, C.M.; Sun, J.J.; Kim, S.M.; Jung, J.H.; Lee, S.Y.; Choi, W.W.; Hong, J.W.; Park, S.U.; Jung, W.S.; Moon, S.K.; et al. The effect of Uhwangchungsimwon on heart rate variability of healthy subjects. J. Int. Korean Med. 2007, 28, 717–726, (In Korean, English abstract). [Google Scholar]
- Ahn, I.H.; Kim, S.G. A clinical study on the Woohwangchungsim-won pills, suspension. J. Int. Korean Med. 1991, 12, 1–18, (In Korean, English abstract). [Google Scholar]
- Oh, H.M.; Lee, J.S.; Kim, S.W.; Oh, Y.T.; Kim, W.Y.; Lee, S.B.; Cho, Y.R.; Jeon, Y.J.; Cho, J.H.; Son, C.G. Uwhangchungsimwon, a standardized herbal drug, exerts an anti-depressive effect in a social isolation stress-induced mouse model. Front. Pharmacol. 2019, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.K.; Hwang, E.W. Experimental studies on the effect of Woohwangchungsimwon and Sohaphyangwon. Kyunghee Med. 1990, 6, 220–237, (In Korean, English Abstract). [Google Scholar]
- Korean Dementia Association. Dementia—A Clinical Approach, 3rd ed.; Korean Medbook: Seoul, Republic of Korea, 2020. (In Korean) [Google Scholar]
- Rong, X.; Jiang, L.; Qu, M.; Hassan, S.S.U.; Liu, Z. Enhancing therapeutic efficacy of donepezil by combined therapy: A comprehensive review. Curr. Pharm. Des. 2021, 27, 332–344. [Google Scholar] [CrossRef]
- Hwang, S.G.; Park, H. An analysis on prescribing patterns of Alzheimer’s dementia treatment and choline alfoscerate using HIRA claims data. Korean J. Clin. Pharm. 2019, 29, 1–8, (In Korean, English abstract). [Google Scholar] [CrossRef]
- Youn, H.C.; Jeong, H.G. Pharmacotherapy for dementia. J. Korean Med. Assoc. 2018, 61, 758–764, (In Korean, English abstract). [Google Scholar] [CrossRef]
- Zhang, N.; Gordon, M.L. Clinical efficacy and safety of donepezil in the treatment of Alzheimer’s disease in Chinese patients. Clin. Interv. Aging 2018, 13, 1963–1970. [Google Scholar] [CrossRef]
- Meguro, K.; Yamaguchi, S. Decreased behavioral abnormalities after treatment with combined donepezil and yokukansankachimpihange in Alzheimer disease: An observational study. The osaki-tajiri project. Neurol. Ther. 2018, 7, 333–340. [Google Scholar] [CrossRef]
- Gu, C.; Shen, T.; An, H.; Yuan, C.; Zhou, J.; Ye, Q.; Liu, T.; Wang, X.; Zhang, T. Combined therapy of Di-Huang-Yi-Zhi with donepezil in patients with Parkinson’s disease dementia. Neurosci. Lett. 2015, 606, 13–17. [Google Scholar] [CrossRef]
- Maruyama, M.; Tomita, N.; Iwasaki, K.; Ootsuki, M.; Matsui, T.; Nemoto, M.; Okamura, N.; Higuchi, M.; Tsutsui, M.; Suzuki, T.; et al. Benefits of combining donepezil plus traditional Japanese herbal medicine on cognition and brain perfusion in Alzheimer’s disease: A 12-week observer-blind, donepezil monotherapy controlled trial. J. Am. Geriatr. Soc. 2006, 54, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, R.; Zhao, C.; Tang, X.; Lu, A.; Bian, Z. Placebo design in WHO-registered trials of Chinese herbal medicine need improvements. BMC Complement. Altern. Med. 2019, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, R.M.; Stephan, B.C.; Dening, T.; Brayne, C. Instruments to measure behavioural and psychological symptoms of dementia. Int. J. Methods Psychiatr. Res. 2014, 23, 69–98. [Google Scholar] [CrossRef]
- Choi, S.H.; Na, D.L.; Kwon, H.M.; Yoon, S.J.; Jeong, J.H.; Ha, C.K. The Korean version of the neuropsychiatric inventory: A scoring tool for neuropsychiatric disturbance in dementia patients. J. Korean Med. Sci. 2000, 15, 609–615. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S.A. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308, (In Korean, English abstract). [Google Scholar]
- Seo, E.H. Neuropsychological assessment of dementia and cognitive disorders. J. Korean Neuropsychiatr. Assoc. 2018, 57, 2–11, (In Korean, English abstract). [Google Scholar] [CrossRef]
- Cano, S.J.; Posner, H.B.; Moline, M.L.; Hurt, S.W.; Swartz, J.; Hsu, T.; Hobart, J.C. The ADAS-cog in Alzheimer’s disease clinical trials: Psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1363–1368. [Google Scholar] [CrossRef]
- Lee, M.H.; Yoon, E.K. A cross-validation of the Korean version of the revised memory and behavioral problems checklist (K-RMBPC): Exploratory and confirmatory analyses. Korean J. Soc. Welf. 2007, 59, 65–88. (In Korean) [Google Scholar]
- Lynch, C.A.; Walsh, C.; Blanco, A.; Moran, M.; Coen, R.F.; Walsh, J.B.; Lawlor, B.A. The clinical dementia rating sum of box score in mild dementia. Dement. Geriatr. Cogn. Disord. 2006, 21, 40–43. [Google Scholar] [CrossRef]
- Eisdorfer, C.; Cohen, D.; Paveza, G.J.; Ashford, J.W.; Luchins, D.J.; Gorelick, P.B.; Hirschman, R.S.; Freels, S.A.; Levy, P.S.; Semla, T.P.; et al. An empirical evaluation of the global deterioration scale for staging Alzheimer’s disease. Am. J. Psychiatry 1992, 149, 190–194. [Google Scholar] [PubMed]
- Chin, J.; Park, J.; Yang, S.J.; Yeom, J.; Ahn, Y.; Baek, M.J.; Ryu, H.J.; Lee, B.H.; Han, N.E.; Ryu, K.H.; et al. Re-standardization of the Korean-Instrumental Activities of Daily Living (K-IADL): Clinical usefulness for various neurodegenerative diseases. Dement. Neurocogn. Disord. 2018, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, J.H.; Ko, H.J.; Ku, H.M.; Kwon, E.J.; Shin, J.Y.; Ahn, I.S.; Chung, S.H.; Kim, D.K. The standardization of the geriatric quality of life scale-dementia (GQOL-D). J. Korean Geriatr. Soc. 2004, 8, 151–164, (In Korean, English abstract). [Google Scholar]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.A. The geriatric depression scale (GDS). Best Pract. Nurs. Care Older Adults 2012, 4, 4. [Google Scholar]
- Kwon, S.M.; Lim, Y.J. The state-trait anxiety inventory, trait version: Examination of a method factor. Korean Soc. Sci. J. 2007, 34, 105–122. [Google Scholar]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Serra, L.; Bruschini, M.; Di Domenico, C.; Mancini, M.; Gabrielli, G.B.; Bonarota, S.; Caltagirone, C.; Cercignania, M.; Marra, C.; Bozzali, M. Behavioral psychological symptoms of dementia and functional connectivity changes: A network-based study. Neurobiol. Aging 2020, 94, 196–206. [Google Scholar] [CrossRef]
- Yu, L.; Lin, S.M.; Zhou, R.Q.; Tang, W.J.; Huang, P.X.; Dong, Y.; Wang, J.; Yu, Z.H.; Chen, J.L.; Wei, L. Chinese herbal medicine for patients with mild to moderate Alzheimer disease based on syndrome differentiation: A randomized controlled trial. J. Chin. Integr. Med. 2012, 10, 766–776. [Google Scholar] [CrossRef]
- Okahara, K.; Ishida, Y.; Hayashi, Y.; Inoue, T.; Tsuruta, K.; Takeuchi, K.; Yoshimuta, H.; Kiue, K.; Ninomiya, Y.; Kawano, J.; et al. Effects of Yokukansan on behavioral and psychological symptoms of dementia in regular treatment for Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 532–536. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.; Shin, I.J.; Park, J.; Bang, S.Y.; Yi, C.; Kim, M.A.; Lew, J.H.; Suh, J.W.; Maeng, S. Woohwangcheongsimwon prevents high-fat diet-induced memory deficits and induces SIRT1 in mice. J. Med. Food 2018, 21, 167–173. [Google Scholar] [CrossRef]
- Baek, J.S.; Kim, J.W.; Hwang, E.W. The effect of Woohwangchungsimwon on the learning and memory in NOS inhibitor treated rats in Morris water maze. J. Orient. Neuroanat. 1999, 10, 115–126, (In Korean, English abstract). [Google Scholar]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Paterson, R.W.; Warren, J.D.; Zetterberg, H.; O’Brien, J.T.; Fox, N.C.; Halliday, G.M.; Schott, J.M. Biomarkers in dementia: Clinical utility and new directions. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chae, M.S.; Lee, S.M.; Jeong, D.; Lee, B.C.; Lee, J.H.; Kim, Y.; Chang, S.T.; Hwang, K.S. Wafer-scale high-resolution patterning of reduced graphene oxide films for detection of low concentration biomarkers in plasma. Sci. Rep. 2016, 6, 31276. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Cho, E.; Shin, H.J.; Park, J.H.; Hwang, K.S. Enhancing the sensitivity of a micro-diaphragm resonating sensor by effectively positioning the mass on the membrane. Sci. Rep. 2015, 5, 17069. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Lee, J.H.; Shin, S.D.; Mar, W.C. The comparison of histological effects of musk containing and civet containing WoohwangChungSimWon on the cerebral ischemia. J. Appl. Pharmacol. 2000, 8, 255–261, (In Korean, English abstract). [Google Scholar]
- Choi, E.W.; Kim, K.N.; Shin, S.D.; Cho, M.H.; Mar, W.C. The comparative effects of civet-containing and musk-containing WooHwangChungSimWon on the central nervous system. Yakhak Hoeji 2000, 44, 470–477, (In Korean, English Abstract). [Google Scholar]
| Herbal Name | Scientific Name | Amount (mg) |
|---|---|---|
| Dioscorea Rhizome | Dioscorea batatas Decne | 263 |
| Glycyrrhiza | Glycyrrhiza glabra L. | 188 |
| Ginseng | Panax ginseng C.A Mey | 94 |
| Cat-Tail | Typha orientalis C. Presl | 94 |
| Massa Medicata Fermentata | 94 | |
| Glycine Semen Germinatum | Glycine max subsp. soja (Siebold and Zucc.) H. Ohashi | 66 |
| Cinnamon Bark | Cinnamomum cassia (Nees and T. Nees) J. Presl | 66 |
| Glue | Equus asinus L. | 66 |
| Peony Root | Paeonia lactiflora Pall. | 56 |
| Liriope Tuber | Liriope platyphylla F.T. Wang and Tang | 56 |
| Scutellaria Root | Scutellaria baicalensis Georgi | 56 |
| Angelica Gigas Root | Angelica gigas Nakai | 56 |
| Saposhnikovia Root | Saposhnikovia divaricata (Turcz.) Schischk | 56 |
| Atractylodes Rhizome White | Atractylis japonica (Koidz. Ex Kitam.) Kitag. | 56 |
| Bupleurum Root | Bupleurum falcatum L. | 47 |
| Platycodon Root | Platycodon grandiflorum A. | 47 |
| Apricot Kernel | Prunus armeniaca L. | 47 |
| Hoelen | Poria cocos Wolf | 47 |
| Cnidium Rhizome | Cnidium officinale Makino | 47 |
| Oriental Bezoar | Bostaurus Linne var. domesticus Gmelin. | 45 |
| Gazelle Horn | Saiga tatarica L. | 38 |
| Civet | Viverra zibetha L. | 114 |
| Borneolum | Dryobalanops aromatica C.F. Gaertn | 38 |
| Ampelopsis Radix | Ampelopsis japonica (Thumb.) Makino | 28 |
| Ginger | Zingiber officinale Roscoe | 28 |
| Mel | Acalypha indica Radoszkowski | 3117 |
| Aurum | quantum satis |
| Study Period | |||||
|---|---|---|---|---|---|
| Screening | Treatment Period | Closing | |||
| Trial Schedule | Visit 1 | Visit 2 (Week 0) | Visit 3 (Week 8) | Visit 4 (Week 16) | Visit 5 (Week 24) |
| Informed consent, assigned screening number, demographics, and medical history | ● | ||||
| 12-lead ECG, Chest X-ray, B-MRI † | ● | ||||
| Inclusion/Exclusion criteria | ● | ● | |||
| Randomization | ● | ||||
| Changed medical history | ● | ● | ● | ● | |
| Clinical trial drug administration | ● | ● | ● | ||
| Compliance | ● | ● | ● | ||
| K-MMSE ‡, NPI ‡ | ● | ● | ● | ● | ● |
| ADAS-cog, SNSB, EQ-5D, Economic evaluation | ● | ● | |||
| GDetS, K-IADL, GQOL, R-MBPC | ● | ● | ● | ||
| CDR ‡, BMI | ● | ● | ● | ● | |
| PSQI, GDepS, STAI | ● | ● | ● | ● | |
| Pattern Identification, TCI, and Sa-sang | ● | ||||
| Human derivatives study (urine, saliva) | ● | ● | ● | ● | ● |
| fMRI (n = 20) | ● | ● | |||
| Physical examination, vital signs | ● | ● | ● | ● | ● |
| Laboratory test § | ● | ● | ● | ||
| Adverse reactions | ● | ● | ● | ||
| Blinding maintenance test | ● | ||||
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.G.; Hyun, M.-S.; Park, S.G.; Cho, E.; Kim, J.; Choi, H.-K.; Son, K.-L.; Lim, C.-Y.; Kim, K.K.; Koo, B.S. Efficacy and Safety of Woohwangchungsimwon Combined with Donepezil in Behavioral and Psychological Symptoms of Dementia in Patients with Probable Alzheimer’s Disease: Study Protocol for a Randomized Controlled Trial. Healthcare 2023, 11, 2036. https://doi.org/10.3390/healthcare11142036
Kim MG, Hyun M-S, Park SG, Cho E, Kim J, Choi H-K, Son K-L, Lim C-Y, Kim KK, Koo BS. Efficacy and Safety of Woohwangchungsimwon Combined with Donepezil in Behavioral and Psychological Symptoms of Dementia in Patients with Probable Alzheimer’s Disease: Study Protocol for a Randomized Controlled Trial. Healthcare. 2023; 11(14):2036. https://doi.org/10.3390/healthcare11142036
Chicago/Turabian StyleKim, Man Gi, Mi-Suk Hyun, Sang Gu Park, Eun Cho, Jinsik Kim, Hyung-Kyoon Choi, Kyung-Lak Son, Chi-Yeon Lim, Kwang Ki Kim, and Byung Soo Koo. 2023. "Efficacy and Safety of Woohwangchungsimwon Combined with Donepezil in Behavioral and Psychological Symptoms of Dementia in Patients with Probable Alzheimer’s Disease: Study Protocol for a Randomized Controlled Trial" Healthcare 11, no. 14: 2036. https://doi.org/10.3390/healthcare11142036
APA StyleKim, M. G., Hyun, M.-S., Park, S. G., Cho, E., Kim, J., Choi, H.-K., Son, K.-L., Lim, C.-Y., Kim, K. K., & Koo, B. S. (2023). Efficacy and Safety of Woohwangchungsimwon Combined with Donepezil in Behavioral and Psychological Symptoms of Dementia in Patients with Probable Alzheimer’s Disease: Study Protocol for a Randomized Controlled Trial. Healthcare, 11(14), 2036. https://doi.org/10.3390/healthcare11142036







