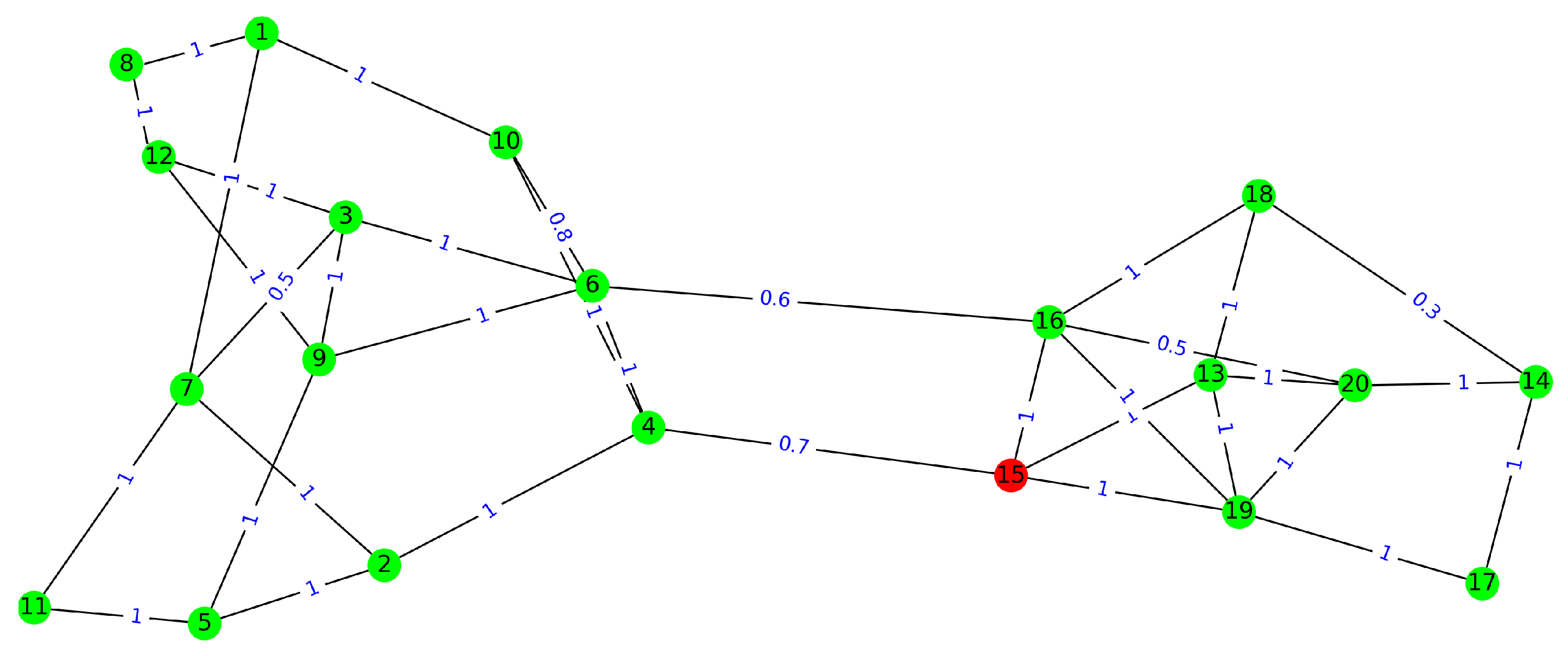
Personnel scheduling during a pandemic requires special handling to ensure employee safety while continuing regular operations. Since the virus that spreads COVID-19, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is transmitted through individual contacts [
22], the World Health Organization [
23] suggested several measures to control the spread of the virus, including social distancing, testing, and vaccination.
This section proposes two MINLP models to solve the personnel scheduling problem during disease outbreaks, particularly the COVID-19 pandemic. We aim to derive a presence strategy to find the optimal schedule of employees (i.e., working remotely or at the workplace) and a testing strategy to determine the testing days of the employees. We consider an organization with n employees who are in contact with each other and have to be allocated in a discrete-time horizon, (i.e., a week, ) such that the risk of infection in the workplace is minimized. The proposed models compute the probability of infection for each employee under two different cases. The first model assumes that the employees comply with the testing protocols following the suggested testing days. The second model does not impose strict regulations on testing, so the employees perform tests arbitrarily during the evaluated time horizon. The notation and assumptions considered in the proposed models are listed below.
3.1. Computing the Probability of Infection in a Graph-Based Approach
In this subsection, we propose a graph-based approach to compute the probability of infection in the workplace. Let
be the probability of infection for
at the end of the working day
d, for
and
. The objective function is to minimize the expected risk of infection inside the facilities, which is directly related to minimizing the probability of infection of employees. Thus,
To compute the probability , we present a two-step procedure. In the first step, we calculate an intermediate probability () based on a recursive formula that incorporates the presence and testing strategies for the employees. As mentioned in the assumptions, the initial probability of infection is set to , which means a risk of infection based on the number of incidences reported in the organization’s neighborhood. In the second step, we calculate the final probability by considering the graph of connectivity among the employees.
Step 1: If an employee with a probability of infection
p performs a test and the result is negative, the infection probability will reduce to
, where
is the probability of false negativity for the tests. Now, let the binary variable
indicate
is tested on day
d before coming to the workplace (i.e.,
) or not (i.e.,
). So, the updating recursive formula can be written as
Equation (
2) works well when the employees follow the testing strategy. If they do not follow this strategy, we can apply a random testing strategy, that is, assuming a testing probability
for each employee. For example, if the employees take two tests in five days, the Probability of testing per day is
. If
performs a test at day
d with probability
, then we can use the following equation for applying the effect of tests and computing
.
Therefore, regarding the fact that the employees follow a recommended testing strategy, Equation (
2), or a random test strategy, Equation (
3), we apply the effect of performing the tests and update the probability of infection for all employees. Then, we update the probabilities of infection for the employees based on their contacts.
Step 2: If
comes to the workplace on day
d (i.e.
), and with the probability of
contacts with
(suppose
as well), then the probability of infection for him/her can be updated as:
where
is the probability of infection for
in the case that
is infected. For the sake of simplicity, we only assume two possible values for
, whether the employee is vaccinated or not.
denotes the effect of contact with
. Thus, by applying all possible contacts
may have during day
d, the infection probability at the end of the day can be computed as follows:
For the sake of simplicity, let us denote the two-step procedure by a function based on the related parameters if the employees follow the recommended testing strategy:
and, if they follow a random testing strategy:
where
is the infection probability for the employees, and
and
are the presence indicator and testing indicators for all the employees in the day
d, respectively.
3.2. Personnel Scheduling and Testing Strategy Models
In this section, we define two MINLP models considering the probability-of-infection equations defined in
Section 3.1. Model 1 assumes that the employees follow a recommended testing strategy, and Model 2 is based on the fact that employees do not follow the testing protocols. Thus, they test themselves randomly with a probability of
for each day. We also consider a set of constraints. We keep the model simple and easy to understand. In practice, different organizations may have their specific limits and constraints, which can be added to the model. Here, we consider two families of upper and lower bound constraints: the first family of constraints is related to satisfying the on-site tasks in the organization. They are defined as:
where
is the
subset of employees such that at least
number of them have to present at the workplace on day
d. As an example, assume the task of registration and deregistration of citizens in the municipality. In the usual situation, it may need, for example, five employees present at office in order to carry out the related tasks. However, in the pandemic, the municipality may reduce the minimum necessary employees to two employees per day. The second family of constraints refers to capacity limitations in the workplace. In fact, during the COVID-19 pandemic, there were regulations on the maximum number of employees who could be simultaneously (in a day) present in the workplace. As an example, assume there is an office that usually has five employees on staff where, due to the pandemic and the maintenance of safe social distance, only three of them can be working simultaneously. So, we can model them as follows:
Note that in real-world applications, scheduling can be conducted for short intervals, such as a week, i.e., working days. This is essential due to a higher rate of change in the incidence level, which plays a basic role in initializing background risk . As a result, in order to keep the model straightforward, we do not include the time-related constraints that may apply to lengthy scheduling intervals.
Model 1 would result in a lower risk of infection compared to Model 2, but it requires the employees to follow the recommended testing strategy. In contrast, Model 2 depicts a more flexible testing scheme, in which the employees apply the offered tests randomly during the scheduling period. The models are defined as follows:
refers to the test capacity for ; the maximum number of available test kits for in a period of D days. This capacity may be the same for all employees or distributed among the employees according to the number of connections each employee has. Model 1 has two decision variables, presence scheduling, , and testing schedule, , for and . So, the model will (optimally) derive which employees to allocate in the workplace and when, and on which days to perform the tests. If in an organization, the employees do not follow the suggested testing strategy, and they use the tests arbitrarily during the scheduling period, Model 1 will not fit with that organization. In this case, the following model, which assumes the tests can be used by the employees with a probability, is a better match for that organization.
Both Model 1 and Model 2 are MINLP and, like the general scheduling problem [
24] with hard constraints, are NP-hard. Furthermore, considering the upper bound and lower bound constraints of the problem, finding even a feasible solution that satisfies the constraints is an intractable problem. The main difficulty of the models is updating the risk of infection for the employees after daily contacts, Equation (
5). It is an exponential equation and impossible for most algorithms to cope with. Therefore, in the following, we present an efficient simplification to handle this issue.
It is worthwhile to mention, both models require the contact network among the employees. That means the (average) contact rate between any pair of employees should be known. Further, the models assume a risk of infection for the employee when they work from home, which may differ in practice. Indeed, the infection risk varies from employee to employee, depending on their connection with family members and friends. Finally, the models do not consider the absence of employees, which may result from sick leaves. This works well when the rate of sick leaves does not affect workflows, and if it is not the case, the constraints on the minimum number of on-site employees should be satisfied with a confident threshold.
Relaxation The term
in Equation (
5) is the only exponential equation of the proposed models. As explained, this term is used for updating the infection risk of an employee after his/her daily contacts. In practice, the value of this term is so small (based on the data and experiments, that it is on the order of
. So, to relax the models and remove this exponential term, we use the linear Taylor expansion of the formula,
. Thus, the simplified approximation of Equation (
5) can be written as below:
To evaluate the accuracy of this simplification, we compared the above equation with Equation (
5) using the parameters reported in the example presented in
Section 5.1. The comparison showed that the equations result in almost the same values, with a precision on the order of
on average. Thus, it is a suitable linear approximation in practice.