Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection Process
2.4. Data Collection Process
2.5. Data Analysis
3. Results
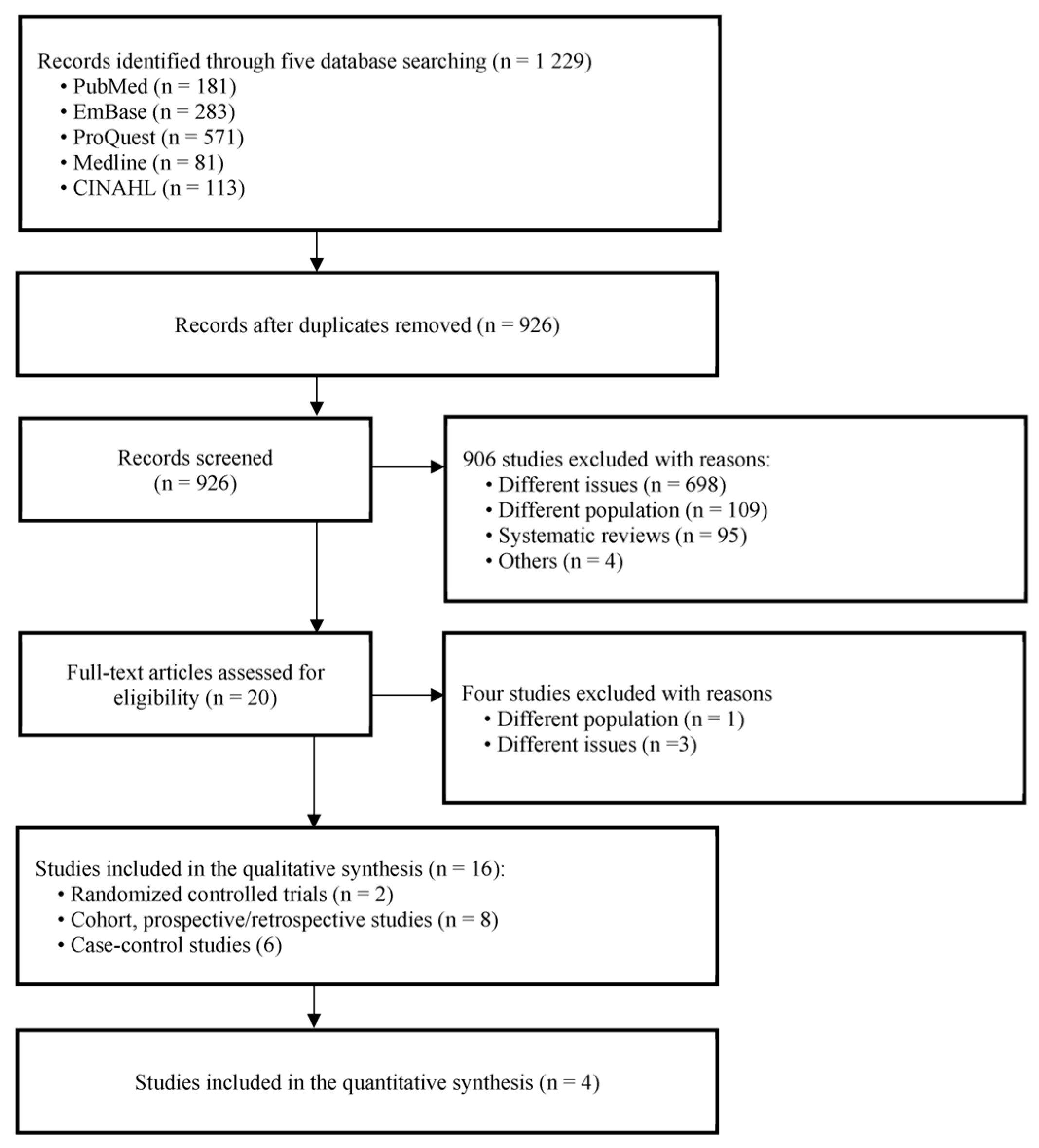
3.1. Literature Search and Characteristics of the Included RCTs
3.2. Predictors of Returning Driving Post-Stroke
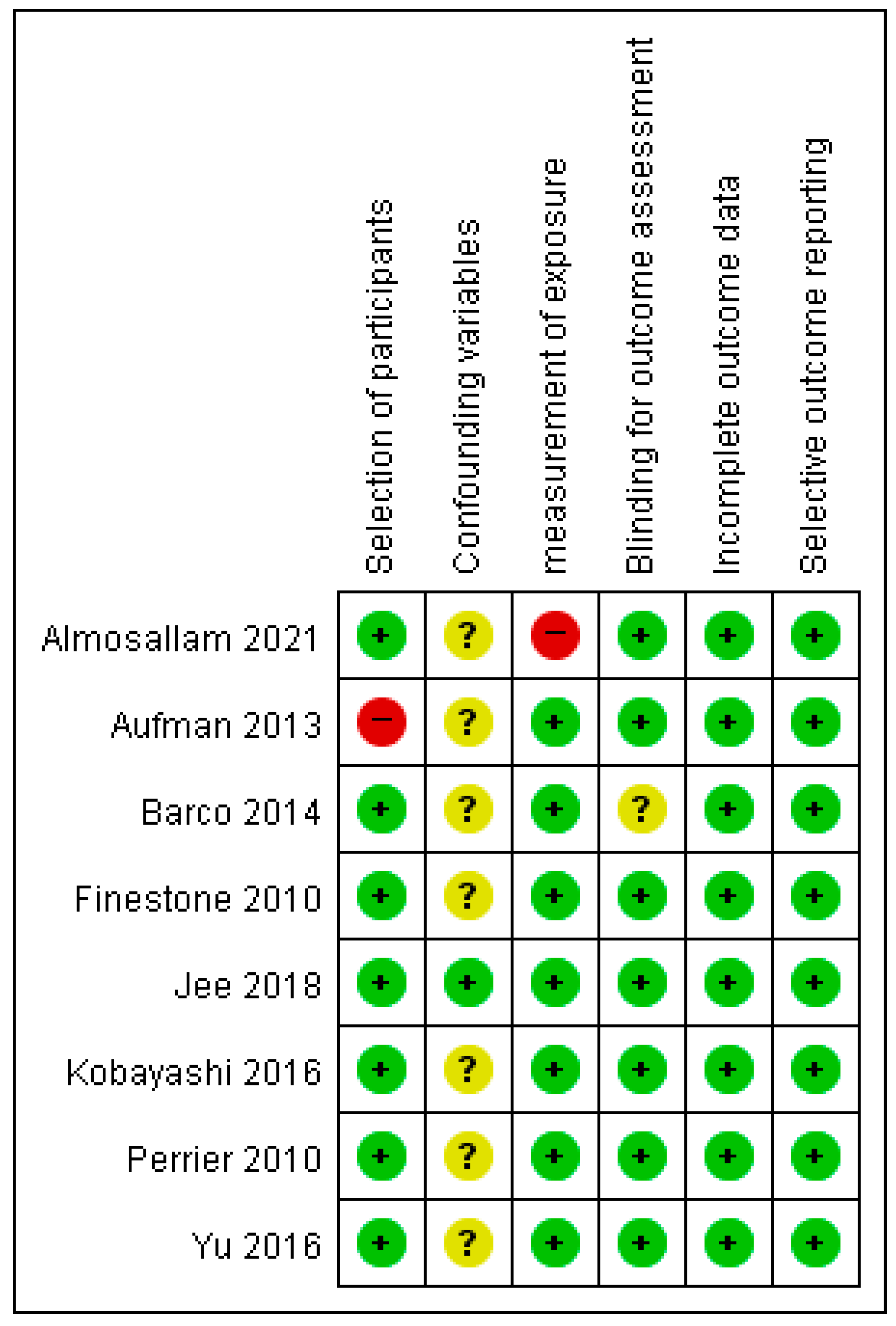
3.3. Risk of Bias in All Included Studies for Predictors to Return to Driving after Stroke
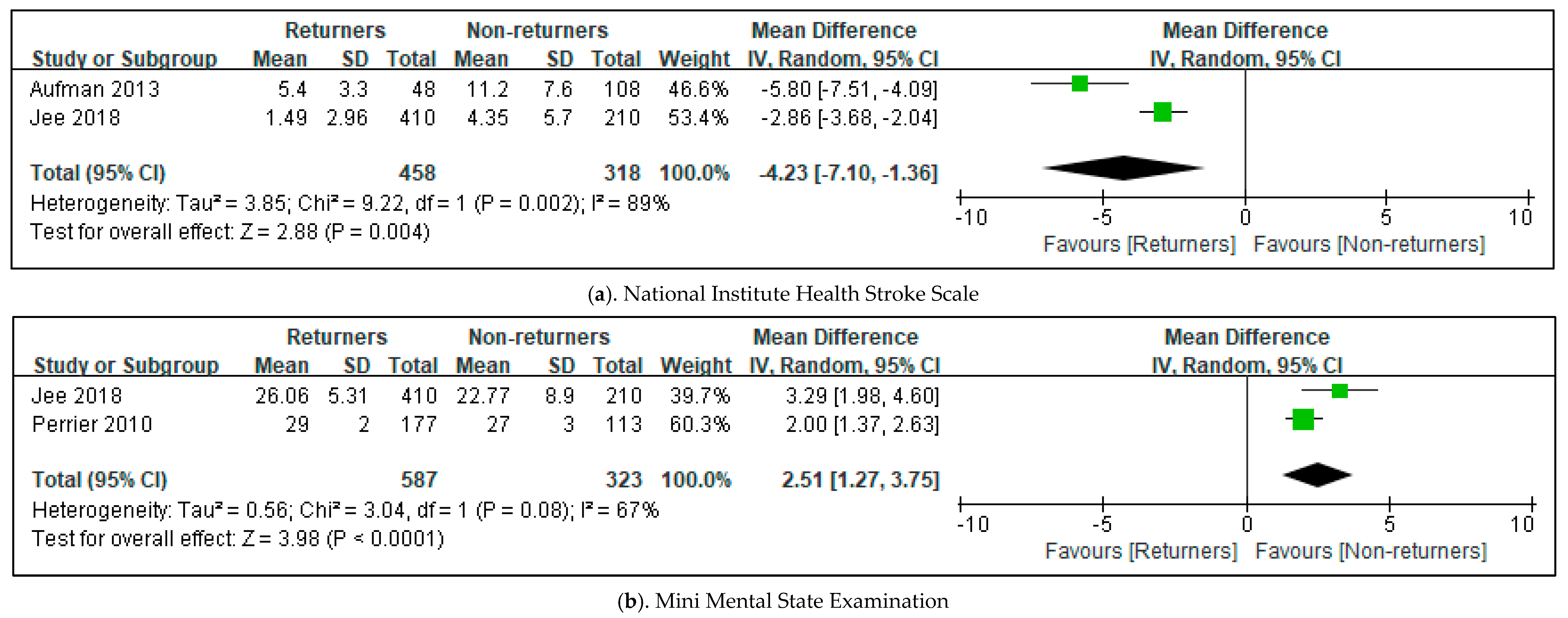
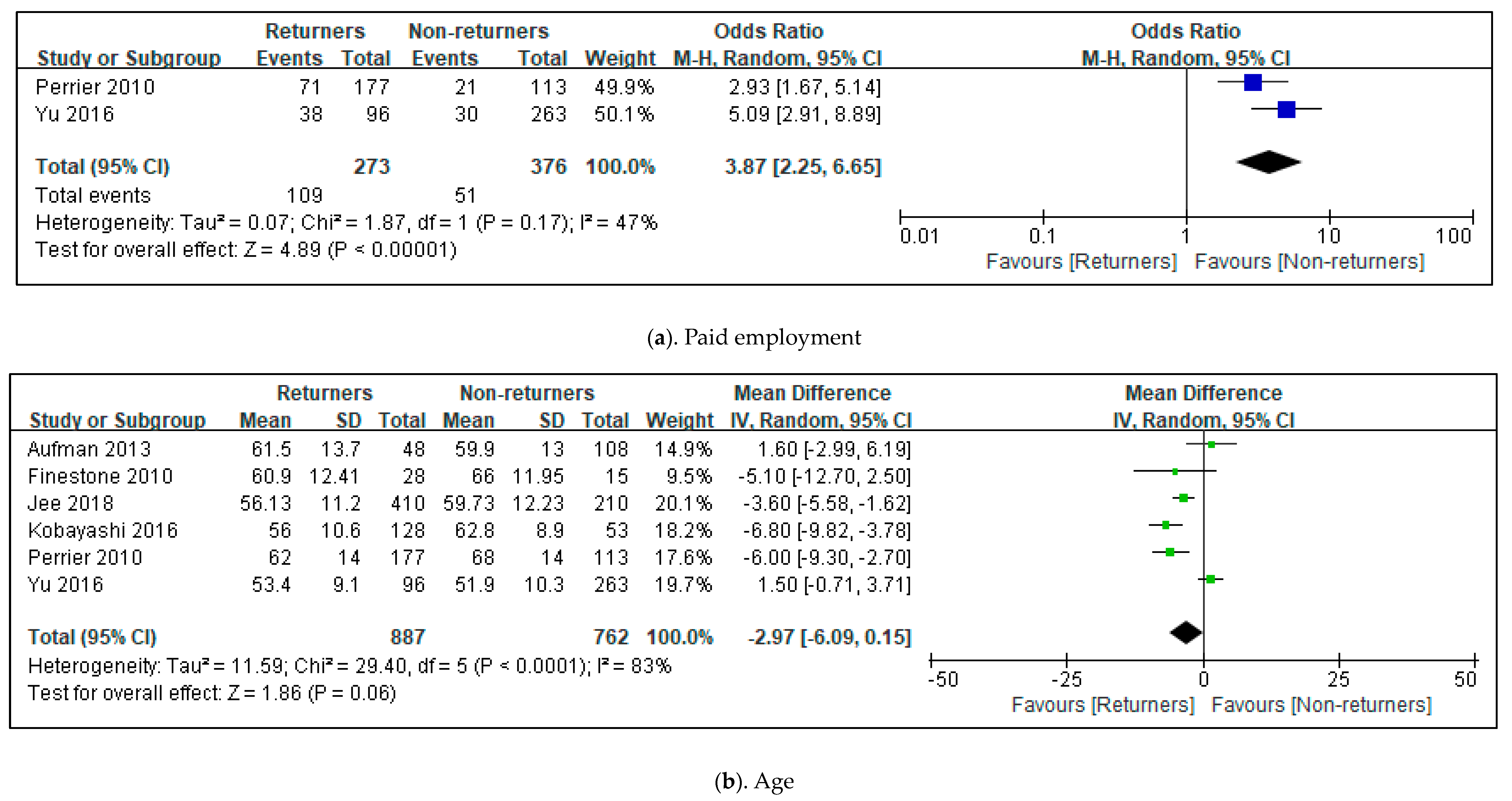
3.4. Effects in All Included Studies for Predictors to Return Driving after Stroke
3.5. Comparison of Stroke Survivors’ Driving Performance with Healthy Controls
3.6. Risk of Bias for Stroke Survivors’ Driving Performance with Healthy Controls
3.7. Effects of Driving Simulator Training for Stroke Patients
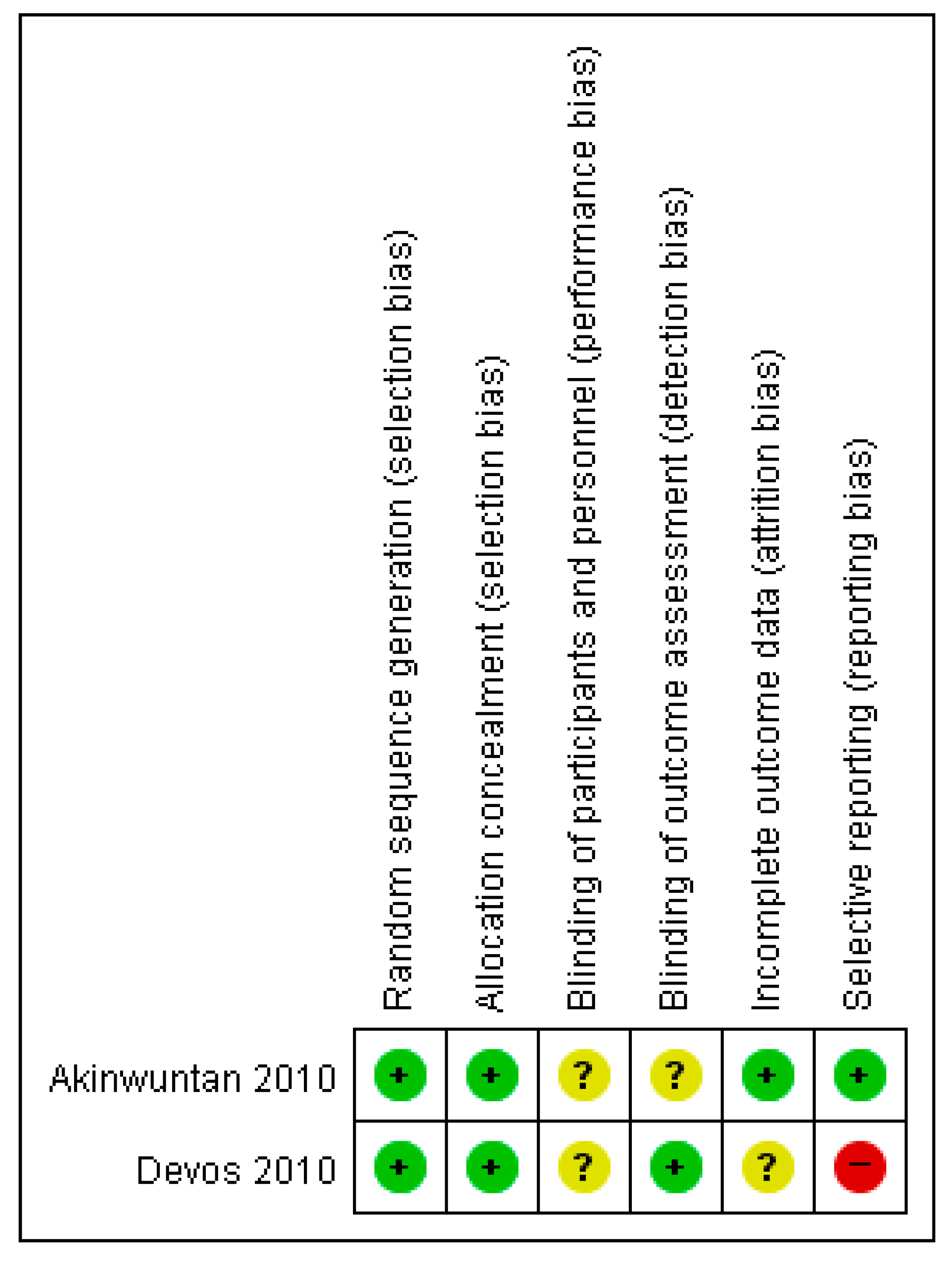
3.8. Risk of Bias of RCT Studies of Driving Stimulator Training for Stroke Patients
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aufman, E.L.; Bland, M.D.; Barco, P.P.; Carr, D.B.; Lang, C.E. Predictors of Return to Driving After Stroke. Am. J. Phys. Med. Rehabil. 2013, 92, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.G.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Han, E.Y.; et al. Prediction of Return to Driving after First-Ever Stroke in Korea: The KOSCO Study. J. Rehabil. Med. 2018, 50, 800–805. [Google Scholar] [CrossRef] [Green Version]
- Blane, A.; Falkmer, T.; Lee, H.C.; Dukic Willstrand, T. Investigating Cognitive Ability and Self-Reported Driving Performance of Post-Stroke Adults in a Driving Simulator. Top. Stroke Rehabil. 2018, 25, 44–53. [Google Scholar] [CrossRef]
- Finestone, H.M.; Guo, M.; O’Hara, P.; Greene-Finestone, L.; Marshall, S.C.; Hunt, L.; Biggs, J.; Jessup, A. Driving and Reintegration into the Community in Patients After Stroke. PM R 2010, 2, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Groeger, J.A.; Murphy, G. Driving and Cognitive Function in People with Stroke and Healthy Age-Matched Controls. Neuropsychol. Rehabil. 2022, 32, 1075–1098. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, M.; Vidmar, G.; Oberstar, K.; Zupan, A. Simple and Choice Reaction Times of Healthy Adults and Patients after Stroke during Simulated Driving. Int. J. Rehabil. Res. 2019, 42, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Lodha, N.; Patel, P.; Shad, J.M.; Casamento-Moran, A.; Christou, E.A. Cognitive and Motor Deficits Contribute to Longer Braking Time in Stroke. J. Neuroeng. Rehabil. 2021, 18, 7. [Google Scholar] [CrossRef]
- Park, M.O. Development of a Web-Based Mini-Driving Scene Screening Test (MDSST) for Clinical Practice in Driving Rehabilitation. Int. J. Environ. Res. Public Health 2022, 19, 3582. [Google Scholar] [CrossRef]
- Devos, H.; Akinwuntan, A.E.; Nieuwboer, A.; Ringoot, I.; Van Berghen, K.; Tant, M.; Kiekens, C.; De Weerdt, W. Effect of Simulator Training on Fitness-to-Drive after Stroke: A 5-Year Follow-up of a Randomized Controlled Trial. Neurorehabil. Neural Repair 2010, 24, 843–850. [Google Scholar] [CrossRef]
- Barco, P.P.; Wallendorf, M.J.; Snellgrove, C.A.; Ott, B.R.; Carr, D.B. Predicting Road Test Performance in Drivers with Stroke. Am. J. Occup. Ther. 2014, 68, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Muhunthan, J.; Lindley, R.; Glozier, N.; Jan, S.; Anderson, C.; Li, Q.; Hackett, M.L. Driving in Stroke Survivors Aged 18–65 Years: The Psychosocial Outcomes in StrokE (POISE) Cohort Study. Int. J. Stroke 2016, 11, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Chan, M.L. Stroke Recurrence among Stroke Patients Referred for Driving Assessment and Rehabilitation: A Cohort Study. J. Cardiovasc. Dev. Dis. 2023, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Stratton, L.; Parsons, J.; Tisbury, S.; Waterworth, S.; Starkey, N. Clinician Knowledge of Driving Restrictions Following a Stroke Event. N. Z. Med. J. 2021, 134, 46–56. [Google Scholar]
- Mårdh, S.; Mårdh, P.; Anund, A. Driving Restrictions Post-stroke: Physicians’ Compliance with Regulations. Traffic. Inj. Prev. 2017, 18, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.M.; Smead, J.M.; Cameron, I.D. Retrospective Cohort Study of Accident Outcomes for Individuals Who Have Successfully Undergone Driver Assessment Following Stroke. Aust. Occup. Ther. J. 2012, 59, 56–62. [Google Scholar] [CrossRef]
- Petzold, A.; Korner-Bitensky, N.; Rochette, A.; Teasell, R.; Marshall, S.; Perrier, M.J. Driving Poststroke: Problem Identification, Assessment Use, and Interventions Offered by Canadian Occupational Therapists. Top. Stroke Rehabil. 2010, 17, 371–379. [Google Scholar] [CrossRef]
- Murie-Fernandez, M.; Iturralde, S.; Cenoz, M.; Casado, M.; Teasell, R. Driving Ability after a Stroke: Evaluation and Recovery. Neurol. Engl. Ed. 2014, 29, 161–167. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 9781119536628. [Google Scholar]
- Almosallam, A.; Qureshi, A.Z.; Ullah, S.; Alibrahim, A. Return to Driving Post Stroke; Patients’ Perspectives and Challenges in Saudi Arabia. Top. Stroke Rehabil. 2022, 29, 192–200. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Omokute, Y.; Mitsuyama, A.; Takaoka, Y.; Takama, C.; Watanabe, Y. Predictors of Track Test Performance in Drivers with Stroke. Turk. Neurosurg. 2017, 27, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Perrier, M.J.; Korner-Bitensky, N.; Mayo, N.E. Patient Factors Associated with Return to Driving Poststroke: Findings From a Multicenter Cohort Study. Arch. Phys. Med. Rehabil. 2010, 91, 868–873. [Google Scholar] [CrossRef]
- Hitosugi, M.; Takehara, I.; Watanabe, S.; Hayashi, Y.; Tokudome, S. Support for Stroke Patients in Resumption of Driving: Patient Survey and Driving Simulator Trial. Int. J. Gen. Med. 2011, 4, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, C.; Rapport, L.J.; Bryer, R.C.; Casey, J. Self-Evaluation of Driving Simulator Performance after Stroke. Top. Stroke Rehabil. 2011, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, A.; Ratcliffe, J.; George, S. Evaluation of Driving Confidence in Post-Stroke Older Drivers in South Australia. Australas. J. Ageing 2014, 33, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Ryu, Y.U.; Lee, H.C. Steering Accuracy and Sitting Symmetry during Simulated Driving in Drivers with Chronic Stroke. Eur. J. Mol. Clin. Med. 2020, 7, 3988–3994. [Google Scholar]
- Akinwuntan, A.E.; Devos, H.; Verheyden, G.; Baten, G.; Kiekens, C.; Feys, H.; De Weerdt, W. Retraining Moderately Impaired Stroke Survivors in Driving-Related Visual Attention Skills. Top. Stroke Rehabil. 2010, 17, 328–336. [Google Scholar] [CrossRef]
- Wouters, A.; Nysten, C.; Thijs, V.; Lemmens, R. Prediction of Outcome in Patients with Acute Ischemic Stroke Based on Initial Severity and Improvement in the First 24 H. Front. Neurol. 2018, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Lyden, P. Using the National Institutes of Health Stroke Scale. Stroke 2017, 48, 513–519. [Google Scholar] [CrossRef]
- Mistry, E.A.; Yeatts, S.; de Havenon, A.; Mehta, T.; Arora, N.; De Los Rios La Rosa, F.; Starosciak, A.K.; Siegler, J.E.; Mistry, A.M.; Yaghi, S.; et al. Predicting 90-Day Outcome After Thrombectomy: Baseline-Adjusted 24-Hour NIHSS Is More Powerful Than NIHSS Score Change. Stroke 2021, 52, 2547–2553. [Google Scholar] [CrossRef]
- Ooi, H.K.; Chai, S.C.; Kadar, M. Effects of Pressure Garment on Spasticity and Function of the Arm in the Early Stages after Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2020, 34, 515–523. [Google Scholar] [CrossRef]
- Weaver, N.A.; Kancheva, A.K.; Lim, J.-S.; Biesbroek, J.M.; Wajer, I.M.H.; Kang, Y.; Kim, B.J.; Kuijf, H.J.; Lee, B.-C.; Lee, K.-J.; et al. Post-Stroke Cognitive Impairment on the Mini-Mental State Examination Primarily Relates to Left Middle Cerebral Artery Infarcts. Int. J. Stroke 2021, 16, 981–989. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, S.; Fan, Z.; Li, Z.; He, F.; Lin, C.; Topatana, W.; Yan, Y.; Liu, Z.; Chen, Y.; et al. Evaluation of the Mini-Mental State Examination and the Montreal Cognitive Assessment for Predicting Post-Stroke Cognitive Impairment During the Acute Phase in Chinese Minor Stroke Patients. Front. Aging Neurosci. 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Wicht, C.A.; Chavan, C.F.; Annoni, J.-M.; Balmer, P.; Aellen, J.; Humm, A.M.; Crettaz von Roten, F.; Spierer, L.; Medlin, F. Predictors for Returning to Paid Work after Transient Ischemic Attack and Minor Ischemic Stroke. J. Pers. Med. 2022, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.; Churilov, L.; Collier, J.M.; Carvalho, L.B.; Borschmann, K.; Moodie, M.; Thijs, V.; Bernhardt, J. Factors Associated with Paid Employment 12 Months after Stroke in A Very Early Rehabilitation Trial (AVERT). Ann. Phys. Rehabil. Med. 2022, 65, 101565. [Google Scholar] [CrossRef] [PubMed]

| First Author (Year) | Aim | Study Design, Follow-Up Period | Population (% Female) Subgroup (Age) | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Almosallam (2021) [19] | To explore the factors involved in return to driving among stroke survivors | Prospective | 100 (0%) R, 7; NR, 87; ND, 6 | Age Marital status | R = NR on age and marital status |
| Aufman (2013) [1] | To identify which patients with acute stroke will and will not return to driving at admission to an inpatient rehabilitation hospital | Retrospective | 198 (46.5%) ND, 42 (64.1 ± 14.0) NR, 108 (59.9 ± 13.0) R, 48 (61.5 ± 13.7) | NIHSS, SBT, Motricity index, ARAT, BBS, Mesulam, CBS Woodcock-Johnson, Boston Naming Test, FIM cognition, FIM walking, FIM UE dressing | R > NR on FIM, NIHSS, Motricity index, BBS, CBS, and Woodcock-Johnson |
| Barco (2014) [10] | To develop a brief screening battery to predict the on-road performance of drivers who had experienced a stroke | Cross-sectional observational research | 72 (46.0%) Pass group, 45 (55.8 ± 13.8) Fail group, 27 (65.1 ± 10.6) | Visual acuity, 9-hole peg test, grip strength, Brake reaction time, Short Blessed Test, SMT, CDT, TMT-A, TMT-B, NIHSS, MFVPT, mWURT | Pass group > Fail group on visuospatial skills, psychomotor speed, attention TMT Part A and SMT |
| Finestone (2010) [4] | To investigate the relationship between driving vs. not driving and community integration after stroke | Prospective study, 1 year | 43 (Not reported) NR 15 (69.1 ± 11.88) R 28 (65.3 ± 12.25) | FIM, CIRS, BDI-II, Self-rated health, Health problems interfere with activities, RNLI score | R > ND on age when learned to drive and FIM, CIRS, self-rated health, and RNLI scores |
| Jee (2018) South Korea [2] | To predict which patients with first-ever stroke will return to driving for the 1-year period after a stroke | Multicenter prospective cohort study, 1 year | 620 (14.8%) R, 410 (56.1 ± 11.2) NR, 210 (59.7 ± 12.2) | NIHSS, FAC, mRS, K-FAST, K-MMSE, FMA | R > NR on Male patients, education about return to driving, lower mRS, and higher FMA at 7 days after stroke |
| Kobayashi (2016) [20] | To predict outcomes of driving tests in patients with stroke who wish to resume driving | Cross-sectional study | 181 (15.5%) Capable drivers 128 (56.0 ± 10.6) Incapable drivers 53 (62.8 ± 8.9) | TMT-A, TMT-B, MMSE, DS, TS, VCT, ADT, SDMT, MUT, PASAT, PST | Capable drivers > Incapable drivers on SDMT |
| Perrier (2010) [21] | To estimate the extent to which body structure function, activity, and context explain driving resumption at 1 year | Cohort study | 290 (32.5%) R, 177 (62 ± 14) NR, 113 (68 ± 14) | CNS score, type of stroke, side of lesion, HUI vision, MMSE, SIS memory, SF-36 mental health, SF-36 emotion, SIS, age, sex, paid employment, number of children, live along | R > NR on SIS (memory, hand, mobility, and strength), SF-36 physical function, and MMSE |
| Yu (2016) [11] | To determine the frequency and predictors of return to driving within 1 month of acute stroke | Cohort study | 359 (33.4%) R, 96 (53.4 ± 9.1) NR, 263 (51.9 ± 10.3) | Job-related driving, recall, education, paid employment, TICS, HADS, JCQ, FAI, SF-36 vitality score | R > NR on paid employment |
| First Author (Year) | Aim | Study Design, Exposure | Population (% Female) Subgroup | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Blane (2017) [3] | To assess the cognition, self-rated performance, and estimation of task demand in a driving simulator with post-stroke drivers and controls | Between-groups comparison group design, driving simulator | Stroke driver 40 (20%, 66 ± 9) Controls 43 (18.6%, 67 ± 8) | Mean speed, speed variability, headway, lateral lane position, steering input, hazard perception speed variability, braking total time to stop, braking distance from reaction, total breaking distance, TLX |
|
| Hitosugi (2011) [22] | To establish an effective support program for stroke patients who wish to resume driving | Cross-sectional study driving simulator | Stroke driver 24 (16.7%, 55.2 ± 12.9) Health 20 (45.0%, 28.3 ± 7.6) | Rate of success and reaction times in the braking task, braking times |
|
| Lodha (2021) [7] | To determine the impact of cognitive and motor impairments on braking time in chronic stroke | Cross-sectional study, driving simulator | Stroke driver 20 (60%, 64.4 ± 14.8) Health 20 (55%, 67.5 ± 8.4) | Divided attention, selective attention, processing speed, plantarflexion force, dorsiflexion force, root mean squared error, braking time |
|
| McKay (2011) [23] | To compare the accuracy of stroke survivors’ self-evaluation of driving with that of healthy controls | Cross-sectional study driving simulator | Stroke driver 30 (50%, 54.3 ± 9.1) Health 30 (60%, 48.5 ± 13.0) | TMT-B, SDMT, COWAT, NAB-Judgment, driving simulator |
|
| McNamara (2013) [24] | To determine whether self-perceived driving confidence levels are lower in the post-stroke driving population than their aged-matched non-stroke driving peers | Cross-sectional study, Telephone interview | Stroke driver 40 (37.5%72 ± 5.2) Health 114 (50.9%, 65 ± 12.2) | ADSES, DHQ |
|
| Shin (2020) [25] | To investigate the characteristics of sitting symmetry and steering accuracy of stroke drivers compared to healthy drivers | Cross-sectional study, driving simulator | Stroke 15 (49.5 ± 9.5) Health 15 (50.1 ± 9.9) | Steering accuracy, searing symmetry |
|
| First Author (Year) | Aim | No. of Participants | Intervention | Therapeutic Intensity | Comparison | Outcome Measures | Summary of Results |
|---|---|---|---|---|---|---|---|
| Akinwuntan (2010) [26] | To investigate the effects of 2 training programs on performance in the UFOV, a validated test of driving-related visual attention skills | 69 EG (n = 33, 55 ± 12) CG (n = 36, 54 ± 11) | Simulator training (STISIM Drive system) | 1 h, 15 sessions | Cognitive therapy (non-simulator-based training of cognitive skills) | UFOV, | EG = CG |
| Devos (2010) [9] | To determine the effect of simulator vs. cognitive rehabilitation therapy on fitness-to-drive in RCTs 5 years after stroke To investigate the differences in clinical characteristics between drivers and non-drivers at 5-years post-stroke with a particular focus on depression | 83 EG (n = 42, 58 ± 12) CG (n = 41, 59 ± 12) | Simulator training | 1 h, 15 sessions | Cognitive therapy | Visual tests (binocular acuity and kinetic vision) Neuropsychological assessment (SDSA, UFOV) On-road tests (TRIP) Others (HADS, BI, number of kilometers driven per year, number of self-reported traffic tickets and accidents, driving status) | EG > CG post-training EG > CG at 6 months EG = CG 5 years later |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Song, C.-S. Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis. Healthcare 2023, 11, 1637. https://doi.org/10.3390/healthcare11111637
Hwang S, Song C-S. Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis. Healthcare. 2023; 11(11):1637. https://doi.org/10.3390/healthcare11111637
Chicago/Turabian StyleHwang, Sujin, and Chiang-Soon Song. 2023. "Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis" Healthcare 11, no. 11: 1637. https://doi.org/10.3390/healthcare11111637
APA StyleHwang, S., & Song, C.-S. (2023). Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis. Healthcare, 11(11), 1637. https://doi.org/10.3390/healthcare11111637






