Indexing of Speckle Tracking Longitudinal Strain of Right Ventricle to Body Surface Area Does Not Improve Its Efficiency in Diagnosis and Mortality Risk Stratification in Patients with Acute Pulmonary Embolism
Abstract
1. Introduction
2. Material and Methods
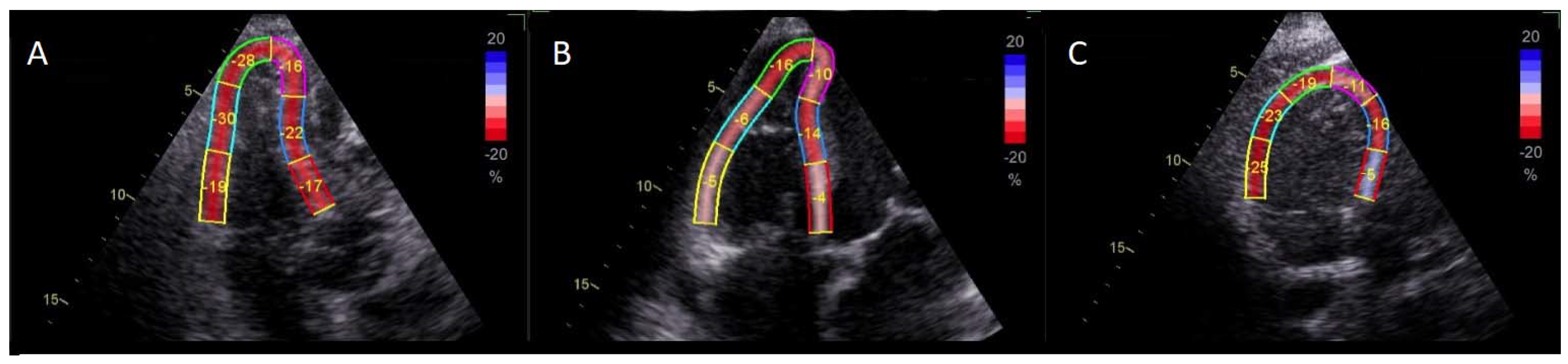
2.1. Methodology
2.2. Statistical Analysis
3. Results
3.1. Course of the Study
3.2. Echocardiographic Parameters
3.3. Analysis of Predictors of 30-Day All-Cause Mortality
4. Discussion
5. Conclusions
6. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstantinides, S.V.; Meyer, G.; Bueno, H.; Galié, N.; Gibbs, J.S.R.; Ageno, W.; Agewall, S.; Almeida, A.G.; Andreotti, F.; Barbato, E.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Oleksiuk-Bójko, M.; Lisowska, A. Venous Thromboembolism: Why Is It Still a Significant Health Problem? Adv. Med. Sci. 2022, 68, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ciurzyński, M.; Kurzyna, M.; Kopeć, G.; Błaszczak, P.; Chrzanowski, Ł.; Kamiński, K.; Mizia-Stec, K.; Mularek-Kubzdela, T.; Biederman, A.; Zieliński, D.; et al. An Expert Opinion of the Polish Cardiac Society Working Group on Pulmonary Circulation on Screening for Chronic Thromboembolic Pulmonary Hypertension Patients after Acute Pulmonary Embolism: Update. Kardiol. Pol. 2022, 80, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Wiliński, J.; Chukwu, O.; Ciuk, K.; Borek, R.; Skwarek, A. Clinical and Linguistic Validation of a Polish Version of the Pulmonary Embolism Quality of Life Questionnaire: A Disease-Specific Quality of Life Questionnaire for Patients after Acute Pulmonary Embolism. Kardiol. Pol. 2021, 79, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Bĕlohlávek, J.; Dytrych, V.; Linhart, A. Pulmonary Embolism, Part I: Epidemiology, Risk Factors and Risk Stratification, Pathophysiology, Clinical Presentation, Diagnosis and Nonthrombotic Pulmonary Embolism. Exp. Clin. Cardiol. 2013, 18, 129–138. [Google Scholar] [PubMed]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mahmoudpour, S.H.; Planquette, B.; Sanchez, O.; Konstantinides, S.V.; Meyer, G. Prognostic Value of Right Ventricular Dysfunction or Elevated Cardiac Biomarkers in Patients with Low-Risk Pulmonary Embolism: A Systematic Review and Meta-Analysis. Eur. Heart J. 2019, 40, 902–910A. [Google Scholar] [CrossRef]
- Pruszczyk, P.; Konstantinides, S. Where to Treat Patients with Acute Pulmonary Embolism? Kardiol. Pol. 2020, 78, 15–19. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Terluk, A.D.; Kritharides, L.; Chow, V.; Chia, E.-M.; Byth, K.; Mussap, C.J.; Ng, A.C.C.; Thomas, L. Right Ventricular Speckle Tracking Strain Echocardiography in Patients with Acute Pulmonary Embolism. Int. J. Cardiovasc. Imaging 2020, 36, 865–872. [Google Scholar] [CrossRef]
- Platz, E.; Hassanein, A.H.; Shah, A.; Goldhaber, S.Z.; Solomon, S.D. Regional Right Ventricular Strain Pattern in Patients with Acute Pulmonary Embolism. Echocardiography 2012, 29, 464–470. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-H.; Park, K.-I.; Kim, M.J.; Kim, J.H.; Ahn, M.S.; Choi, S.W.; Jeong, J.-O.; Seong, I.-W. A Comparison of Different Techniques of Two-Dimensional Speckle-Tracking Strain Measurements of Right Ventricular Systolic Function in Patients with Acute Pulmonary Embolism. J. Cardiovasc. Ultrasound 2014, 22, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Støylen, A.; Mølmen, H.E.; Dalen, H. Left Ventricular Global Strains by Linear Measurements in Three Dimensions: Interrelations and Relations to Age, Gender and Body Size in the HUNT Study. Open Heart 2019, 6, e001050. [Google Scholar] [CrossRef]
- Menting, M.E.; McGhie, J.S.; Koopman, L.P.; Vletter, W.B.; Helbing, W.A.; van den Bosch, A.E.; Roos-Hesselink, J.W. Normal Myocardial Strain Values Using 2D Speckle Tracking Echocardiography in Healthy Adults Aged 20 to 72 Years. Echocardiography 2016, 33, 1665–1675. [Google Scholar] [CrossRef]
- Aristizábal-Duque, C.H.; Fernández Cabeza, J.; Blancas Sánchez, I.M.; Delgado Ortega, M.; Aparicio Martinez, P.; Romero-Saldaña, M.; Fonseca Del Pozo, F.J.; Pan, M.; Ruiz Ortiz, M.; Mesa-Rubio, M.D. The Assessment of Myocardial Longitudinal Strain in a Paediatric Spanish Population Using a New Software Analysis. J. Clin. Med. 2022, 11, 3272. [Google Scholar] [CrossRef] [PubMed]
- Kai, T.; Wang, M.; Grimm, R.A.; Rodriguez, L.L.; Collier, P.; Griffin, B.P.; Popović, Z.B. Defining the Reference Range for Right Ventricular Systolic Strain by Echocardiography in Healthy Subjects: A Meta-Analysis. PLoS ONE 2021, 16, e0256547. [Google Scholar] [CrossRef]
- Wiliński, J.; Skwarek, A.; Borek, R.; Chukwu, O.; Ciuk, K.; Stolarz-Skrzypek, K.; Rajzer, M. Subcostal Echocardiographic Assessment of Tricuspid Annular Kick (SEATAK): A Novel Independent Predictor of 30-Day Mortality in Patients with Acute Pulmonary Embolism. Kardiol. Pol. 2022, 80, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Wiliński, J.; Skwarek, A.; Borek, R.; Chukwu, O.; Ciuk, K.; Stolarz-Skrzypek, K.; Rajzer, M. Right Ventricular Wall Thickness Indexed to Body Surface Area as an Echocardiographic Predictor of Acute Pulmonary Embolism in High-Risk Patients. Kardiol. Pol. 2022, 80, 205–207. [Google Scholar] [CrossRef]
- Recommended Reading on Echocardiography-European Association of Cardiovascular Imaging (EACVI). Available online: https://www.escardio.org/Guidelines/Recommended-Reading/Cardiovascular-Imaging/Echocardiography (accessed on 31 July 2018).
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Tadic, M.; Nita, N.; Schneider, L.; Kersten, J.; Buckert, D.; Gonska, B.; Scharnbeck, D.; Reichart, C.; Belyavskiy, E.; Cuspidi, C.; et al. The Predictive Value of Right Ventricular Longitudinal Strain in Pulmonary Hypertension, Heart Failure, and Valvular Diseases. Front. Cardiovasc. Med. 2021, 8, 698158. [Google Scholar] [CrossRef]
- Hamada-Harimura, Y.; Seo, Y.; Ishizu, T.; Nishi, I.; Machino-Ohtsuka, T.; Yamamoto, M.; Sugano, A.; Sato, K.; Sai, S.; Obara, K.; et al. Incremental Prognostic Value of Right Ventricular Strain in Patients with Acute Decompensated Heart Failure. Circ. Cardiovasc. Imaging 2018, 11, e007249. [Google Scholar] [CrossRef]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome Prediction by Quantitative Right Ventricular Function Assessment in 575 Subjects Evaluated for Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; Gavazzoni, M.; Sciatti, E.; Dallapellegrina, L.; Bernardi, N.; Raddino, R.; Fiorina, C.; Adamo, M.; Metra, M. Right Ventricular Deformation and Right Ventricular-Arterial Coupling in Patients with Heart Failure Due to Severe Aortic Stenosis Undergoing TAVI: Long-Term Results. Am. J. Cardiovasc. Dis. 2020, 10, 150–163. [Google Scholar] [PubMed]
- Prihadi, E.A.; Van Der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients with Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef]
- Fine, N.M.; White, J.A.; Jimenez-Zepeda, V.; Howlett, J.G. Determinants and Prognostic Significance of Serial Right Heart Function Changes in Patients with Cardiac Amyloidosis. Can. J. Cardiol. 2020, 36, 432–440. [Google Scholar] [CrossRef]
- Tadic, M.; Baudisch, A.; Haßfeld, S.; Heinzel, F.; Cuspidi, C.; Burkhardt, F.; Escher, F.; Attanasio, P.; Pieske, B.; Genger, M. Right Ventricular Function and Mechanics in Chemotherapy- and Radiotherapy-Naïve Cancer Patients. Int. J. Cardiovasc. Imaging 2018, 34, 1581–1587. [Google Scholar] [CrossRef]
- Van Kessel, M.; Seaton, D.; Chan, J.; Yamada, A.; Kermeen, F.; Butler, T.; Sabapathy, S.; Morris, N. Prognostic Value of Right Ventricular Free Wall Strain in Pulmonary Hypertension Patients with Pseudo-Normalized Tricuspid Annular Plane Systolic Excursion Values. Int. J. Cardiovasc. Imaging 2016, 32, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Tsugu, T.; Kawakami, T.; Kataoka, M.; Endo, J.; Kohno, T.; Itabashi, Y.; Fukuda, K.; Murata, M. Preoperative Right Ventricular Strain Predicts Sustained Right Ventricular Dysfunction after Balloon Pulmonary Angioplasty in Patients with Chronic Thromboembolic Pulmonary Hypertension. Echocardiography 2020, 37, 2040–2047. [Google Scholar] [CrossRef]
- Diana, G.; Locorotondo, G.; Manfredonia, L.; Graziani, F.; Lombardo, A.; Lanza, G.A.; Pedicino, D.; Liuzzo, G.; Massetti, M.; Crea, F. Subclinical Dysfunction of Remote Myocardium Is Related to High NT-ProBNP and Affects Global Contractility at Follow-up, Independently of Infarct Area. Front. Cardiovasc. Med. 2022, 9, 997821. [Google Scholar] [CrossRef]
- Ji, M.; Wu, W.; He, L.; Gao, L.; Zhang, Y.; Lin, Y.; Qian, M.; Wang, J.; Zhang, L.; Xie, M.; et al. Right Ventricular Longitudinal Strain in Patients with Heart Failure. Diagnostics 2022, 12, 445. [Google Scholar] [CrossRef]
- Sugiura, E.; Dohi, K.; Onishi, K.; Takamura, T.; Tsuji, A.; Ota, S.; Yamada, N.; Nakamura, M.; Nobori, T.; Ito, M. Reversible Right Ventricular Regional Non-Uniformity Quantified by Speckle-Tracking Strain Imaging in Patients with Acute Pulmonary Thromboembolism. J. Am. Soc. Echocardiogr. 2009, 22, 1353–1359. [Google Scholar] [CrossRef]
- Ramberg, E.; Olausson, M.; Jørgensen, T.B.S.; Nepper, M.L.; Bhardwaj, P.; Binko, T.S.; Petersen, J.R.; Fornitz, G.G. Right Atrial and Ventricular Function Evaluated with Speckle Tracking in Patients with Acute Pulmonary Embolism. Am. J. Emerg. Med. 2017, 35, 136–143. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, Y.S.; Kim, Y.J.; Lee, I.S.; Kim, J.H.; Lee, J.-H.; Choi, S.W.; Jeong, J.-O.; Seong, I.-W. Differentiation between Acute and Chronic Cor Pulmonales with Midventricular Systolic Strain of the Right Ventricle in the Emergency Department. Heart Vessel. 2011, 26, 435–439. [Google Scholar] [CrossRef]
- Dahhan, T.; Siddiqui, I.; Tapson, V.F.; Velazquez, E.J.; Sun, S.; Davenport, C.A.; Samad, Z.; Rajagopal, S. Clinical and Echocardiographic Predictors of Mortality in Acute Pulmonary Embolism. Cardiovasc. Ultrasound 2016, 14, 44. [Google Scholar] [CrossRef]
- Lee, K.; Kwon, O.; Lee, E.J.; Sin, M.J.; Lee, J.S.; Lee, S.; Kang, D.H.; Song, J.K.; Song, J.M. Prognostic Value of Echocardiographic Parameters for Right Ventricular Function in Patients with Acute Non-Massive Pulmonary Embolism. Heart Vessel. 2019, 34, 1187–1195. [Google Scholar] [CrossRef]
- Kanar, B.G.; Göl, G.; Oğur, E.; Kavas, M.; Ataş, H.; Mutlu, B. Assessment of Right Ventricular Function and Relation to Mortality after Acute Pulmonary Embolism: A Speckle Tracking Echocardiography-Based Study. Echocardiography 2019, 36, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Vitarelli, A.; Barillà, F.; Capotosto, L.; D’Angeli, I.; Truscelli, G.; De Maio, M.; Ashurov, R. Right Ventricular Function in Acute Pulmonary Embolism: A Combined Assessment by Three-Dimensional and Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sokalskis, V.; Peluso, D.; Jagodzinski, A.; Sinning, C. Added Clinical Value of Applying Myocardial Deformation Imaging to Assess Right Ventricular Function. Echocardiography 2017, 34, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, H.; Steinvil, A.; Aviram, G.; Moaad, S.; Sharon, A.; Bendet, A.; Biner, S.; Shacham, Y.; Sherez, J.; Megidish, R.; et al. Re-Appraisal of Echocardiographic Assessment in Patients with Pulmonary Embolism: Prospective Blinded Long-Term Follow-Up. Isr. Med. Assoc. J. 2020, 11, 688–695. [Google Scholar] [PubMed]
- Pruszczyk, P.; Skowrońska, M.; Ciurzyński, M.; Kurnicka, K.; Lankeit, M.; Konstantinides, S. Assessment of Pulmonary Embolism Severity and the Risk of Early Death. Pol. Arch. Intern. Med. 2021, 131, 16134. [Google Scholar] [CrossRef]
| All Subjects (n = 167) | Patients with No PE (n = 79) | Patients with PE (n = 88) | p | Survivors (n = 76) | Non-Survivors (n = 12) | p | |
|---|---|---|---|---|---|---|---|
| Gender [male] | 76 (45.51%) | 32 (40.51%) | 44 (50%) | 0.36 | 41 (53.95%) | 3 (25%) | 0.12 |
| Age [years] | 69 (59.50–79.08) | 70 (61.21–78.51) | 69 (58.14–79.37) | 0.95 | 66 (56.75–77.50) | 74.5 (69–89.25) | 0.01 |
| BMI [kg/m2] | 27.55 (23.84–31.08) | 26.61 (22.39–29.47) | 28.22 (25.97–31.25) | 0.02 | 28.34 (26.07–31.25) | 27.3 (23.93–30.99) | 0.46 |
| Arterial hypertension | 102 (61.08%) | 50 (63.29%) | 52 (59.09%) | 0.64 | 46 (60.53%) | 6 (50%) | 0.49 |
| Hyperlipidemia | 72 (43.11%) | 39 (49.37%) | 33 (37.5%) | 0.08 | 29 (38.16%) | 4 (33.33%) | 1 |
| Diabetes mellitus | 38 (22.75%) | 18 (22.78%) | 20 (22.73%) | 0.72 | 17 (22.37%) | 3 (25.00%) | 1 |
| Coronary artery disease | 44 (26.35%) | 28 (35.44%) | 16 (18.18%) | 0.01 | 15 (19.74%) | 1 (8.33%) | 0.69 |
| Chronic heart failure | 51 (30.54%) | 30 (37.97%) | 21 (23.86%) | 0.01 | 18 (23.68%) | 3 (25%) | 0.76 |
| Atrial fibrillation (present or prior) | 21 (12.57%) | 10 (12.66%) | 11 (12.50%) | 0.28 | 9 (11.84%) | 2 (16.67%) | 0.64 |
| Chronic lung disease | 17 (10.18%) | 10 (12.66%) | 7 (7.95%) | 0.09 | 6 (7.89%) | 1 (8.33%) | 1 |
| Active malignancy | 38 (22.75%) | 19 (24.05%) | 19 (21.59%) | 0.67 | 16 (21.05%) | 3 (25.00%) | 0.69 |
| Acute infection | 65 (38.92%) | 31 (39.24%) | 34 (38.64%) | 0.29 | 28 (36.84%) | 6 (50.00%) | 0.38 |
| PESI [pts] | 94 (79–116) | 94 (79–117) | 94 (78–115) | 0.42 | 90 (70–107) | 136 (113–173) | <0.001 |
| Troponin T [pg/mL] | 21.51 (11.53–45.75) | 21.74 (13.14–48.81) | 20.87 (10.73–45.61) | 0.56 | 18.84 (9.20–44.57) | 70.29 (28.62–136.08) | 0.01 |
| NT-proBNP [pg/mL] | 811.00 (196.00–3119.00) | 878.00 (229.00–3950.00) | 627.50 (152.00–2878.00) | 0.30 | 548.00 (142.00–2631.00) | 2984.00 (1711.00–7791.00) | 0.02 |
| D-dimer [ng/mL] | 3697.00 (1990.00–6998.00) | 2898.00 (1721.00–4805.00) | 4792.00 (2657.00–7801.00) | <0.001 | 4792.00 (2348.00–7801.00) | 4737.00 (4280.00–7499.00) | 0.62 |
| Creatinine clearance [mL/min] | 82.00 (62.12–104.47) | 81.55 (60.25–102.60) | 83.3 (67.07–106.12) | 0.47 | 84.40 (69.35–104.97) | 70.65 (42.50–132.15) | 0.54 |
| Patients with No PE (n = 79) | Patients with PE (n = 88) | p | Survivors (n = 76) | Non-Survivors (n = 12) | p | |
|---|---|---|---|---|---|---|
| RVTD [mm] | 38.25 (35.17–42.43) | 40.11 (37.06–43.16) | 0.048 | 41.35 (37.49–43.25) | 38.11 (35.75–42.23) | 0.30 |
| LVTD [mm] | 43.65 (38.22–49.85) | 44.33 (39.05–47.09) | 0.98 | 44.21 (40.16–48.68) | 40.29 (35.51–44.09) | 0.06 |
| RVTD/LVTD | 0.88 (0.77–1.03) | 0.93 (0.84–1.05) | 0.11 | 0.93 (0.83–1.04) | 1 (0.89–1.08) | 0.38 |
| Act [ms] | 95.04 (74.11–111.82) | 72.41 (57.75–96.22) | <0.001 | 74.16 (58.75–98.88) | 59.07 (47.50–72.51) | 0.07 |
| TRPG [mm Hg] | 2.72 (2.30–3.16) | 2.74 (2.31–3.13) | 0.86 | 2.75 (2.36–3.11) | 2.72 (2.11–3.25) | 0.94 |
| TAPSE [mm] | 22.14 (17.09–25.76) | 21.45 (17.55–24.25) | 0.78 | 22.07 (18.19–25.25) | 18.50 (15.75–20.04) | 0.06 |
| TASV TDI [cm/s] | 16.33 (12.11–20.44) | 15.20 (13.07–19.23) | 0.87 | 15.23 (13.17–18.51) | 19.04 (14.52–20.55) | 0.14 |
| McConnell sign | 1 (1.27%) | 10 (11.36%) | 0.01 | 9 (11.84%) | 1 (8.33%) | 1 |
| LVEF [%] | 56.31 (48.52–65.04) | 56.15 (50.41–63.17) | 0.93 | 55.52 (50.07–63.44) | 56.22 (48.54–62.56) | 0.95 |
| RV FAC [%] | 46.58 (37.14–51.36) | 40.26 (31.03–48.33) | 0.31 | 40.26 (29.69–49.17) | 39.13 (37.16–41.10) | 0.81 |
| LVGLS [%] | 16.90 (13.31–19.75) | 17.20 (14.02–19.85) | 0.49 | 17.21 (14.52–19.65) | 16.61 (13.07–20.95) | 0.93 |
| RVOT PLAX [mm] | 30.00 (27.00–32.00) | 30.00 (28.50–34.00) | 0.22 | 31.00(28.00–34.00) | 28.00 (26.00–33.00) | 0.13 |
| RVOT SAX diastolic [mm] | 32.00 (28.00–35.75) | 32.00 (29.00–35.00) | 0.76 | 32.00 (29.00–36.00) | 30 (29.00–32.50) | 0.10 |
| RVOT SAX systolic [mm] | 20.00 (17.00–25.75) | 19.50 (16.00–24.00) | 0.82 | 20.00 (16.00–25.00) | 19 (16.00–22.75) | 0.53 |
| Segments | Patients with No PE (n = 79) | Patients with PE (n = 88) | p | Survivors (n = 76) | Non-Survivors (n = 12) | p |
|---|---|---|---|---|---|---|
| RV strain original values [%] | ||||||
| RVFW basal | 23.00 (16.00–25.00) | 21.00 (14.00–26.00) | 0.45 | 21.00 (16.75–27.00) | 13.00 (9.50–21.25) | 0.03 |
| RVFW mid | 22.00 (17.50–28.00) | 20.00 (14.75–25.00) | 0.03 | 20.00 (16.00–25.00) | 15 (10.75–23.50) | 0.18 |
| RVFW apical | 18.00 (12.00–25.00) | 18.00 (14.00–23.25) | 0.84 | 18.00 (14.00–23.25) | 17.00 (8.00–21.25) | 0.41 |
| SEP basal | 16.00 (10.00–19.00) | 17.00 (11.00–21.00) | 0.10 | 17.00 (11.75–21.00) | 13.5 (8.25–16.25) | 0.049 |
| SEP mid | 18.00 (13.00–21.00) | 17.00 (13.75–20.50) | 0.78 | 17.00 (14.00–22.00) | 15.00 (12.50–20.00) | 0.32 |
| SEP apical | 16.00 (11.50–22.00) | 16.00 (13.75–22.25) | 0.40 | 16.00 (14.00–22.00) | 16.50 (6.75–23.00) | 0.58 |
| RVFW-average of 3 segments | 19.67 (14.83–25.83) | 18.5 (15.33–22.92) | 0.43 | 19 (15.67–22.92) | 16.67 (10.75–22.58) | 0.32 |
| RVGLS | 18.50 (14.17–22.08) | 17.75 (14.46–21.5) | 0.64 | 18 (14.88–21.12) | 16 (10.62–22.58) | 0.23 |
| RV strain indexed to BSA [%/m2] | ||||||
| RVFW basal | 11.52 (8.88–14.65) | 11.26 (7.53–13.59) | 0.13 | 11.49 (7.85–13.67) | 7.34 (4.9–10.87) | 0.08 |
| RVFW mid | 12.27 (9.02–15.76) | 10.12 (7.4–12.96) | 0.01 | 10.17 (7.71–12.92) | 7.29 (5.63–13.86) | 0.37 |
| RVFW apical | 10.16 (6.71–13.39) | 9.39 (6.89–11.99) | 0.36 | 9.39 (7.15–11.89) | 8.65 (4.95–12.08) | 0.56 |
| SEP basal | 8.36 (5.49–10.53) | 8.47 (5.72–10.77) | 0.53 | 8.53 (6.11–10.98) | 7.06 (4.7–9.79) | 0.23 |
| SEP mid | 9.92 (7.28–11.98) | 8.56 (6.82–11.34) | 0.24 | 8.51 (6.96–11.34) | 9.02 (5.93–10.9) | 0.97 |
| SEP apical | 8.65 (6.04–12.23) | 8.55 (6.6–11.78) | 0.96 | 8.55 (6.66–11.78) | 9.12 (3.25–12.09) | 0.77 |
| RVFW-average of 3 segments | 10.20 (7.89–14.38) | 9.43 (7.51–12.03) | 0.12 | 9.67 (7.63–11.88) | 8.62 (5.86–12.37) | 0.61 |
| RVGLS | 9.71 (7.71–12.59) | 8.81 (7.5–11.51) | 0.22 | 8.81 (7.61–11.51) | 8.51 (5.85–11.55) | 0.66 |
| AUC | p | Youden Cut-off | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Age [years] | 0.74 (0.61, 0.87) | 0.004 | 66 | 1 (0.74–1) | 0.49 (0.37–0.6) |
| BMI [kg/m2] | 0.63 (0.54, 0.72) | 0.002 | 24.7 | 0.82 (0.72–0.89) | 0.44 (0.33–0.56) |
| PESI [pts] | 0.88 (0.80, 0.96) | <0.001 | 100 | 1 (0.74–1) | 0.7 (0.59–0.8) |
| D-dimer [pg/mL] | 0.66 (0.57, 0.75) | <0.001 | 3559 | 0.7 (0.58–0.79) | 0.6 (0.48–0.71) |
| Troponin T [ng/mL] | 0.78 (0.60, 0.95) | 0.005 | 66 | 0.62 (0.24–0.91) | 0.88 (0.78–0.94) |
| NT-proBNP [pg/mL] | 0.75 (0.58, 0.92) | 0.01 | 1120 | 0.89 (0.52–1) | 0.62 (0.5–0.73) |
| RVFW basal LS [%] | 0.70 (0.54, 0.86) | 0.02 | −14 | 0.58 (0.28–0.85) | 0.78 (0.67–0.86) |
| SEP basal LS [%] | 0.68 (0.49, 0.86) | 0.02 | −15 | 0.5 (0.21–0.79) | 0.67 (0.55–0.77) |
| RVFW mid LS [%] | 0.60 (0.51, 0.69) | 0.01 | −21 | 0.61 (0.5–0.72) | 0.54 (0.43–0.66) |
| RVFW mid LS/ BSA [%/m2] | 0.62 (0.54, 0.71) | 0.003 | −14 | 0.84 (0.75–0.91) | 0.39 (0.28–0.51) |
| Act [ms] | 0.67 (0.59, 0.75) | <0.001 | 67 | 0.48 (0.37–0.59) | 0.82 (0.72–0.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiliński, J.; Skwarek, A.; Borek, R.; Medygrał, M.; Chrzan, I.; Lechowicz-Wilińska, M.; Chukwu, O. Indexing of Speckle Tracking Longitudinal Strain of Right Ventricle to Body Surface Area Does Not Improve Its Efficiency in Diagnosis and Mortality Risk Stratification in Patients with Acute Pulmonary Embolism. Healthcare 2023, 11, 1629. https://doi.org/10.3390/healthcare11111629
Wiliński J, Skwarek A, Borek R, Medygrał M, Chrzan I, Lechowicz-Wilińska M, Chukwu O. Indexing of Speckle Tracking Longitudinal Strain of Right Ventricle to Body Surface Area Does Not Improve Its Efficiency in Diagnosis and Mortality Risk Stratification in Patients with Acute Pulmonary Embolism. Healthcare. 2023; 11(11):1629. https://doi.org/10.3390/healthcare11111629
Chicago/Turabian StyleWiliński, Jerzy, Anna Skwarek, Radosław Borek, Michał Medygrał, Iwona Chrzan, Marta Lechowicz-Wilińska, and Ositadima Chukwu. 2023. "Indexing of Speckle Tracking Longitudinal Strain of Right Ventricle to Body Surface Area Does Not Improve Its Efficiency in Diagnosis and Mortality Risk Stratification in Patients with Acute Pulmonary Embolism" Healthcare 11, no. 11: 1629. https://doi.org/10.3390/healthcare11111629
APA StyleWiliński, J., Skwarek, A., Borek, R., Medygrał, M., Chrzan, I., Lechowicz-Wilińska, M., & Chukwu, O. (2023). Indexing of Speckle Tracking Longitudinal Strain of Right Ventricle to Body Surface Area Does Not Improve Its Efficiency in Diagnosis and Mortality Risk Stratification in Patients with Acute Pulmonary Embolism. Healthcare, 11(11), 1629. https://doi.org/10.3390/healthcare11111629







