Chronic Low Back Pain and Incident Transient Ischemic Attack and Stroke in General Practices in Germany
Abstract
1. Introduction
2. Methods
2.1. Ethics Approval and Consent to Participate
2.2. Database
2.3. Population
2.4. Outcome
2.5. Covariates
2.6. Statistical Analyses
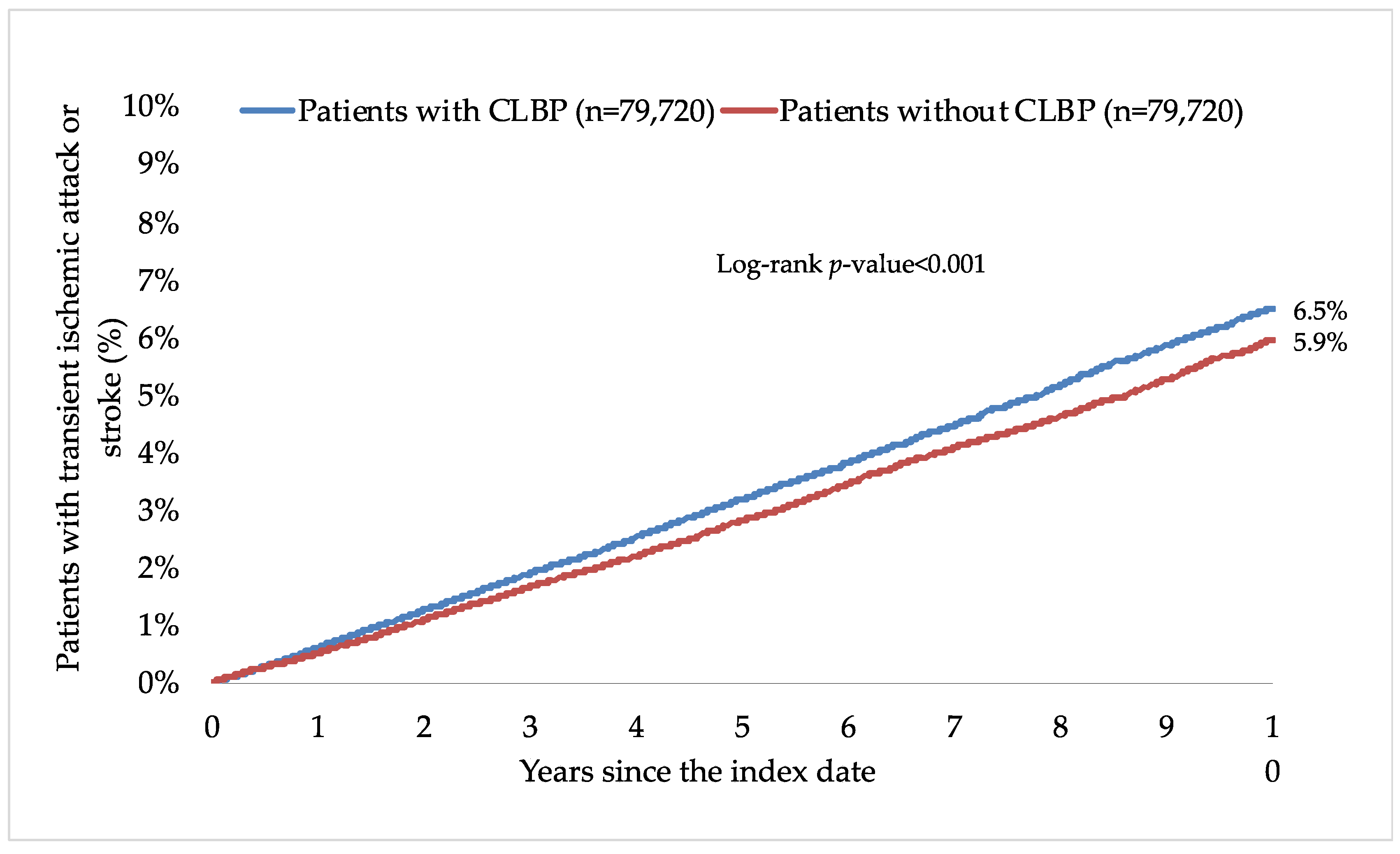
3. Results
4. Discussion
4.1. Main Findings
4.2. Interpretation of Findings
4.3. Clinical Implications and Directions for Future Research
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The Definition of Stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Attanasi, C.; Cecchini, W.; Marazzi, A.; Capobianco, S.V.; Santilli, V. Chronic Low Back Pain and Postural Rehabilitation Exercise: A Literature Review. J. Pain Res. 2019, 12, 95–107. [Google Scholar] [CrossRef]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M.X. Prevalence of Chronic Low Back Pain: Systematic Review. Rev. Saude Publica 2015, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Su, Y.-C.; Luk, H.-N.; Wang, J.-H.; Hsu, C.-Y.; Lin, S.-Z. Increased Risk of Strokes in Patients with Chronic Low Back Pain (CLBP): A Nationwide Population-Based Cohort Study. Clin. Neurol. Neurosurg 2020, 192, 105725. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.D.; Folsom, A.R.; Blair, S.N. Physical Activity and Stroke Risk: A Meta-Analysis. Stroke 2003, 34, 2475–2481. [Google Scholar] [CrossRef]
- Orr, L.C.; George, S.Z.; Simon, C.B. Association between Physical Activity and Pain Processing in Adults with Chronic Low Back Pain Compared to Pain-Free Controls. J. Back Musculoskelet. Rehabil. 2017, 30, 575–581. [Google Scholar] [CrossRef]
- Liao, C.-C.; Shih, C.-C.; Yeh, C.-C.; Chang, Y.-C.; Hu, C.-J.; Lin, J.-G.; Chen, T.-L. Impact of Diabetes on Stroke Risk and Outcomes: Two Nationwide Retrospective Cohort Studies. Medicine 2015, 94, e2282. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Sørgjerd, E.P.; Åsvold, B.O.; Zwart, J.-A. Is Chronic Low Back Pain a Risk Factor for Diabetes? The Nord-Trøndelag Health Study. BMJ Open Diabetes Res. Care 2018, 6, e000569. [Google Scholar] [CrossRef]
- Fernandez, M.; Ordoñana, J.R.; Hartvigsen, J.; Ferreira, M.L.; Refshauge, K.M.; Sánchez-Romera, J.F.; Pinheiro, M.B.; Simpson, S.J.; Hopper, J.L.; Ferreira, P.H. Is Chronic Low Back Pain Associated with the Prevalence of Coronary Heart Disease When Genetic Susceptibility Is Considered? A Co-Twin Control Study of Spanish Twins. PLoS ONE 2016, 11, e0155194. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Bartz, T.M.; Kamel, H.; Soliman, E.Z.; Howard, V.; Psaty, B.M.; Okin, P.M.; Safford, M.M.; Elkind, M.S.V.; Longstreth, W.T. Silent Myocardial Infarction and Subsequent Ischemic Stroke in the Cardiovascular Health Study. Neurology 2021, 97, e436–e443. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.J.; Slater, M.A.; Patterson, T.L.; Grant, I.; Garfin, S.R. Prevalence, Onset, and Risk of Psychiatric Disorders in Men with Chronic Low Back Pain: A Controlled Study. Pain 1991, 45, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, M.J.; Kubzansky, L.D.; Thurston, R.C. Prospective Study of Anxiety and Incident Stroke. Stroke 2014, 45, 438–443. [Google Scholar] [CrossRef]
- Barlinn, K.; Kepplinger, J.; Puetz, V.; Illigens, B.M.; Bodechtel, U.; Siepmann, T. Exploring the Risk-Factor Association between Depression and Incident Stroke: A Systematic Review and Meta-Analysis. Neuropsychiatr. Dis. Treat. 2014, 11, 1–14. [Google Scholar] [CrossRef]
- Varas-Lorenzo, C.; Riera-Guardia, N.; Calingaert, B.; Castellsague, J.; Pariente, A.; Scotti, L.; Sturkenboom, M.; Perez-Gutthann, S. Stroke Risk and NSAIDs: A Systematic Review of Observational Studies. Pharmacoepidemiol. Drug Saf. 2011, 20, 1225–1236. [Google Scholar] [CrossRef]
- Gore, M.; Tai, K.-S.; Sadosky, A.; Leslie, D.; Stacey, B.R. Use and Costs of Prescription Medications and Alternative Treatments in Patients with Osteoarthritis and Chronic Low Back Pain in Community-Based Settings. Pain Pract. 2012, 12, 550–560. [Google Scholar] [CrossRef]
- Trajkova, S.; d’Errico, A.; Soffietti, R.; Sacerdote, C.; Ricceri, F. Use of Antidepressants and Risk of Incident Stroke: A Systematic Review and Meta-Analysis. Neuroepidemiology 2019, 53, 142–151. [Google Scholar] [CrossRef]
- Mathew, J.; Singh, S.B.; Garis, S.; Diwan, A.D. Backing up the Stories: The Psychological and Social Costs of Chronic Low-Back Pain. Int. J. Spine Surg. 2013, 7, e29–e38. [Google Scholar] [CrossRef]
- Andersen, K.K.; Olsen, T.S. Married, Unmarried, Divorced, and Widowed and the Risk of Stroke. Acta. Neurol. Scand. 2018, 138, 41–46. [Google Scholar] [CrossRef]
- Gallo, W.T.; Bradley, E.H.; Falba, T.A.; Dubin, J.A.; Cramer, L.D.; Bogardus, S.T.; Kasl, S.V. Involuntary Job Loss as a Risk Factor for Subsequent Myocardial Infarction and Stroke: Findings from the Health and Retirement Survey. Am. J. Ind. Med. 2004, 45, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Shmagel, A.; Foley, R.; Ibrahim, H. Epidemiology of Chronic Low Back Pain in US Adults: National Health and Nutrition Examination Survey 2009–2010. Arthritis Care Res. 2016, 68, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Coggon, D.; Ntani, G.; Walker-Bone, K.; Palmer, K.T.; Felli, V.E.; Harari, R.; Barrero, L.H.; Felknor, S.A.; Gimeno, D.; Cattrell, A.; et al. Epidemiological Differences between Localized and Nonlocalized Low Back Pain. Spine 2017, 42, 740–747. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.-W.C.; Chenot, J.-F.; van Tulder, M.; Koes, B.W. Clinical Practice Guidelines for the Management of Non-Specific Low Back Pain in Primary Care: An Updated Overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Bongaerts, B.; Carius, H.-J.; Kruppert, S.; Kostev, K. Basic Characteristics and Representativeness of the German Disease Analyzer Database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Delitto, A.; George, S.Z.; Van Dillen, L.; Whitman, J.M.; Sowa, G.; Shekelle, P.; Denninger, T.R.; Godges, J.J.; Orthopaedic Section of the American Physical Therapy Association. Low Back Pain. J. Orthop. Sport. Phys. Ther. 2012, 42, A1–A57. [Google Scholar] [CrossRef]
- Schrepf, A.; Phan, V.; Clemens, J.Q.; Maixner, W.; Hanauer, D.; Williams, D.A. ICD-10 Codes for the Study of Chronic Overlapping Pain Conditions in Administrative Databases. J. Pain 2020, 21, 59–70. [Google Scholar] [CrossRef]
- Jacob, L.; Koyanagi, A.; Smith, L.; Shin, J.I.; Haro, J.M.; Garthe, T.; Kostev, K. Prevalence of and Factors Associated with Long-Term Sick Leave in Working-Age Adults with Chronic Low Back Pain in Germany. Int. Arch. Occup. Environ. Health 2022, 95, 1549–1556. [Google Scholar] [CrossRef]
- Shiozawa, M.; Kaneko, H.; Itoh, H.; Morita, K.; Okada, A.; Matsuoka, S.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Michihata, N.; et al. Association of Body Mass Index with Ischemic and Hemorrhagic Stroke. Nutrients 2021, 13, 2343. [Google Scholar] [CrossRef]
- Hsu, P.-S.; Lin, H.-H.; Li, C.-R.; Chung, W.-S. Increased Risk of Stroke in Patients with Osteoarthritis: A Population-Based Cohort Study. Osteoarthr. Cartil. 2017, 25, 1026–1031. [Google Scholar] [CrossRef]
- Hsu, W.-T.; Esmaily-Fard, A.; Lai, C.-C.; Zala, D.; Lee, S.-H.; Chang, S.-S.; Lee, C.-C. Antipsychotics and the Risk of Cerebrovascular Accident: A Systematic Review and Meta-Analysis of Observational Studies. J. Am. Med. Dir. Assoc. 2017, 18, 692–699. [Google Scholar] [CrossRef]
- Andersen, K.K.; Olsen, T.S.; Dehlendorff, C.; Kammersgaard, L.P. Hemorrhagic and Ischemic Strokes Compared: Stroke Severity, Mortality, and Risk Factors. Stroke 2009, 40, 2068–2072. [Google Scholar] [CrossRef]
- Price, A.J.; Wright, F.L.; Green, J.; Balkwill, A.; Kan, S.W.; Yang, T.O.; Floud, S.; Kroll, M.E.; Simpson, R.; Sudlow, C.L.M.; et al. Differences in Risk Factors for 3 Types of Stroke: UK Prospective Study and Meta-Analyses. Neurology 2018, 90, e298–e306. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Z.; Wang, Y.; Cicuttini, F.M.; Hughes, H.J.; Chou, L.; Urquhart, D.M.; Ong, P.X.; Hussain, S.M. Association between Inflammatory Biomarkers and Nonspecific Low Back Pain: A Systematic Review. Clin. J. Pain 2020, 36, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Esenwa, C.C.; Elkind, M.S. Inflammatory Risk Factors, Biomarkers and Associated Therapy in Ischaemic Stroke. Nat. Rev. Neurol. 2016, 12, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.R.; Long, D.L.; Howard, G.; McClure, L.A.; Zakai, N.A.; Jenny, N.S.; Kissela, B.M.; Safford, M.M.; Howard, V.J.; Cushman, M. C-Reactive Protein and Stroke Risk in Blacks and Whites: The REasons for Geographic And Racial Differences in Stroke Cohort. Am. Heart J. 2019, 217, 94–100. [Google Scholar] [CrossRef]
- Martinic-Popovic, I.; Simundic, A.-M.; Dukic, L.; Lovrencic-Huzjan, A.; Popovic, A.; Seric, V.; Basic-Kes, V.; Demarin, V. The Association of Inflammatory Markers with Cerebral Vasoreactivity and Carotid Atherosclerosis in Transient Ischaemic Attack. Clin. Biochem. 2014, 47, 182–186. [Google Scholar] [CrossRef]
- Last, A.R.; Hulbert, K. Chronic Low Back Pain: Evaluation and Management. Am. Fam. Physician 2009, 79, 1067–1074. [Google Scholar] [CrossRef]
- Pettersson, N.; Kragsbjerg, F.; Hamrin, A.; Bergman, S.; Forsblad-d’Elia, H.; Karling, P. Increased Chronic Pain in Patients with Ulcerative Colitis Is Mostly Associated to Increased Disease Activity. A Cross-Sectional Case-Control Study. Scand. J. Gastroenterol. 2020, 55, 1193–1199. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Chen, J.-H.; Muo, C.-H.; Chang, Y.-J.; Sung, F.-C.; Hsu, C.Y. Increased Risk of Ischaemic Stroke amongst Patients with Chronic Osteomyelitis: A Population-Based Cohort Study in Taiwan. Eur. J. Neurol. 2015, 22, 633–639. [Google Scholar] [CrossRef]
- Tanislav, C.; Trommer, K.; Labenz, C.; Kostev, K. Inflammatory Bowel Disease as a Precondition for Stroke or TIA: A Matter of Crohn’s Disease Rather than Ulcerative Colitis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105787. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ligero, M.; Moral-Munoz, J.A.; Failde, I.; Dueñas, M. Physical Activity Levels in Adults with Chronic Low Back Pain: A National Survey in the General Spanish Population. J. Rehabil. Med. 2023, 55, jrm00366. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Heck, J.E.; Sandler, D.P.; Ritz, B.; Chen, H.; Krause, N. Occupational and Leisure-Time Physical Activity Differentially Predict 6-Year Incidence of Stroke and Transient Ischemic Attack in Women. Scand. J. Work Environ. Health 2019, 45, 267–279. [Google Scholar] [CrossRef] [PubMed]


| Variable | Patients with CLBP (n = 79,720) | Patients without CLBP (n = 79,720) | p-Value * |
|---|---|---|---|
| Age at the index date (in years) | |||
| Mean (standard deviation) | 52.1 (16.5) | 52.1 (16.5) | 0.632 |
| ≤40 | 25.2 | 25.1 | 0.066 |
| 41–50 | 21.4 | 21.4 | |
| 51–60 | 23.1 | 22.7 | |
| 61–70 | 14.1 | 14.6 | |
| >70 | 16.2 | 16.2 | |
| Sex | |||
| Female | 51.5 | 51.5 | 1.000 |
| Male | 48.5 | 48.5 | |
| Number of medical consultations per year during the follow-up, mean (standard deviation) | 8.7 (3.4) | 8.6 (3.4) | 0.064 |
| Number of years of follow-up, mean (standard deviation) | 6.6 (3.9) | 6.6 (3.9) | 1.000 |
| Chronic physical and psychiatric conditions diagnosed prior to the end of follow-up † | |||
| Essential hypertension | 56.0 | 57.2 | <0.001 |
| Disorders of lipoprotein metabolism and other lipidemias | 39.4 | 39.4 | 0.782 |
| Osteoarthritis | 38.7 | 29.2 | <0.001 |
| Depression | 36.2 | 31.5 | <0.001 |
| Diabetes mellitus | 22.7 | 24.9 | <0.001 |
| Ischemic heart diseases | 19.4 | 19.1 | 0.162 |
| Overweight and obesity | 18.6 | 16.9 | <0.001 |
| Anxiety disorders | 14.6 | 12.7 | <0.001 |
| Cancer | 13.9 | 14.5 | <0.001 |
| Chronic kidney disease and unspecified kidney failure | 8.6 | 8.7 | 0.281 |
| Atrial fibrillation and flutter | 8.4 | 9.3 | <0.001 |
| Drugs prescribed prior to the end of follow-up | |||
| Nonsteroidal anti-inflammatory drugs | 89.3 | 65.0 | <0.001 |
| Antidepressants | 30.6 | 26.3 | <0.001 |
| Antipsychotics | 9.6 | 9.3 | 0.046 |
| Incidence in Patients with CLBP * | Incidence in Patients without CLBP * | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| TIA and stroke † | ||||
| Overall population | 6.9 | 6.1 | 1.28 (1.22–1.35) | <0.001 |
| Age ≤40 years | 1.3 | 1.4 | 0.96 (0.76–1.20) | 0.687 |
| Age 41–50 years | 3.7 | 3.3 | 1.24 (1.07–1.43) | 0.005 |
| Age 51–60 years | 6.0 | 5.5 | 1.26 (1.13–1.42) | <0.001 |
| Age 61–70 years | 10.9 | 9.6 | 1.29 (1.16–1.43) | <0.001 |
| Age >70 years | 19.1 | 16.2 | 1.34 (1.23–1.45) | <0.001 |
| Female sex | 6.3 | 6.2 | 1.25 (1.17–1.34) | <0.001 |
| Male sex | 7.4 | 6.0 | 1.31 (1.22–1.42) | <0.001 |
| By type of TIA and stroke ‡ | ||||
| TIA | 2.5 | 1.9 | 1.42 (1.30–1.55) | <0.001 |
| Hemorrhagic stroke | 0.6 | 0.6 | 1.03 (0.87–1.22) | 0.746 |
| Ischemic stroke | 2.1 | 1.8 | 1.32 (1.20–1.45) | <0.001 |
| Unspecified stroke | 1.8 | 1.7 | 1.16 (1.05–1.28) | 0.003 |
| By type of low back pain § | ||||
| Lumbago with sciatica | 7.2 | 6.5 | 1.27 (1.18–1.38) | <0.001 |
| Low back pain | 6.6 | 5.8 | 1.28 (1.20–1.37) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, L.; Smith, L.; Koyanagi, A.; Haro, J.M.; Shin, J.I.; Tanislav, C.; Schnitzler, A.; Kostev, K. Chronic Low Back Pain and Incident Transient Ischemic Attack and Stroke in General Practices in Germany. Healthcare 2023, 11, 1499. https://doi.org/10.3390/healthcare11101499
Jacob L, Smith L, Koyanagi A, Haro JM, Shin JI, Tanislav C, Schnitzler A, Kostev K. Chronic Low Back Pain and Incident Transient Ischemic Attack and Stroke in General Practices in Germany. Healthcare. 2023; 11(10):1499. https://doi.org/10.3390/healthcare11101499
Chicago/Turabian StyleJacob, Louis, Lee Smith, Ai Koyanagi, Josep Maria Haro, Jae Il Shin, Christian Tanislav, Alexis Schnitzler, and Karel Kostev. 2023. "Chronic Low Back Pain and Incident Transient Ischemic Attack and Stroke in General Practices in Germany" Healthcare 11, no. 10: 1499. https://doi.org/10.3390/healthcare11101499
APA StyleJacob, L., Smith, L., Koyanagi, A., Haro, J. M., Shin, J. I., Tanislav, C., Schnitzler, A., & Kostev, K. (2023). Chronic Low Back Pain and Incident Transient Ischemic Attack and Stroke in General Practices in Germany. Healthcare, 11(10), 1499. https://doi.org/10.3390/healthcare11101499









