Hospital Admission Profile Related to Inner Ear Diseases in England and Wales
Abstract
1. Introduction
2. Methods
2.1. Data Source and the Population
2.2. Statistical Analysis
3. Results
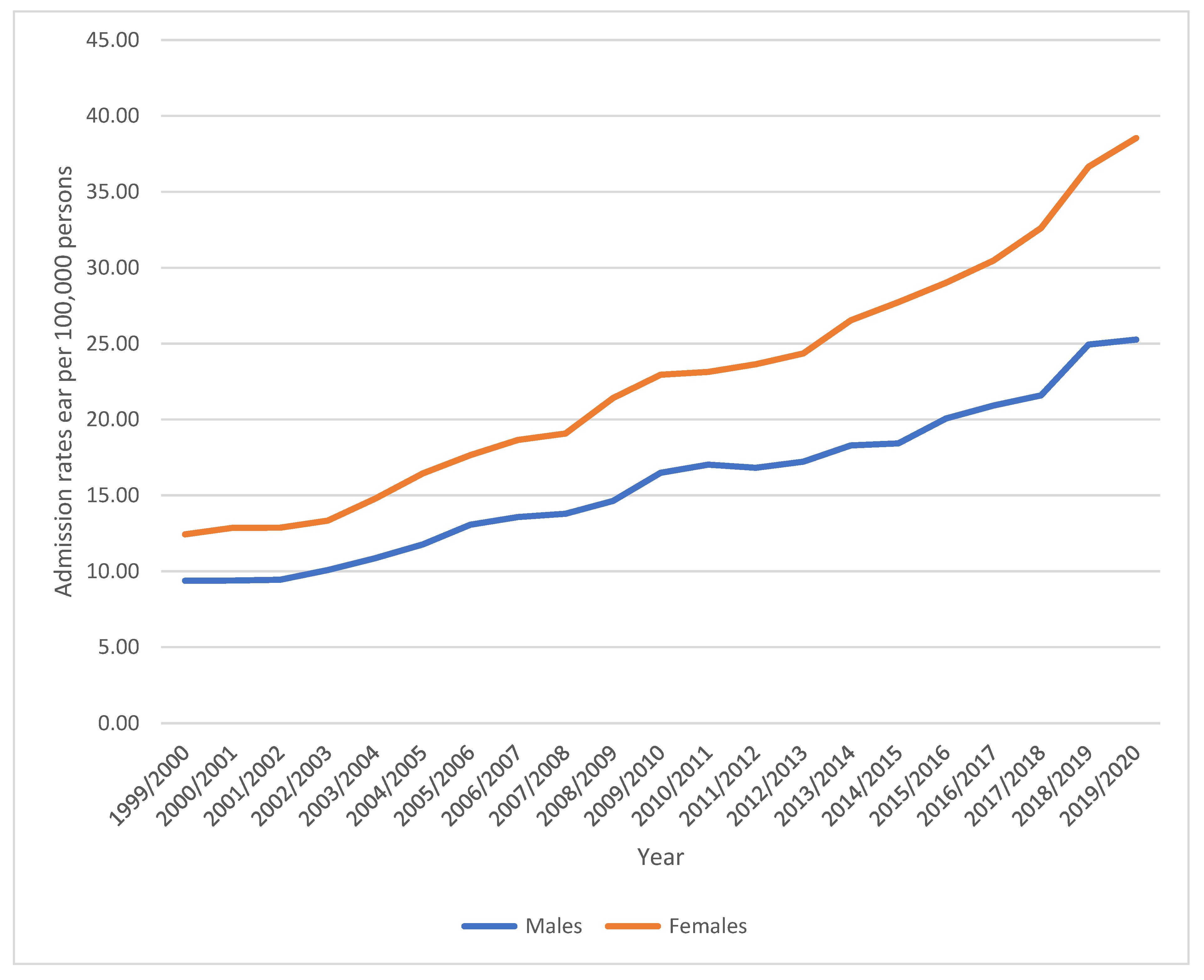
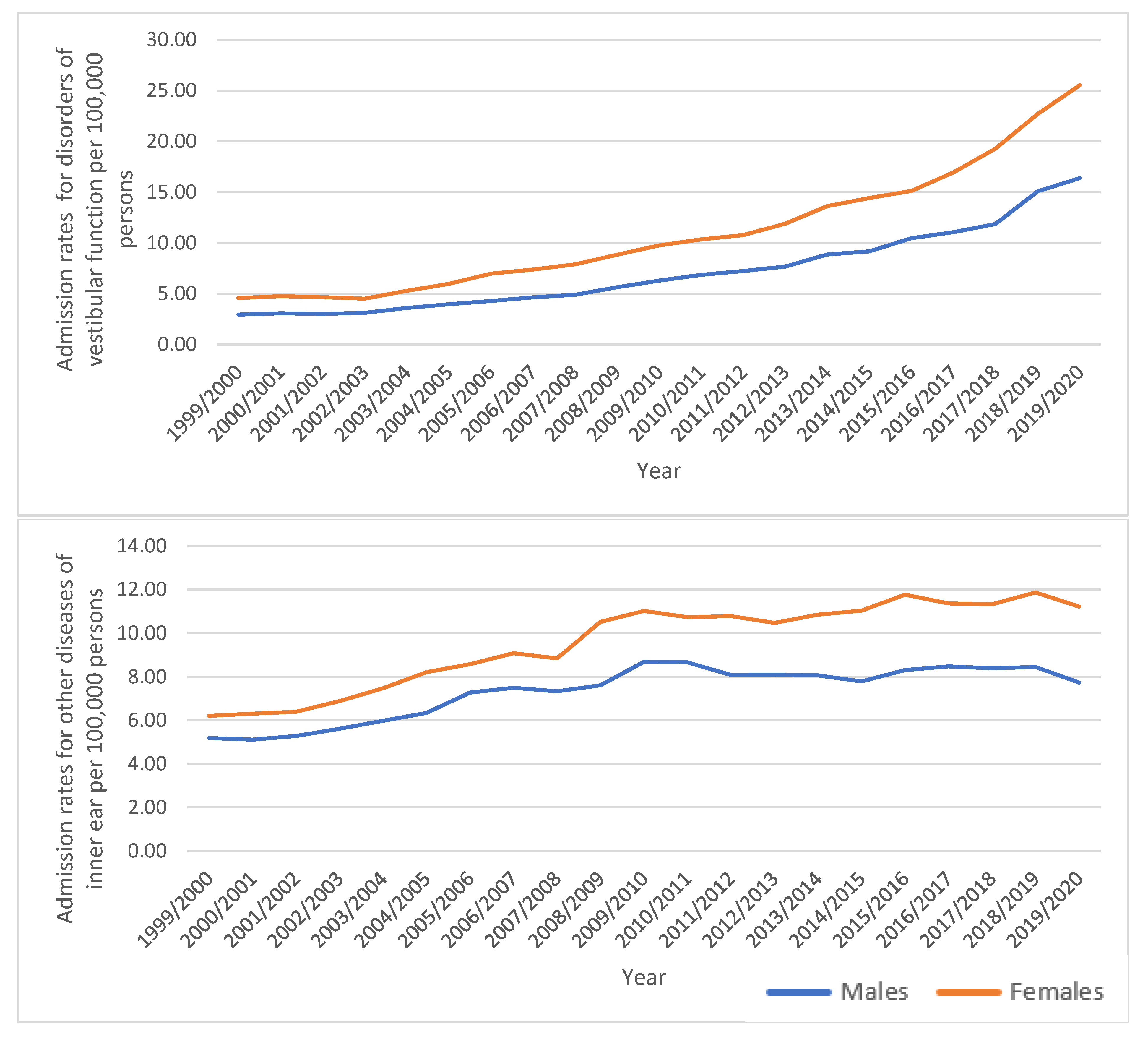
3.1. Diseases of the Inner Ear Admission Rate by Gender
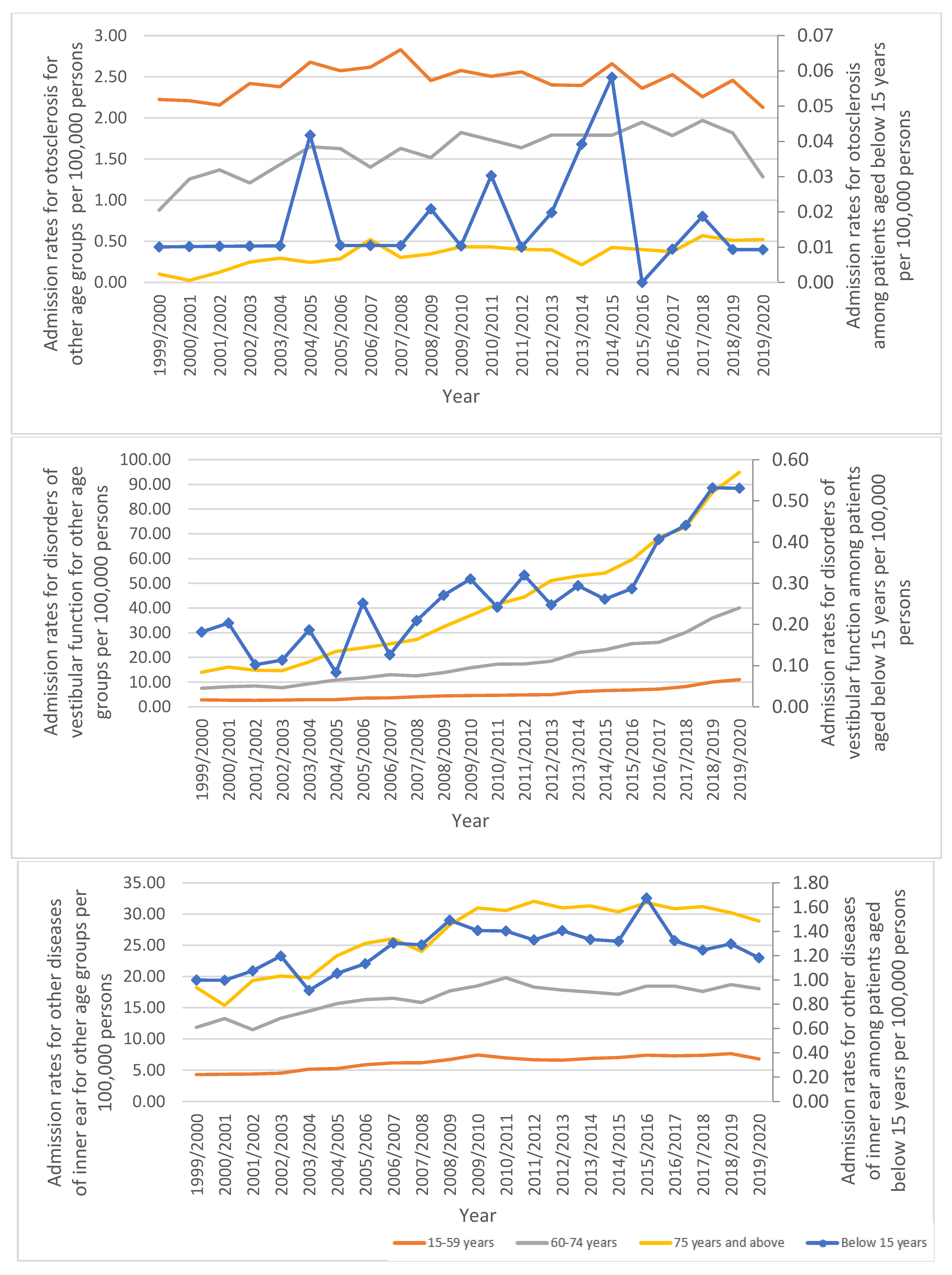
3.2. Diseases of the Inner Ear Admission Rate by Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, R. Diseases of the Ear, Nose and Throat; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nesterova, A.P.; Klimov, E.A.; Zharkova, M.; Sozin, S.; Sobolev, V.; Ivanikova, N.V.; Shkrob, M.; Yuryev, A. Diseases of the ear. In Disease Pathways: An Atlas of Human Disease Signaling Pathways; Nesterova, A.P., Klimov, E.A., Zharkova, M., Sozin, S., Sobolev, V., Ivanikova, N.V., Shkrob, M., Yuryev, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–325. [Google Scholar]
- Ciuman, R.R. Inner ear symptoms and disease: Pathophysiological understanding and therapeutic options. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2013, 19, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Deafness and Hearing Loss. Available online: https://www.who.int/health-topics/hearing-loss#tab=tab_1 (accessed on 6 February 2023).
- Kim, D.K. Nanomedicine for Inner Ear Diseases: A Review of Recent In Vivo Studies. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Graydon, K.; Waterworth, C.; Miller, H.; Gunasekera, H. Global burden of hearing impairment and ear disease. J. Laryngol. Otol. 2019, 133, 18–25. [Google Scholar] [CrossRef]
- Pirodda, A.; Cicero, A.F.; Borghi, C. Kidney disease and inner ear impairment: A simpler and closer pathogenic analogy? Intern. Emerg. Med. 2012, 7, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef]
- Mustafa Ali, M.K.; Naser, A.Y.; AbuAlhommos, A.; Al-Daghastani, T.; Alrawashdeh, H.; Mustafa Ali, S.; Alwafi, H.; Alqurashi, M.M.; Basha Ahmed, A.H.; Albarqi, H. Hospital Admissions Secondary to Diseases of the Blood, Blood-Forming Organs, and Immune System in England and Wales. Cureus 2022, 14, e30179. [Google Scholar] [CrossRef]
- Naser, A.Y.; Alwafi, H.; Hemmo, S.I.; Alrawashdeh, H.M.; Alqahtani, J.S.; Alghamdi, S.M.; Mustafa Ali, M.K. Trends in Hospital Admissions Due to Neoplasms in England and Wales between 1999 and 2019: An Ecological Study. Int. J. Environ. Res. Public Health 2022, 19, 8054. [Google Scholar] [CrossRef]
- Naser, A.Y.; Dahmash, E.Z.; Alqahtani, J.S.; Alsairafi, Z.K.; Alsaleh, F.M.; Alwafi, H. Trends in Hospital Admissions for Mental, Behavioural and Neurodevelopmental Disorders in England and Wales between 1999 and 2019: An Ecological Study. Healthcare 2022, 10, 2191. [Google Scholar] [CrossRef]
- Sweiss, K.; Naser, A.Y.; Alrawashdeh, H.M.; Alharazneh, A. Hospital admissions due to vasomotor and allergic rhinitis in England and Wales between 1999 and 2019: An ecological study. Ir. J. Med. Sci. 2023, 192, 349–355. [Google Scholar] [CrossRef]
- Health and Social Care Information Centre (HSCIC). Hospital Episode Statistics. Available online: http://content.digital.nhs.uk/hes (accessed on 13 January 2021).
- NHS Wales Informatics Service. Annual PEDW Data Tables. Available online: http://www.infoandstats.wales.nhs.uk/page.cfm?pid=41010&orgid=869 (accessed on 13 January 2021).
- ICD10 Codes. ICD-10-CM Codes. Available online: https://www.aapc.com/codes/icd-10-codes-range/ (accessed on 22 July 2022).
- Hülse, R.; Biesdorf, A.; Hörmann, K.; Stuck, B.; Erhart, M.; Hülse, M.; Wenzel, A. Peripheral Vestibular Disorders: An Epidemiologic Survey in 70 Million Individuals. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2019, 40, 88–95. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Radtke, A.; von Brevern, M.; Lezius, F.; Feldmann, M.; Lempert, T. Burden of dizziness and vertigo in the community. Arch. Intern. Med. 2008, 168, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Wang, T.C.; Chuang, L.J.; Chen, M.H.; Wang, P.C. Epidemiology of vertigo: A National Survey. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2011, 145, 110–116. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Lempert, T. Vertigo: Epidemiologic aspects. Semin. Neurol. 2009, 29, 473–481. [Google Scholar] [CrossRef]
- Wenzel, A.; Eck, S.; Hülse, K.; Rohr, K.; Hörmann, K.; Umbreit, C.; Hülse, M.; Hülse, R. Development of a new software and test setup for analyzing hVOR in very young children by vHIT. J. Vestib. Res. Equilib. Orientat. 2017, 27, 155–162. [Google Scholar] [CrossRef]
- Ozono, Y.; Kitahara, T.; Fukushima, M.; Michiba, T.; Imai, R.; Tomiyama, Y.; Nishiike, S.; Inohara, H.; Morita, H. Differential diagnosis of vertigo and dizziness in the emergency department. Acta Oto-Laryngol. 2014, 134, 140–145. [Google Scholar] [CrossRef]
- Kozin, E.D.; Sethi, R.K.; Remenschneider, A.K.; Kaplan, A.B.; Del Portal, D.A.; Gray, S.T.; Shrime, M.G.; Lee, D.J. Epidemiology of otologic diagnoses in United States emergency departments. Laryngoscope 2015, 125, 1926–1933. [Google Scholar] [CrossRef]
- Young, A.S. Disorders of the inner-ear balance organs and their pathways. In Handbook of Clinical Neurology Balance, Gait, and Falls; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 385–401. [Google Scholar]
- Renner, V.; Geißler, K.; Boeger, D.; Buentzel, J.; Esser, D.; Hoffmann, K.; Jecker, P.; Mueller, A.; Radtke, G.; Axer, H.; et al. Inpatient Treatment of Patients Admitted for Dizziness: A Population-Based Healthcare Research Study on Epidemiology, Diagnosis, Treatment, and Outcome. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2017, 38, e460–e469. [Google Scholar] [CrossRef]
- Bae, C.H.; Na, H.G.; Choi, Y.S. Current diagnosis and treatment of vestibular neuritis: A narrative review. J. Yeungnam Med. Sci. 2022, 39, 81–88. [Google Scholar] [CrossRef]
- Chen, C.C.; Cho, H.S.; Lee, H.H.; Hu, C.J. Efficacy of Repositioning Therapy in Patients With Benign Paroxysmal Positional Vertigo and Preexisting Central Neurologic Disorders. Front. Neurol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Riga, M.; Bibas, A.; Xenellis, J.; Korres, S. Inner ear disease and benign paroxysmal positional vertigo: A critical review of incidence, clinical characteristics, and management. Int. J. Otolaryngol. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Sweiss, K.; Naser, A.Y.; Samannodi, M.; Alwafi, H. Hospital admissions due to infectious and parasitic diseases in England and Wales between 1999 and 2019: An ecological study. BMC Infect. Dis. 2022, 22, 398. [Google Scholar] [CrossRef] [PubMed]
- Danishyar, A.; Ashurst, J. Acute Otitis Media. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470332/ (accessed on 14 January 2023).
- Crompton, M.; Cadge, B.A.; Ziff, J.L.; Mowat, A.J.; Nash, R.; Lavy, J.A.; Powell, H.R.F.; Aldren, C.P.; Saeed, S.R.; Dawson, S.J. The Epidemiology of Otosclerosis in a British Cohort. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2019, 40, 22–30. [Google Scholar] [CrossRef]
- Batson, L.; Rizzolo, D. Otosclerosis: An update on diagnosis and treatment. JAAPA Off. J. Am. Acad. Physician Assist. 2017, 30, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ekwall, A.; Schrab, J.; Runesson, K.; Magnussons, M. Hospital admission in older persons presenting with dizziness in the Emergency department. Int. Emerg. Nurs. 2018, 37, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Sanchez, J.M.; Lopez-Escamez, J.A. Menière’s disease. Handb. Clin. Neurol. 2016, 137, 257–277. [Google Scholar]
- Penger, M.; Strobl, R.; Grill, E. Country-specific and individual determinants of dizziness in Europe: Results from the Survey of Health Ageing and Retirement in Europe (SHARE). Public Health 2017, 149, 1–10. [Google Scholar] [CrossRef]
- Yang, T.H.; Xirasagar, S.; Cheng, Y.F.; Wu, C.S.; Kuo, N.W.; Lin, H.C. Peripheral Vestibular Disorders: Nationwide Evidence From Taiwan. Laryngoscope 2021, 131, 639–643. [Google Scholar] [CrossRef]
- Choi, H.G.; Kim, S.Y.; Chung, J. The Risk of BPPV, Meniere’s Disease, and Vestibular Neuronitis in Patients with Gout: A Longitudinal Follow-Up Study Using a National Health Screening Cohort. J. Clin. Med. 2022, 12, 185. [Google Scholar] [CrossRef]
- Muelleman, T.; Shew, M.; Subbarayan, R.; Shum, A.; Sykes, K.; Staecker, H.; Lin, J. Epidemiology of Dizzy Patient Population in a Neurotology Clinic and Predictors of Peripheral Etiology. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2017, 38, 870–875. [Google Scholar] [CrossRef]
- Terauchi, M.; Odai, T.; Hirose, A.; Kato, K.; Akiyoshi, M.; Masuda, M.; Tsunoda, R.; Fushiki, H.; Miyasaka, N. Dizziness in peri- and postmenopausal women is associated with anxiety: A cross-sectional study. BioPsychoSoc. Med. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- National Institute of Mental Health. Any Anxiety Disorder. Available online: https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder (accessed on 6 February 2023).
- Dougherty, J.M.; Carney, M.; Hohman, M.H.; Emmady, P.D. Vestibular Dysfunction. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558926/ (accessed on 14 January 2023).
- Yamanaka, T.; Shirota, S.; Sawai, Y.; Murai, T.; Fujita, N.; Hosoi, H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope 2013, 123, 2813–2816. [Google Scholar] [CrossRef]
- Curhan, S.G.; Stankovic, K.; Halpin, C.; Wang, M.; Eavey, R.D.; Paik, J.M.; Curhan, G.C. Osteoporosis, bisphosphonate use, and risk of moderate or worse hearing loss in women. J. Am. Geriatr. Soc. 2021, 69, 3103–3113. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.B.; Lee, C.H.; Kim, H.M. Hearing loss in postmenopausal women with low bone mineral density. Auris Nasus Larynx 2016, 43, 155–160. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. Epidemiology, Burden, and Treatment of Osteoporosis in the United KingdoM; International Osteoporosis Foundation: Nyon, Switzerland, 2021. [Google Scholar]
- Kahveci, O.K.; Demirdal, U.S.; Yücedag, F.; Cerci, U. Patients with osteoporosis have higher incidence of sensorineural hearing loss. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2014, 39, 145–149. [Google Scholar] [CrossRef]
- Mucci, V.; Hamid, M.; Jacquemyn, Y.; Browne, C.J. Influence of sex hormones on vestibular disorders. Curr. Opin. Neurol. 2022, 35, 135–141. [Google Scholar] [CrossRef]
- Gerlier, C.; Hoarau, M.; Fels, A.; Vitaux, H.; Mousset, C.; Farhat, W.; Firmin, M.; Pouyet, V.; Paoli, A.; Chatellier, G.; et al. Differentiating central from peripheral causes of acute vertigo in an emergency setting with the HINTS, STANDING, and ABCD2 tests: A diagnostic cohort study. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2021, 28, 1368–1378. [Google Scholar] [CrossRef]
- Goman, A.M.; Lin, F.R. Prevalence of Hearing Loss by Severity in the United States. Am. J. Public Health 2016, 106, 1820–1822. [Google Scholar] [CrossRef]
- Lin, F.R.; Niparko, J.K.; Ferrucci, L. Hearing loss prevalence in the United States. Arch. Intern. Med. 2011, 171, 1851–1852. [Google Scholar] [CrossRef]
- Chien, W.; Lin, F.R. Prevalence of hearing aid use among older adults in the United States. Arch. Intern. Med. 2012, 172, 292–293. [Google Scholar] [CrossRef]
- Foley, D.M.; Frick, K.D.; Lin, F.R. Association between hearing loss and healthcare expenditures in older adults. J. Am. Geriatr. Soc. 2014, 62, 1188–1189. [Google Scholar] [CrossRef]
- Stucky, S.R.; Wolf, K.E.; Kuo, T. The economic effect of age-related hearing loss: National, state, and local estimates, 2002 and 2030. J. Am. Geriatr. Soc. 2010, 58, 618–619. [Google Scholar] [CrossRef]
- Mohr, P.E.; Feldman, J.J.; Dunbar, J.L.; McConkey-Robbins, A.; Niparko, J.K.; Rittenhouse, R.K.; Skinner, M.W. The societal costs of severe to profound hearing loss in the United States. Int. J. Technol. Assess. Health Care 2000, 16, 1120–1135. [Google Scholar] [CrossRef] [PubMed]
- Access Economics. Listen Hear!: The Economic Impact and Cost of Hearing Loss in Australia. Australian Policy Online. Available online: https://audiology.asn.au/public/1/files/Publications/ListenHearFinal.pdf (accessed on 25 April 2023).
- Mathers, C.D.; Vos, E.T.; Stevenson, C.E.; Begg, S.J. The burden of disease and injury in Australia. Bull. World Health Organ. 2001, 79, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- US Department of Veterans Affairs. Annual Benefits Report. Veterans Benefits Administration. Available online: http://www.benefits.va.gov/REPORTS/abr/ABR_FY2013_Compensation_07172014.pdf (accessed on 25 April 2023).
- Daniell, W.E.; Fulton-Kehoe, D.; Smith-Weller, T.; Franklin, G.M. Occupational hearing loss in Washington state, 1984-1991: II. Morbidity and associated costs. Am. J. Ind. Med. 1998, 33, 529–536. [Google Scholar] [PubMed]
- Tufts, J.B.; Weathersby, P.K.; Rodriguez, F.A. Modeling the Unites States government’s economic cost of noise-induced hearing loss for a military population. Scand. J. Work Environ. Health 2010, 36, 242–249. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef]
- Mener, D.J.; Betz, J.; Genther, D.J.; Chen, D.; Lin, F.R. Hearing loss and depression in older adults. J. Am. Geriatr. Soc. 2013, 61, 1627–1629. [Google Scholar] [CrossRef]
- Kochkin, S. The efficacy of hearing aids in achieving compensation equity in the workplace. Hear. J. 2010, 63, 19–28. [Google Scholar]
| ICD Code * | Description | Percentage from Total Number |
|---|---|---|
| H80 | “Otosclerosis (Otosclerosis involving oval window, nonobliterative, otosclerosis involving oval window, obliterative, cochlear otosclerosis, and unspecified otosclerosis)” | 8.8% |
| H81 | “Disorders of vestibular function (Ménière’s disease, benign paroxysmal vertigo, vestibular neuronitis, other peripheral vertigo, vertigo of central origin, and unspecified disorder of vestibular function)” | 47.6% |
| H83 | “Other diseases of inner ear (Labyrinthitis, labyrinthine fistula, labyrinthine dysfunction, noise effects on inner ear, and unspecified disease of inner ear)” | 43.6% |
| Diseases | Admission Rate in 1999 “per 100,000 Persons (95% CI)” | Admission Rate in 2020 “per 100,000 Persons (95% CI)” | Percentage Change |
|---|---|---|---|
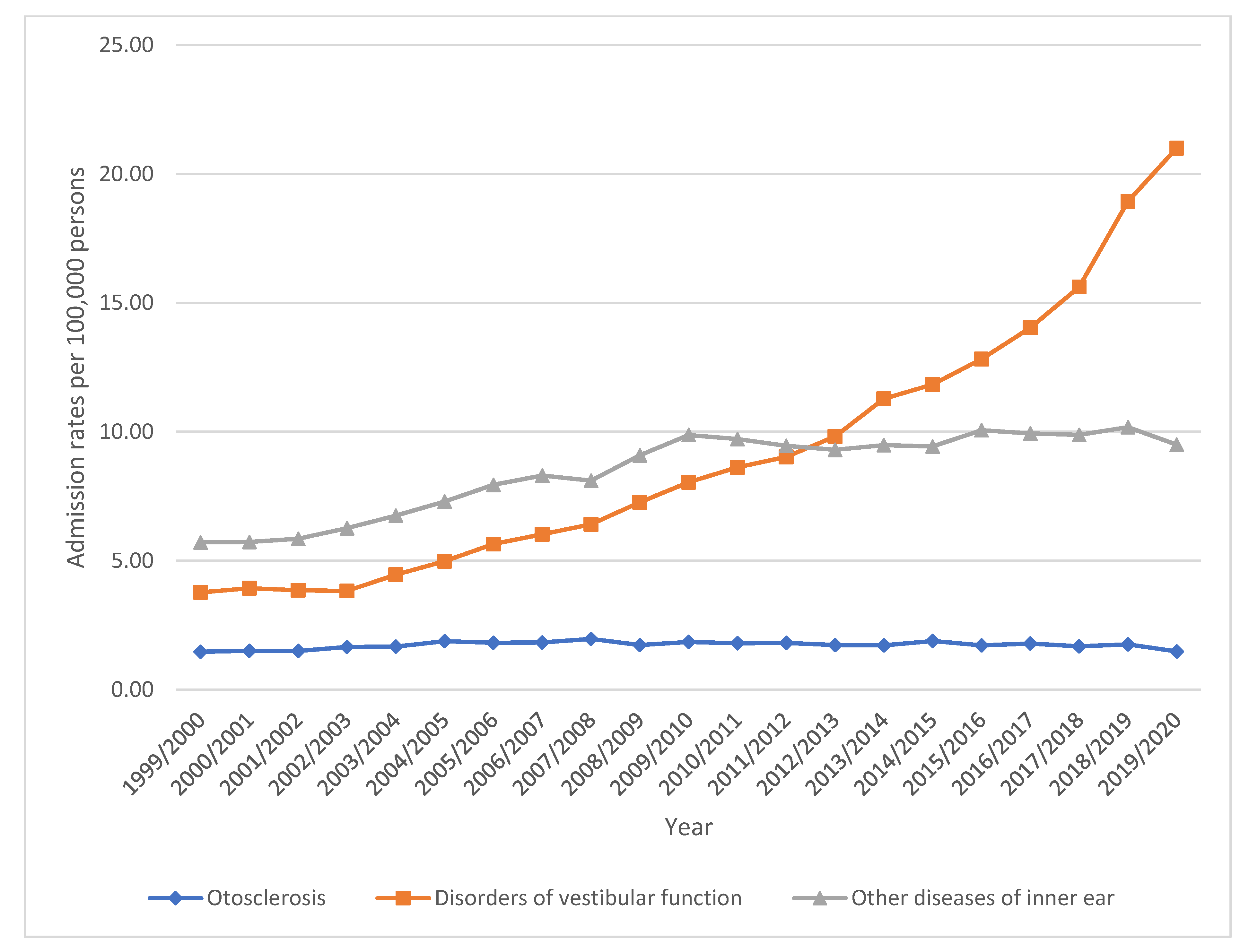
| “Disorders of vestibular function” | 3.77 (3.60–3.93) | 21.00 (20.63–21.37) | 457.5% |
| “Other diseases of inner ear” | 5.71 (5.50–5.91) | 9.50 (9.25–9.75) | 66.5% |
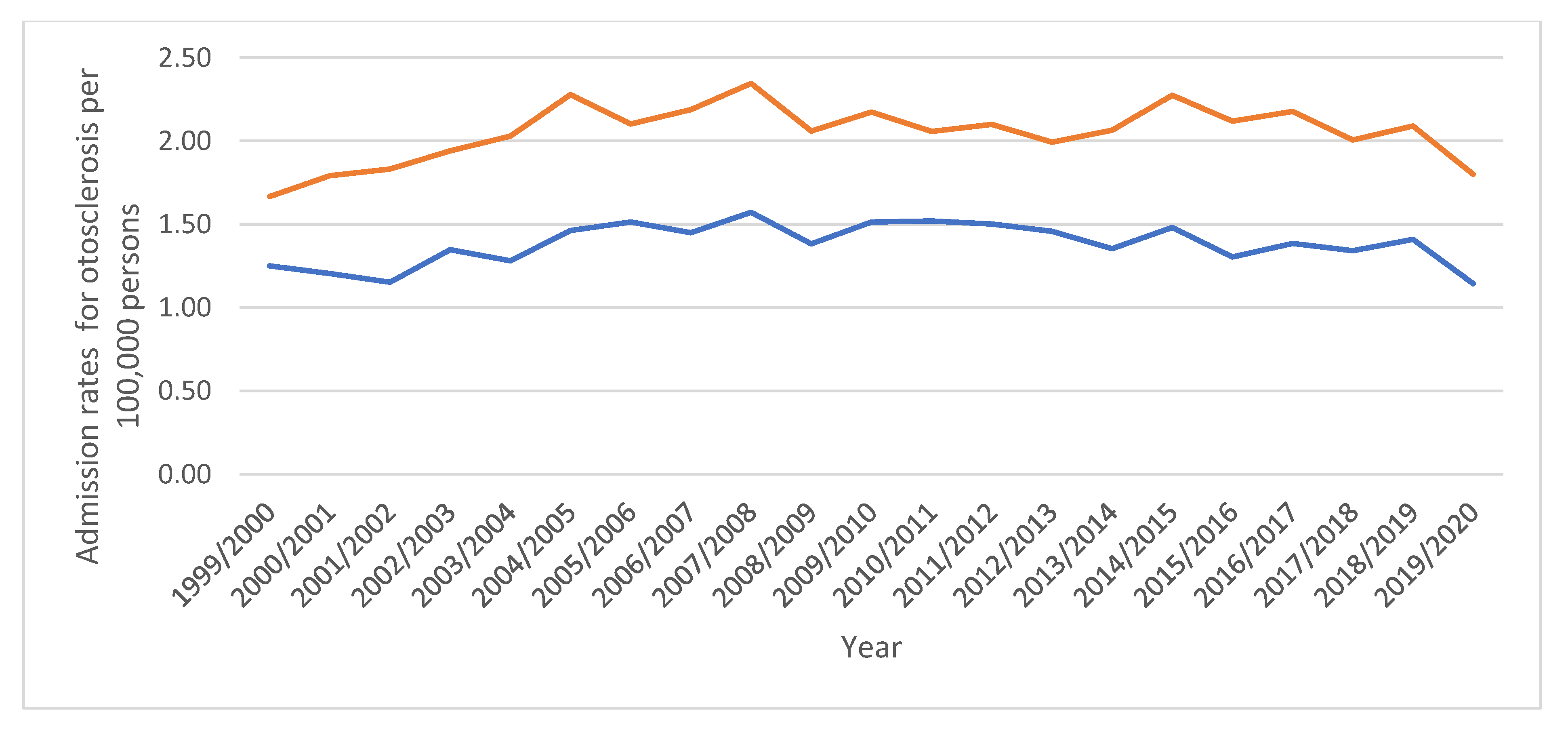
| “Otosclerosis” | 1.47 (1.36–1.57) | 1.48 (1.38–1.57) | 0.7% |
| Diseases | Rate of Diseases in 1999 per 100,000 Persons (95% CI) | Rate of Diseases in 2020 per 100,000 Persons (95% CI) | Percentage from Total Number | Percentage Change |
|---|---|---|---|---|
| “H81.1 Benign paroxysmal vertigo” | 0.58 (0.51–0.64) | 13.11 (12.82–13.40) | 23.2% | 2160.3% |
| “H81.3 Other peripheral vertigo” | 0.19 (0.15–0.23) | 1.08 (1–1.17) | 1.8% | 468.4% |
| “H81.8 Other disorders of vestibular function” | 0.07 (0.05–0.1) | 0.37 (0.32–0.42) | 1.0% | 428.6% |
| “H81.2 Vestibular neuronitis” | 0.69 (0.62–0.76) | 3.12 (2.98–3.26) | 6.8% | 352.2% |
| “H81.9 Unspecified disorder of vestibular function” | 0.22 (0.18–0.26) | 0.82 (0.74–0.89) | 2.2% | 272.7% |
| “H83.8 Other specified diseases of inner ear” | 0.1 (0.08–0.13) | 0.25 (0.21–0.29) | 0.9% | 150.0% |
| “H83.0 Labyrinthitis” | 5.27 (5.07–5.47) | 9.02 (8.78–9.26) | 41.4% | 71.2% |
| “H80.0 Otosclerosis involving oval window, nonobliterative” | 0.05 (0.03–0.07) | 0.07 (0.05–0.10) | 0.3% | 40.0% |
| “H83.1 Labyrinthine fistula” | 0.02 (0.01–0.04) | 0.03 (0.01–0.04) | 0.1% | 50.0% |
| “H80.8 Other otosclerosis” | 0.04 (0.02–0.06) | 0.06 (0.04–0.08) | 0.2% | 50.0% |
| “H81.0 Ménière disease” | 1.91 (1.79–2.03) | 2.37 (2.25–2.50) | 12.0% | 24.1% |
| “H81.4 Vertigo of central origin” | 0.11 (0.08–0.14) | 0.13 (0.10–0.16) | 0.6% | 18.2% |
| “H83.3 Noise effects on inner ear” | 0.01 (0–0.01) | 0.01 (0–0.02) | 0.1% | 0.0% |
| “H83.2 Labyrinthine dysfunction” | 0.08 (0.05–0.10) | 0.08 (0.05–0.10) | 0.4% | 0.0% |
| “H80.1 Otosclerosis involving oval window, obliterative” | 0.02 (0.01–0.03) | 0.02 (0.01–0.03) | 0.1% | 0.0% |
| “H80.9 Unspecified otosclerosis” | 1.32 (1.22–1.42) | 1.3 (1.21–1.39) | 8.0% | −1.5% |
| “H80.2 Cochlear otosclerosis” | 0.03 (0.02–0.05) | 0.02 (0.01–0.03) | 0.1% | −33.3% |
| “H83.9 Unspecified disease of inner ear” | 0.23 (0.19–0.27) | 0.11 (0.09–0.14) | 0.7% | −52.2% |
| “H82.X Unspecified vertiginous syndromes in diseases” | (–) | 0 (0–0.01) | 0.0% | - |
| Age Group | Admission Rate in 1999 “per 100,000 Persons (95% CI)” | Admission Rate in 2020 “per 100,000 Persons (95% CI)” | Percentage Change | Percentage from Total Number |
|---|---|---|---|---|
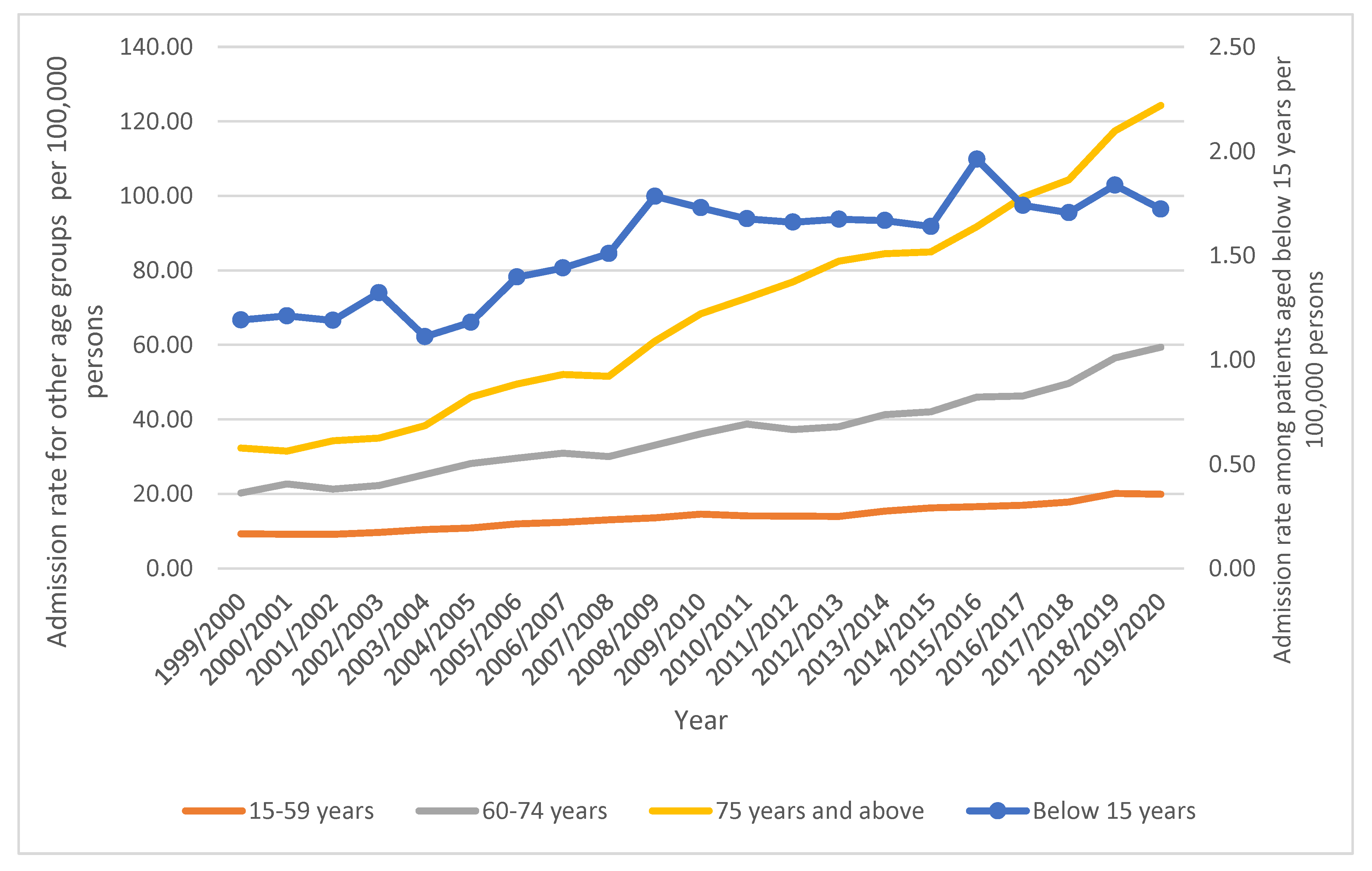
| Below 15 years | 1.19 (0.98–1.41) | 1.72 (1.47–1.97) | 44.6% | 1.42% |
| 15–59 years | 9.30 (8.96–9.63) | 19.93 (19.46–20.40) | 114.3% | 42.31% |
| 60–74 years | 20.24 (19.18–21.30) | 59.38 (57.82–60.94) | 193.4% | 27.42% |
| 75 years and over | 32.29 (30.51–34.07) | 124.28 (121.24–127.31) | 284.0% | 28.84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taybeh, E.O.; Naser, A.Y. Hospital Admission Profile Related to Inner Ear Diseases in England and Wales. Healthcare 2023, 11, 1457. https://doi.org/10.3390/healthcare11101457
Taybeh EO, Naser AY. Hospital Admission Profile Related to Inner Ear Diseases in England and Wales. Healthcare. 2023; 11(10):1457. https://doi.org/10.3390/healthcare11101457
Chicago/Turabian StyleTaybeh, Esra’ O., and Abdallah Y. Naser. 2023. "Hospital Admission Profile Related to Inner Ear Diseases in England and Wales" Healthcare 11, no. 10: 1457. https://doi.org/10.3390/healthcare11101457
APA StyleTaybeh, E. O., & Naser, A. Y. (2023). Hospital Admission Profile Related to Inner Ear Diseases in England and Wales. Healthcare, 11(10), 1457. https://doi.org/10.3390/healthcare11101457








