Large Pelvic Mass in a Female Adolescent: Atypical Presentation and Successful Treatment of Extraskeletal Ewing Sarcoma
Abstract
1. Introduction
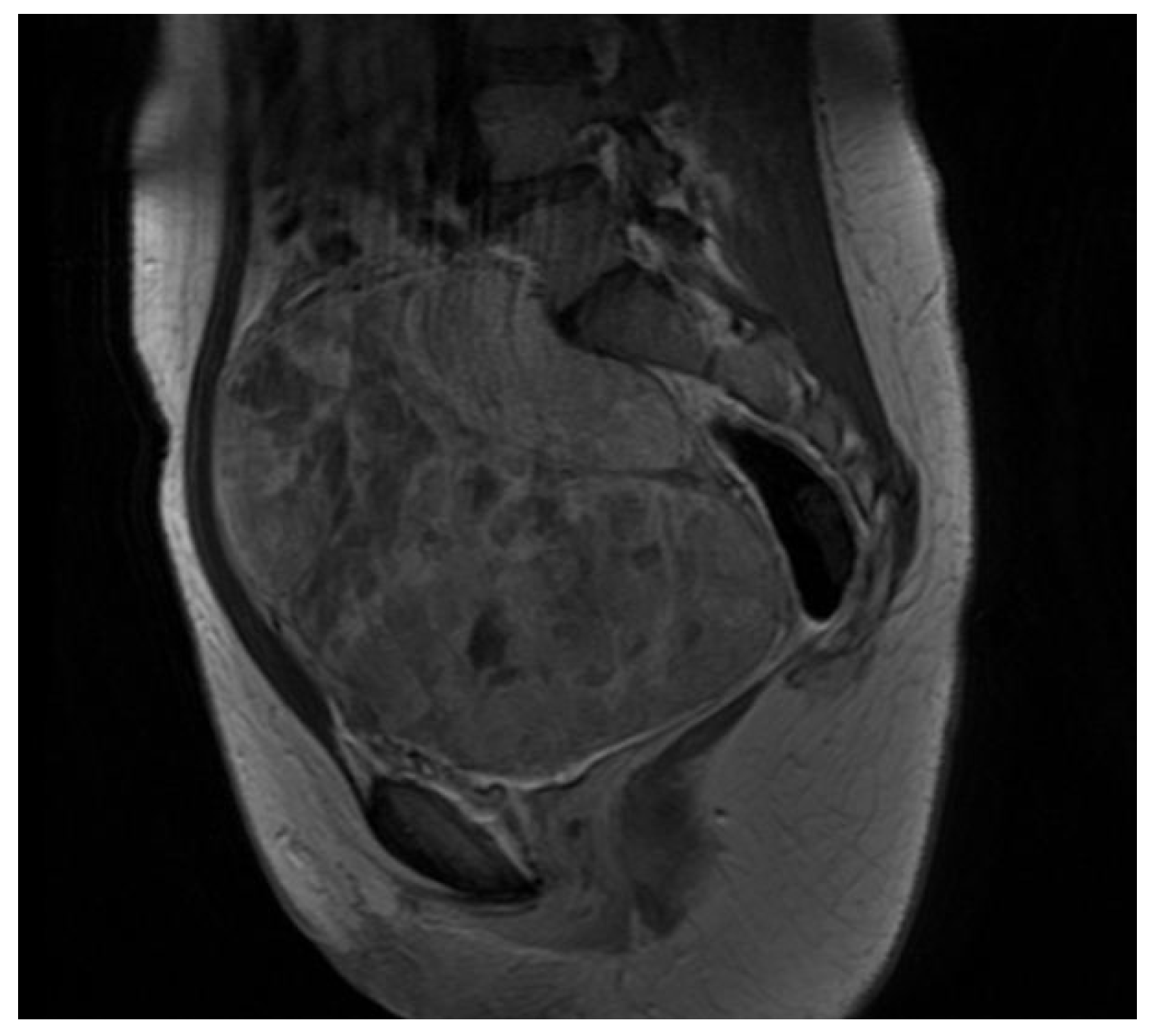
2. Case Presentation
- (1)
- A desmoplastic small round cell tumor (DSRCT), but this was excluded because desmin and WT1 were negative.
- (2)
- Small cell ovarian carcinoma of the hypercalcemic type (OSCCHT), but this was excluded because the ovaries were normal and the tumoral cells were FLI1+ and EMA−.
- (3)
- Metastatic small cells melanoma, but this was also excluded because of the cytokeratin positivity and negativity of HBME-45 and Melan A.
- (4)
- Rhabdomyosarcoma, excludable due to negative muscle markers (desmin, myogenin, myoglobin, MYOD1 and positive anti-cytokeratin antibodies).
- (5)
- Neuroblastoma, excluded due to negative NSE, neurofilament, synaptophysin and positive anti-cytokeratin antibodies.
- (6)
- Juvenile granulosa cell tumor of the ovary, excluded due to the intraoperative findings of normal ovaries and for negative inibin and positive anti-cytokeratin antibodies.
- (7)
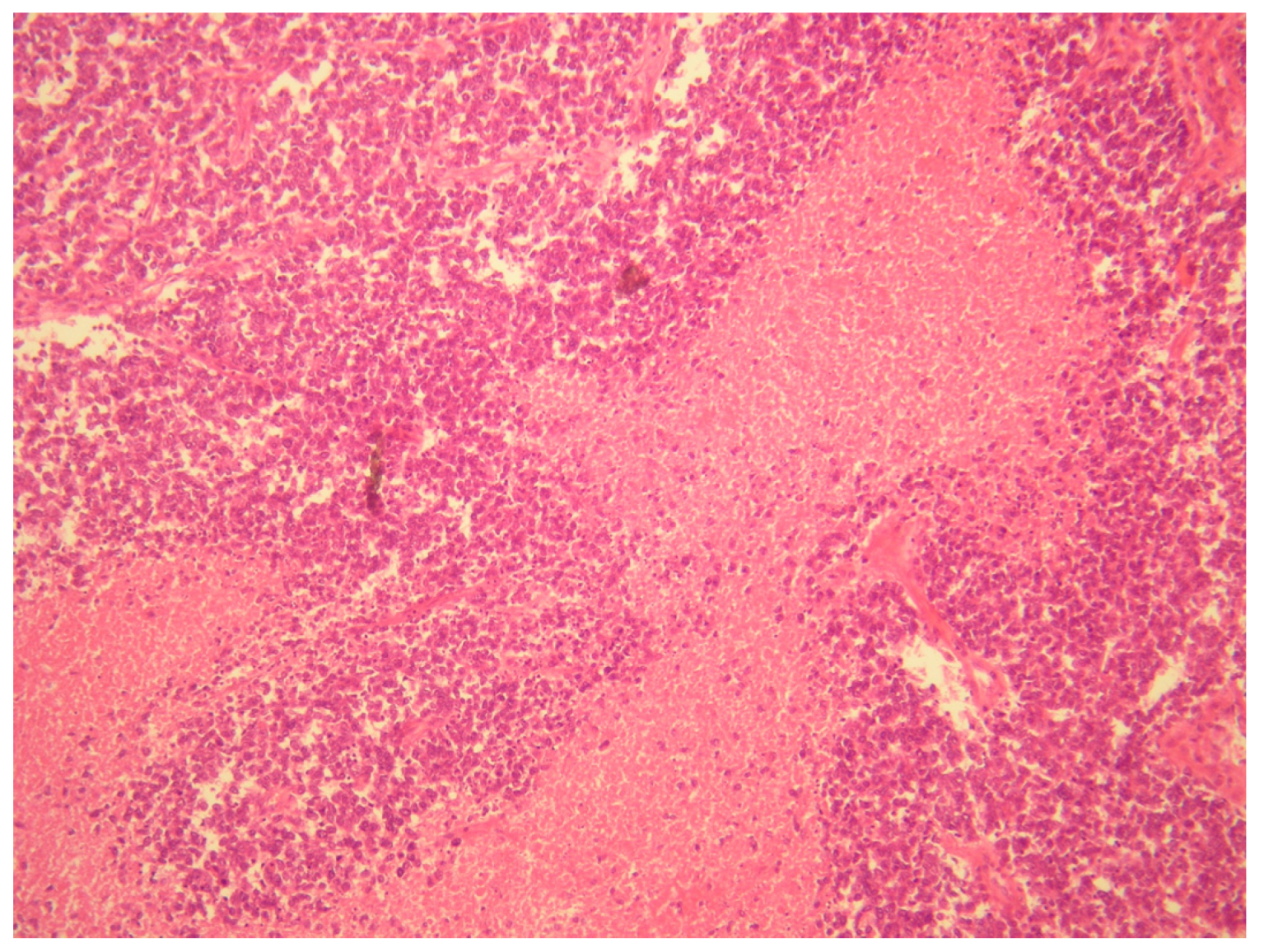
- Ewing sarcoma; this was the main one suspected, both for morphological criteria and for immunohistochemistry, too (positive CD99, cytokeratin, vimentin and FLI1).
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kliegman, R.M.; Behrman, R.E.; Jenson, H.B.; Stanton, B. Nelson Textbook of Pediatrics, 18th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- de Alava, E.; Kawai, A.; Healey, J.H.; Fligman, I.; Meyers, P.A.; Huvos, A.G.; Gerald, W.L.; Jhanwar, S.C.; Argani, P.; Antonescu, C.R.; et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing’s sarcoma. J. Clin. Oncol. 1998, 16, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Delattre, O.; Zucman, J.; Plougastel, B.; Desmaze, C.; Melot, T.; Peter, M.; Kovar, H.; Joubert, I.; De Jong, P.; Rouleau, G.; et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992, 359, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Ellegast, J.M.; Ross, K.N.; Malone, C.F.; Lin, S.; Mabe, N.W.; Dharia, N.V.; Meyer, A.; Conway, A.; Su, A.H.; et al. The ETS transcription factor ETV6 constrains the transcriptional activity of EWS-FLI to promote Ewing sarcoma. Nat. Cell Biol. 2023, 25, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Hense, H.W.; Ahrens, S.; Paulussen, M.; Lehnert, M.; Jürgens, H. Factors associated with tumor volume and primary metastases in Ewing tumors: Results from the (EI)CESS studies. Ann. Oncol. 1999, 10, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Scurr, M.; Judson, I. How to treat the Ewing’s family of sarcomas in adult patients. Oncologist 2006, 11, 65–72. [Google Scholar] [CrossRef]
- Balamuth, N.J.; Womer, R.B. Ewing’s sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef]
- Ahmad, R.; Mayol, B.R.; Davis, M.; Rougraff, B.T. Extraskeletal Ewing’s sarcoma. Cancer 1999, 85, 725–731. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Canc. Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Sánchez-Borrego, R.; Sánchez-Prieto, M. What are the mechanisms of action of the different contraceptive methods to reduce the risk of ovarian cancer? Eur. J. Contracept. Reprod. Health Care 2021, 26, 79–84. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; D’indinosante, M.; Turrini, I.; Giusti, M.; Gullo, G.; Vizzielli, G.; Mattei, A.; Scambia, G.; et al. Fertility Sparing Treatments in Endometrial Cancer Patients: The Potential Role of the New Molecular Classification. Int. J. Mol. Sci. 2021, 22, 12248. [Google Scholar] [CrossRef]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. Int. J. Gynecol. Cancer 2021, 31, 961–982. [Google Scholar] [CrossRef]
- Perelli, F.; Mattei, A.; Scambia, G.; Cavaliere, A.F. Editorial: Methods in gynecological oncology. Front. Oncol. 2023, 13, 1167088. [Google Scholar] [CrossRef]
- Terzic, M.; Rapisarda, A.M.C.; Della Corte, L.; Manchanda, R.; Aimagambetova, G.; Norton, M.; Garzon, S.; Riemma, G.; King, C.R.; Chiofalo, B.; et al. Diagnostic work-up in paediatric and adolescent patients with adnexal masses: An evidence-based approach. J. Obstet. Gynaecol. 2021, 41, 503–515. [Google Scholar] [CrossRef]
- Meyer, J.S.; Nadel, H.R.; Marina, N.; Womer, R.B.; Brown, K.L.; Eary, J.F.; Gorlick, R.; Grier, H.E.; Randall, R.L.; Lawlor, E.R.; et al. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: A report from the Children’s Oncology Group Bone Tumor Committee. Pediatr. Blood Cancer 2008, 51, 163–170. [Google Scholar] [CrossRef]
- Donati, D.; Yin, J.; Di Bella, C.; Colangeli, M.; Bacci, G.; Ferrari, S.; Bertoni, F.; Barbieri, E.; Mercuri, M. Local and distant control in non-metastatic pelvic Ewing’s sarcoma patients. J. Surg. Oncol. 2007, 96, 19–25. [Google Scholar] [CrossRef]
- El Weshi, A.; Allam, A.; Ajarim, D.; Al Dayel, F.; Pant, R.; Bazarbashi, S.; Memon, M. Extraskeletal Ewing’s sarcoma family of tumours in adults: Analysis of 57 patients from a single institution. Clin. Oncol. 2010, 22, 374–381. [Google Scholar] [CrossRef]
- Kallala, R.; Nikkhah, D.; Nix, P.; Woodhead, C. Primary extraskeletal Ewing sarcoma involving the carotid artery: A case report and review of the current literature. Ind. Mark. Manag. 2012, 94, e141–e143. [Google Scholar] [CrossRef]
- Randall, R.L.; Bruckner, J.D.; Papenhausen, M.D.; Thurman, T.; Conrad, E.U., 3rd. Errors in diagnosis and margin determination of soft-tissue sarcomas initially treated at non-tertiary centers. Orthopedics 2004, 27, 209–212. [Google Scholar] [CrossRef]
- Esiashvili, N.; Goodman, M.; Marcus, R.B., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J. Pediatr. Hematol. Oncol. 2008, 30, 425–430. [Google Scholar] [CrossRef]
- Breneman, J.C.; Donaldson, S.S.; Constine, L.; Merchant, T.; Marcus, K.; Paulino, A.C.; Followill, D.; Mahajan, A.; Laack, N.; Esiashvili, N.; et al. The Children’s Oncology Group Radiation Oncology Discipline: 15 Years of Contributions to the Treatment of Childhood Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 860–874. [Google Scholar] [CrossRef]
- Le Deley, M.C.; Paulussen, M.; Lewis, I.; Brennan, B.; Ranft, A.; Whelan, J.; Le Teuff, G.; Michon, J.; Ladenstein, R.; Marec-Bérard, P.; et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. 2014, 32, 2440–2448. [Google Scholar] [CrossRef]
- Lewis, T.B.; Coffin, C.M.; Bernard, P.S. Differentiating Ewing’s sarcoma from other round blue cell tumors using a RT-PCR translocation panel on formalin-fixed paraffin-embedded tissues. Mod. Pathol. 2007, 20, 397–404. [Google Scholar] [CrossRef]
- Tessier, L.; McKechnie, T.; Lee, Y.; Park, L.J.; Gangam, N.; Eskicioglu, C. Laparoscopic ovarian transposition prior to pelvic radiation in young women with anorectal malignancies: A systematic review and meta-analysis of prevalence. Color. Dis. 2023. [Google Scholar] [CrossRef]
- Alur-Gupta, S.; Fruchtman, H.; Paroder, V. Fertility-sparing options for cancer patients. Abdom. Imaging 2023, 48, 1618–1628. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Kado, R.; McCune, W.J. Ovarian protection with gonadotropin-releasing hormone agonists during cyclophosphamide therapy in systemic lupus erythematosus. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 64, 97–106. [Google Scholar] [CrossRef]
- Park, C.-Y.; Jung, S.-Y.; Lee, K.-B.; Yang, S.-H. The feasibility and efficacy of gonadotropin-releasing hormone agonists for prevention of chemotherapy induced ovarian failure in patient with gynecological malignancies. Obstet. Gynecol. Sci. 2014, 57, 478–483. [Google Scholar] [CrossRef]
- Di Mattei, V.E.; Perego, G.; Rancoita, P.M.V.; Taranto, P.; Carnelli, L.; Mangili, G.; Sarais, V.; Bergamini, A.; Candiani, M. Psychological Aspects Associated With Fertility Preservation in Oncology: An Exploratory Study. Front. Psychol. 2020, 11, 608651. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelli, F.; Vizzielli, G.; Cavaliere, A.F.; Restaino, S.; Scambia, G.; Zannoni, G.F.; Arciuolo, D.; Gallotta, V. Large Pelvic Mass in a Female Adolescent: Atypical Presentation and Successful Treatment of Extraskeletal Ewing Sarcoma. Healthcare 2023, 11, 1373. https://doi.org/10.3390/healthcare11101373
Perelli F, Vizzielli G, Cavaliere AF, Restaino S, Scambia G, Zannoni GF, Arciuolo D, Gallotta V. Large Pelvic Mass in a Female Adolescent: Atypical Presentation and Successful Treatment of Extraskeletal Ewing Sarcoma. Healthcare. 2023; 11(10):1373. https://doi.org/10.3390/healthcare11101373
Chicago/Turabian StylePerelli, Federica, Giuseppe Vizzielli, Anna Franca Cavaliere, Stefano Restaino, Giovanni Scambia, Gian Franco Zannoni, Damiano Arciuolo, and Valerio Gallotta. 2023. "Large Pelvic Mass in a Female Adolescent: Atypical Presentation and Successful Treatment of Extraskeletal Ewing Sarcoma" Healthcare 11, no. 10: 1373. https://doi.org/10.3390/healthcare11101373
APA StylePerelli, F., Vizzielli, G., Cavaliere, A. F., Restaino, S., Scambia, G., Zannoni, G. F., Arciuolo, D., & Gallotta, V. (2023). Large Pelvic Mass in a Female Adolescent: Atypical Presentation and Successful Treatment of Extraskeletal Ewing Sarcoma. Healthcare, 11(10), 1373. https://doi.org/10.3390/healthcare11101373






