Deep Learning for the Detection and Classification of Diabetic Retinopathy with an Improved Activation Function
Abstract
1. Introduction
2. Research Background
3. Materials and Methods
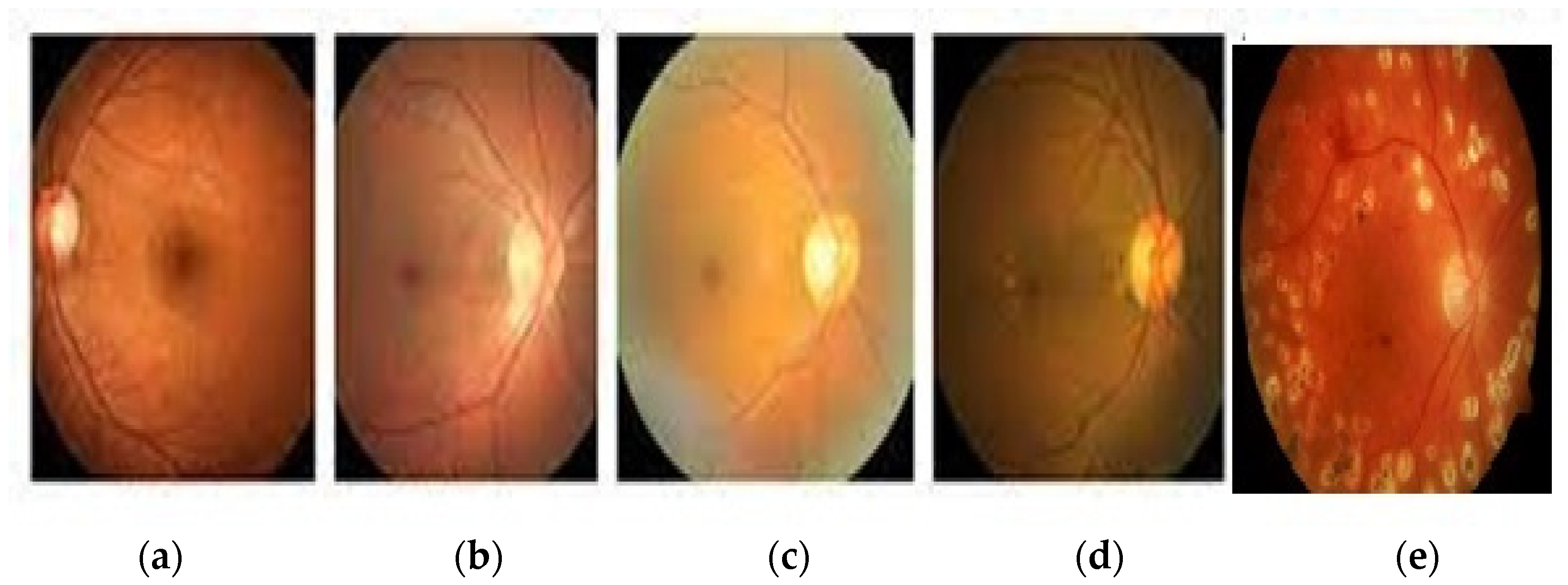
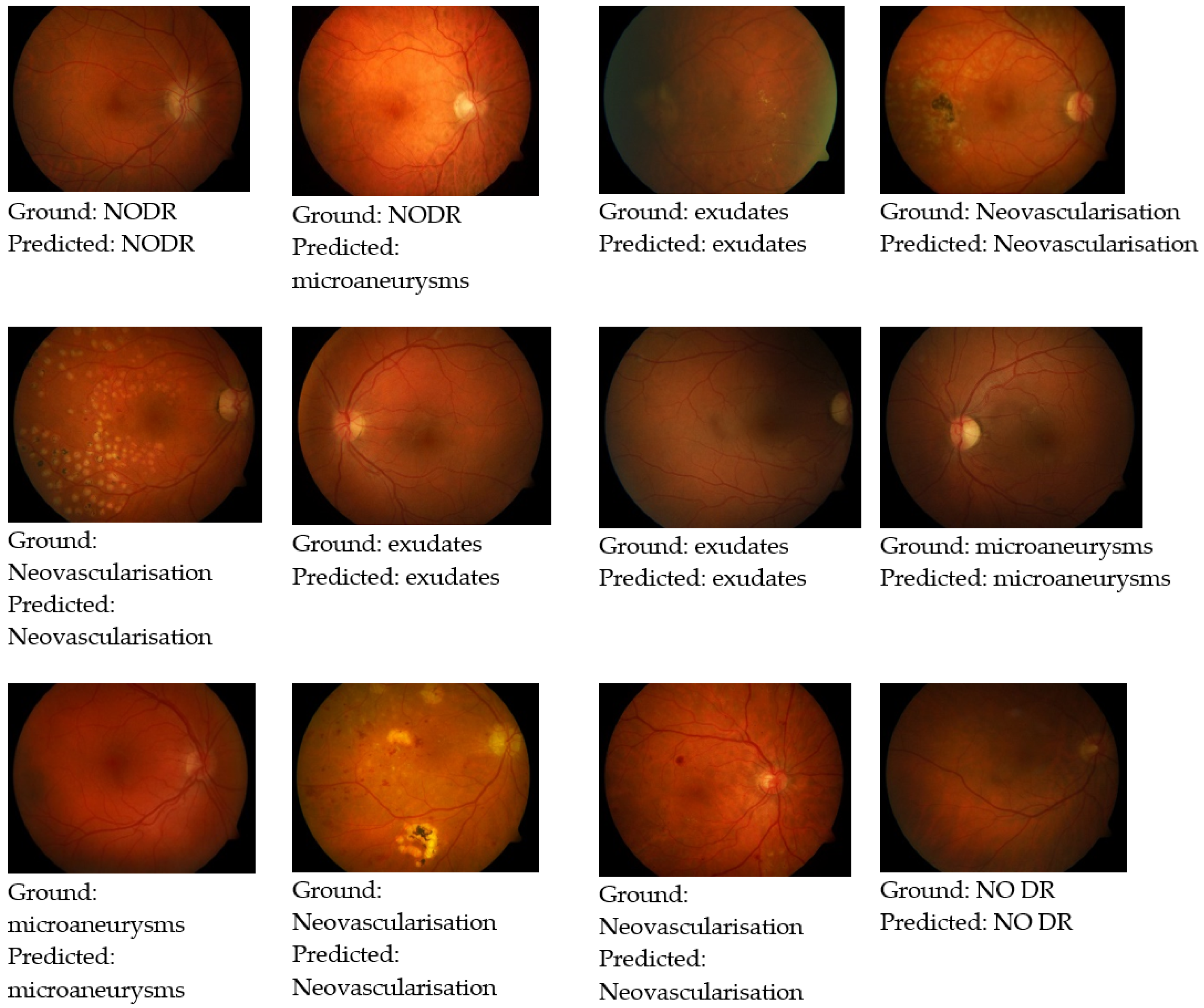
3.1. Dataset
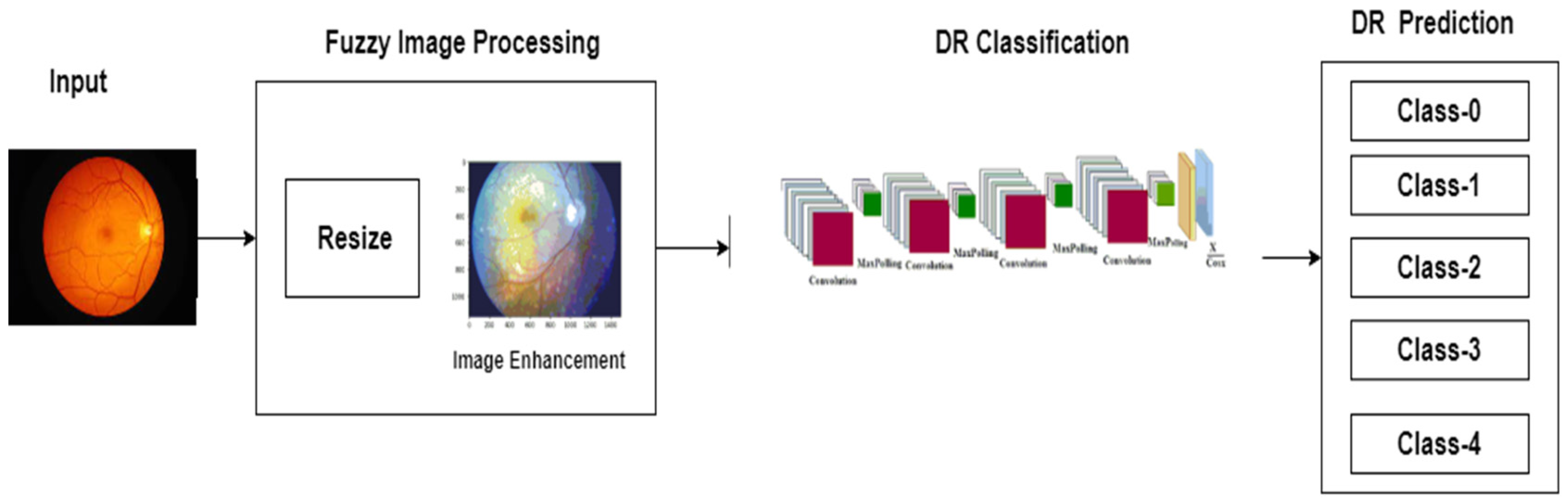
3.2. Image Preprocessing
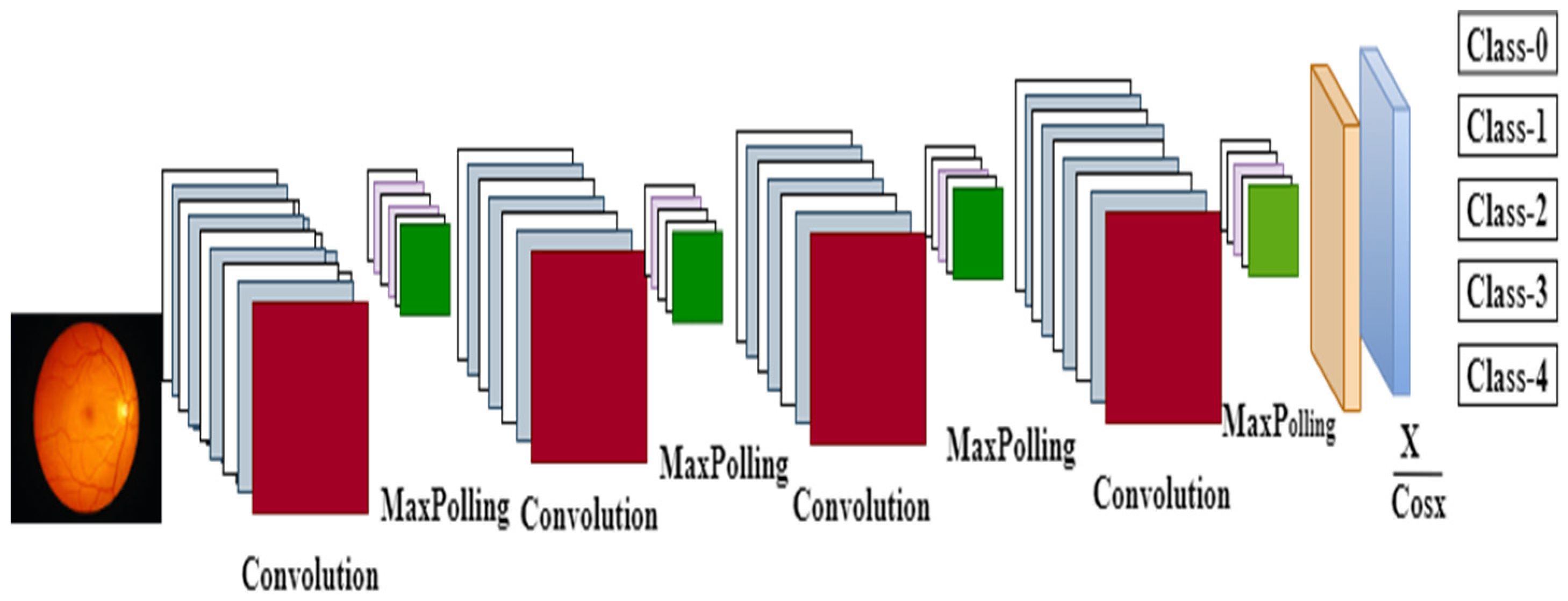
3.3. Improved CNN Model Training
3.3.1. Convolution Layer
3.3.2. Pooling Layer
3.3.3. Activation Function
3.3.4. Fully Connected Layer
- f(0) = 0 and (0) = 1f(x) is derivableProof: f (0−) = f (0+) = 0(0−) = (0+) = 1f(x) is derivable
- When x > 0, f(x) > 0 and (x) = 1Proof: [−1,1]when x > 0, f(x) > 0 and (x) = 1f(x) ==0 < f(x) < x and (x) > 0
- As x → +, f(x) → 0Proof: As x → + f(x) → 0As x → + f(x) → 1
4. Results and Discussion
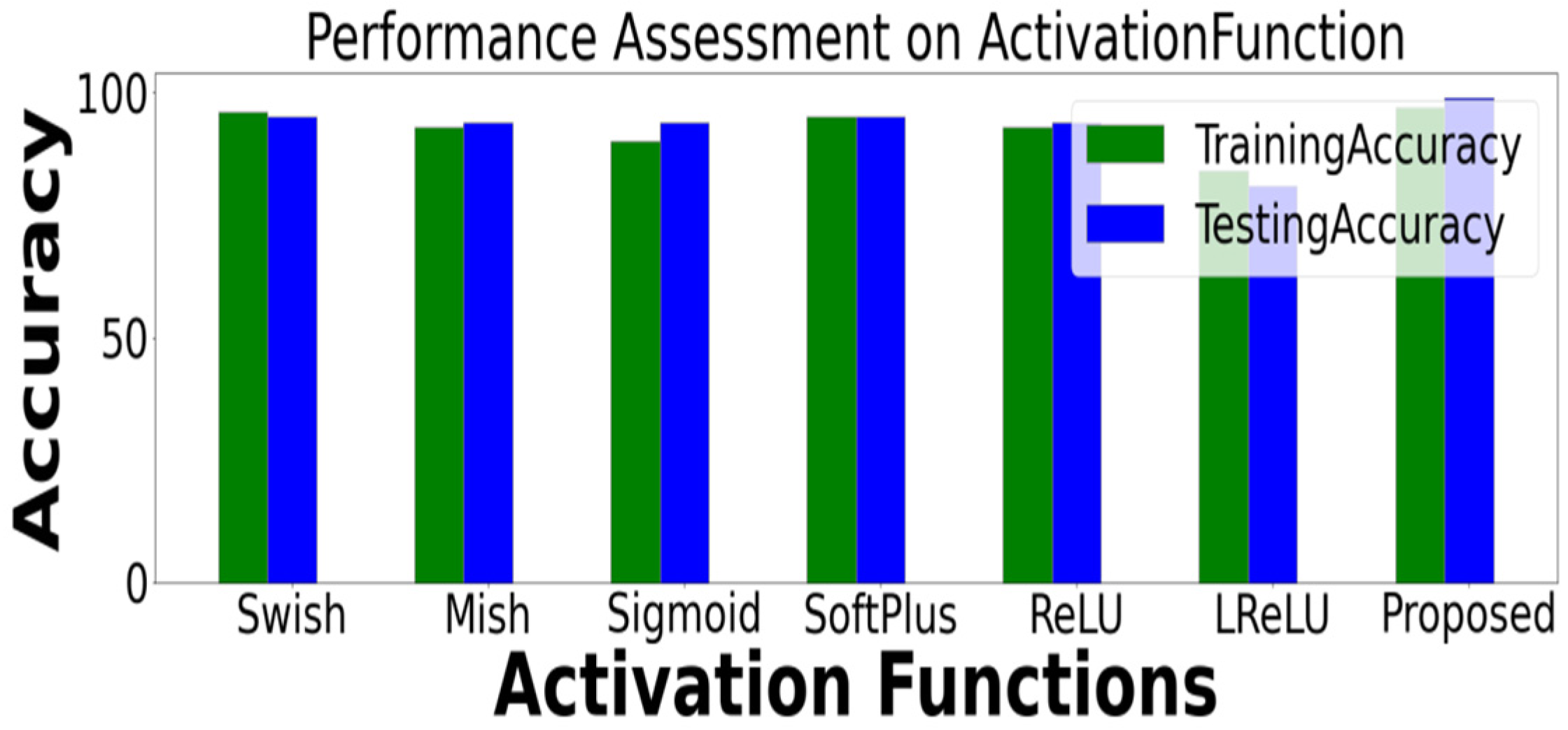
4.1. Accuracy Comparison of Different Activation Functions
4.2. CNN Model Performance Evaluations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 2569. [Google Scholar] [CrossRef]
- Scully, T. Diabetes in numbers. Nature 2012, 485, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fernandez-Loaiza, P.; Sauma, J.; Hernandez-Bogantes, E.; Masis, M. Classification of diabetic retinopathy and diabetic macular Edema. World J. Diabetes 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.M.; O’Neill, W.D.; Penn, R.D.; Blair, N.P.; Shahidi, M. Detection of subclinical diabetic retinopathy by fine structure analysis of retinal images. J. Ophthalmol. 2019, 2019, 5171965. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Rudisill, C.; Egan, C.; Kapetanakis, V.V.; Salas-Vega, S.; Owen, C.G.; Lee, A.; Louw, V.; Anderson, J.; Liew, G.; et al. Automated diabetic retinopathy image assessment software: Diagnostic accuracy and cost-effectiveness compared with human graders. Ophthalmology 2017, 124, 343–351. [Google Scholar] [CrossRef]
- Ozieh, M.N.; Bishu, K.G.; Dismuke, C.E.; Egede, L.E. Trends in Health Care Expenditure in U.S. Adults With Diabetes: 2002–2011. Diabetes Care 2015, 38, 1844–1851. [Google Scholar] [CrossRef]
- Idris, I.; Sellahewa, L.; Simpson, C.; Maharajan, P.; Duffy, J. Grader agreement, and sensitivity and specificity of digital photography in a community optometry-based diabetic eye screening program. Clin. Ophthalmol. 2014, 8, 1345–1349. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Gulshan, V.; Rajan, R.; Widner, K.; Wu, D.; Wubbels, P.; Rhodes, T.; Whitehouse, K.; Coram, M.; Corrado, G.; Ramasamy, K.; et al. Performance of a Deep-Learning Algorithm vs Manual Grading for Detecting Diabetic Retinopathy in India. JAMA Ophthalmol. 2019, 137, 987–993. [Google Scholar] [CrossRef]
- Winder, R.; Morrow, P.; McRitchie, I.; Bailie, J.; Hart, P. Algorithms for digital image processing in diabetic retinopathy. Comput. Med. Imaging Graph. 2009, 33, 608–622. [Google Scholar] [CrossRef]
- Chandrakumar, T.; Kathirvel, R. Classifying diabetic retinopathy using deep learning architecture. Int. J. Eng. Res. Technol. 2016, 5, 19–24. [Google Scholar] [CrossRef]
- Pratt, H.; Coenen, F.; Broadbent, D.M.; Harding, S.P.; Zheng, Y. Convolutional neural networks for diabetic retinopathy. Procedia Comput. Sci. 2016, 90, 200–205. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; Shi, J.; Fang, W.; Li, H.; Wang, X. Zoom-in-net: Deep mining lesions for diabetic retinopathy detection. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Quebec City, QC, Canada, 10–14 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 267–275. [Google Scholar]
- Qummar, S.; Khan, F.G.; Shah, S.; Khan, A.; Shamshirband, S.; Rehman, Z.U.; Khan, I.A.; Jadoon, W. A Deep Learning Ensemble Approach for Diabetic Retinopathy Detection. IEEE Access 2019, 7, 150530–150539. [Google Scholar] [CrossRef]
- Prataprao Bhatkar, A.; Kharat, G.U. Detection of diabetic retinopathy in retinal images using MLP classifier. In Proceedings of the 2015 IEEE International Symposium on Nanoelectronic and Information Systems, Indore, India, 21–23 December 2015; pp. 331–335. [Google Scholar]
- Wan, S.; Liang, Y.; Zhang, Y. Deep convolutional neural networks for diabetic retinopathy detection by image classification. Comput. Electr. Eng. 2018, 72, 274–282. [Google Scholar] [CrossRef]
- Dutta, S.; Manideep, B.C.; Basha, S.M.; Caytiles, R.D.; Iyengar, N.C.S.N. Classification of Diabetic Retinopathy Images by Using Deep Learning Models. Int. J. Grid Distrib. Comput. 2018, 11, 99–106. [Google Scholar] [CrossRef]
- Garc’ıa, G.; Gallardo, J.; Mauricio, A.; L’opez, J.; Del Carpio, C. Detection of diabetic retinopathy based on a convolutional neural network using retinal fundus images. In Proceedings of the International Conference on Artificial Neural Networks, Alghero, Italy, 11–15 September 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 635–642. [Google Scholar]
- DiaretDB0. Available online: https://www.it.lut.fi/project/imageret/diaretdb0/index.html (accessed on 16 December 2022).
- DRIVE. Available online: https://drive.grand-challenge.org/ (accessed on 16 December 2022).
- CHASE. Available online: https://www.idiap.ch/software/bob/docs/bob/bob.db.chasedb1/master/index.html (accessed on 16 December 2022).
- Kaggle. Available online: https://www.kaggle.com/c/diabetic-retinopathy-detection/data (accessed on 16 December 2022).
- Chang, S.L.; Shu, M.G.; Chin, Y.H. Genetic Based fuzzy image filter and its applications to image processing. IEEE Trans. Syst. Man Cybern. 2005, 35, 694–711. [Google Scholar]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multi-ethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Guo, J.; Chen, Y.; Yi, Z.; Zhong, J. Diagnosis of Diabetic retinopathy using deep neural networks. IEEE Access 2018, 7, 3360–3370. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Lamia, A.N.M.; Sarah, H.T. Diabetic retinopathy diagnosis based on convolutional neural networks. J. Phys. Conf. Ser 2021, 1999, 012117. [Google Scholar]
- Eman, A.; Shaker, E.S.; Sherif, B.; Tamer, A.; Mohammed, E. Automatic Diabetic retinopathy grading system based on detecting multiple retinal lesions. IEEE Access 2021, 9, 15939–15960. [Google Scholar]
- Jebaseeli, T.J.; Durai, C.A.D.; Peter, J.D. Retinal Blood vessel segmentation from diabetic retinopathy images using tandem PCNN model and deep learning based SVM. Optik 2019, 199, 163328. [Google Scholar] [CrossRef]
- Erick, O.R.; Aura, C.; Panos, L. ELEMENT: Multimodal retinal vessel segmentation based on a coupled region growing and machine learning approach. IEEE J. Biomed. Health Inform. 2020, 24, 3507–3519. [Google Scholar]
- Mohamed, H.M.; Salman, A.; Fouad, H.; Amir, A.; Ahmed, E.Y. An automatic detection system of diabetic retinopathy using a hybrid inductive machine learning algorithm. Pers. Ubiquitous Comput. 2021, 1, 1–15. [Google Scholar]
- Nneji, G.U.; Cai, J.; Deng, J.; Monday, H.N.; Hossin, M.A.; Nahar, S. Identification of Diabetic retinopathy using weighted fusion deep learning based on dual channel fundus scans. Diagnostics 2022, 12, 540. [Google Scholar] [CrossRef]
- Bhuiyan A; Govindaiah A; Deobhakta A; Hossain M; Rosen R; Smith Automated diabetic retinopathy screening for primary care settings using deep learning. Intell. Based Med. 2021, 5, 100045. [CrossRef]
| Function | Definition | Equation | Limitations |
|---|---|---|---|
| Linear type | The final activation function of the last layer is just a linear function of the first layer of the input, and it can be used in the output layer. | Y = x; −∞ to +∞ | Nonlinearity is difficult to achieve. |
| Binary type | The binary classification is used mainly when inputs exceed thresholds, otherwise, outputs are zero. | 0; if input < threshold, otherwise 1; if input > threshold; Range: {0, 1} | Cannot classify the multiclass problems |
| Nonlinear | |||
| Sigmoid | A small change in input will result in a large change in output. To convert the output into a predictable score, this layer is placed at the end of the model. | 1/(1 + ex); Range:0 to1 or −1 to 1 | During training, a model other than the output layer is invalid due to the vanishing gradients |
| Tanh | It is used as an alternative to the Sigmoid function if the output is other than zero and one. | Tanh(x = (ex − e−x)/(ex + e−x); Range: −1 to +1 | If the weighted sum of the input is very large, then the function gradient becomes very small and close to zero. It has the vanishing gradient problem. |
| ReLu | It is implemented in the hidden layers of the model. It is computationally less expensive and much faster than the tanh and Sigmoid and solves the vanishing gradient problem | max (0, x); if x is positive, output x, otherwise 0; Range: 0 to +∞ | It does not compute the exponentials and the divisions. It overfits more than the Sigmoid function. It does not avoid the exploding gradient problem. |
| Swish | It deals with the vanishing gradient problem. It helps in normalizing the output. The output does not saturate to a maximum value, i.e., the gradient does not become zero. | x.σ(x); Range: −∞ to +∞ | It is computationally more expensive than the Sigmoid. |
| Mish | It is continuously differentiable and nonmonotonic. It is used in the hidden layer. | x.tanh(ln(1 + ex)); Range: −∞ to +∞ | It is computationally more expensive than the ReLu. |
| Activation Function | Epochs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 | |
| Tanh | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 |
| Sigmoid | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Relu | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| LReLu | 0.95 | 0.95 | 0.95 | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| ELU | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| SELU | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Log sin | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Sinc | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 |
| Wave | 0.94 | 0.94 | 0.94 | 0.94 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Rootsig | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| Logsigm | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| Proposed | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 | 0.97 | 0.98 | 0.98 |
| Activation Function | Learning Rates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 × 10−1 | 1 × 10−2 | 1 × 10−3 | 1 × 10−4 | 1 × 10−5 | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 | 1 × 10−9 | |
| Tanh | 0.91 | 0.91 | 0.91 | 0.91 | 0.92 | 0.92 | 0.93 | 0.93 | 0.94 |
| Sigmoid | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.94 | 0.94 | 0.94 |
| Relu | 0.93 | 0.93 | 0.93 | 0.94 | 0.94 | 0.95 | 0.95 | 0.95 | 0.94 |
| LReLu | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| ELU | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| SELU | 0.98 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| Log sin | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.94 |
| Sinc | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.96 | 0.97 | 0.96 |
| Wave | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 |
| Rootsig | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.95 | 0.95 |
| Logsigm | 0.96 | 0.96 | 0.96 | 0.97 | 0.96 | 0.97 | 0.96 | 0.96 | 0.96 |
| Proposed | 0.98 | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 | 0.97 | 0.97 | 0.97 |
| Activation Function | Batch Sizes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | 2048 | |
| Tanh | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 |
| Sigmoid | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Relu | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| LReLu | 0.95 | 0.95 | 0.95 | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| ELU | 0.95 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| SELU | 0.98 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Log sin | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Sinc | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 |
| Wave | 0.94 | 0.94 | 0.94 | 0.94 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Rootsig | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| Logsigm | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| Proposed | 0.98 | 0.98 | 0.98 | 0.99 | 0.98 | 0.98 | 0.97 | 0.97 | 0.97 |
| Database | Model | Accuracy | Sensitivity | Specificity | Precision | F1 Score | AUC | Model Loss |
|---|---|---|---|---|---|---|---|---|
| DIRATEDB0 | Inception-v3 | 92.12 | 94.53 | 95.41 | 92.76 | 95.57 | 0.83 | 0.0029 |
| VGG-19 | 94.92 | 97.56 | 98.34 | 95.25 | 94.77 | 0.73 | 0.0025 | |
| ResNet-50 | 93.54 | 95.27 | 98.32 | 99.43 | 98.42 | 0.89 | 0.0019 | |
| AlexNet | 95.82 | 81.62 | 94.36 | 91.66 | 94.47 | 0.79 | 0.0021 | |
| GoogleNet | 94.08 | 78.36 | 92.42 | 89.22 | 90.39 | 0.78 | 0.0029 | |
| SqueezeNet | 84.52 | 89.46 | 96.86 | 91.38 | 89.33 | 0.70 | 0.0058 | |
| ResNet-152 | 96.64 | 97.96 | 98.79 | 99.53 | 99.15 | 0.93 | 0.0013 | |
| Kaggle | Inception-v3 | 93.63 | 96.34 | 96.74 | 93.63 | 94.52 | 0.89 | 0.0026 |
| VGG-19 | 93.32 | 97.24 | 93.77 | 96.74 | 96.62 | 0.95 | 0.0024 | |
| ResNet-50 | 94.64 | 94.24 | 96.86 | 95.74 | 97.72 | 0.97 | 0.0016 | |
| Alexnet | 96.27 | 87.64 | 96.89 | 97.84 | 98.78 | 0.87 | 0.0020 | |
| GoogleNet | 95.87 | 83.33 | 93.85 | 94.79 | 94.83 | 0.88 | 0.0024 | |
| SqueezeNet | 87.85 | 90.36 | 97.36 | 93.92 | 91.88 | 0.84 | 0.0030 | |
| ResNet-152 | 99.41 | 98.28 | 99.94 | 99.89 | 99.93 | 0.98 | 0.0010 | |
| DRIVE | Inception-v3 | 96.43 | 93.74 | 93.63 | 93.62 | 96.53 | 0.88 | 0.0036 |
| VGG-19 | 92.45 | 93.74 | 94.63 | 98.44 | 97.22 | 0.84 | 0.0047 | |
| ResNet-50 | 92.44 | 93.72 | 95.27 | 94.83 | 95.88 | 0.93 | 0.0023 | |
| AlexNet | 96.74 | 86.89 | 95.84 | 93.83 | 97.62 | 0.74 | 0.0032 | |
| GoogleNet | 93.88 | 77.92 | 95.24 | 85.68 | 93.73 | 0.73 | 0.0034 | |
| SqueezeNet | 86.07 | 86.35 | 93.46 | 93.77 | 90.69 | 0.74 | 0.0046 | |
| ResNet-152 | 97.84 | 98.45 | 99.26 | 99.68 | 99.57 | 0.94 | 0.0015 | |
| CHASE | Inception-v3 | 94.65 | 96.34 | 94.63 | 96.62 | 93.34 | 0.85 | 0.0025 |
| VGG-19 | 93.74 | 94.83 | 95.85 | 93.62 | 96.62 | 0.94 | 0.0027 | |
| ResNet-50 | 93.83 | 93.22 | 96.95 | 95.73 | 94.68 | 0.96 | 0.0028 | |
| AlexNet | 96.62 | 88.74 | 97.83 | 94.38 | 92.67 | 0.84 | 0.0028 | |
| GoogleNet | 92. 58 | 79.48 | 97.28 | 90.82 | 93.73 | 0.84 | 0.0038 | |
| SqueezeNet | 88. 42 | 90.84 | 98.25 | 94.84 | 91.73 | 0.78 | 0.0047 | |
| ResNet-152 | 99.05 | 98.45 | 99.59 | 99.94 | 99.89 | 0.97 | 0.0017 |
| Activation Function | Model | Accuracy | Processing Time | Model Loss |
|---|---|---|---|---|
| SELU | Inception-v3 | 91.82 | 20 | 0.0029 |
| VGG-19 | 91.18 | 22 | 0.0026 | |
| ResNet-50 | 92.17 | 20 | 0.0020 | |
| AlexNet | 93.28 | 20 | 0.0021 | |
| GoogleNet | 92.27 | 19 | 0.0028 | |
| SqueezeNet | 84.94 | 22 | 0.0036 | |
| ResNet-152 | 98.57 | 17 | 0.0015 | |
| ReLu | Inception-v3 | 90.82 | 21 | 0.0028 |
| VGG-19 | 90.83 | 24 | 0.0027 | |
| ResNet-50 | 91.28 | 26 | 0.0026 | |
| AlexNet | 92.72 | 22 | 0.0021 | |
| GoogleNet | 91.26 | 21 | 0.0025 | |
| SqueezeNet | 82.17 | 23 | 0.0032 | |
| ResNet-152 | 95.73 | 19 | 0.0020 | |
| Sigmoid | Inception-v3 | 90.63 | 22 | 0.0034 |
| VGG-19 | 90.37 | 25 | 0.0027 | |
| ResNet-50 | 92.62 | 26 | 0.0021 | |
| AlexNet | 91.63 | 23 | 0.0026 | |
| GoogleNet | 90.68 | 22 | 0.0026 | |
| SqueezeNet | 82.73 | 23 | 0.0036 | |
| ResNet-152 | 95.63 | 20 | 0.0016 | |
| ELU | Inception-v3 | 90.52 | 23 | 0.0029 |
| VGG-19 | 90.26 | 25 | 0.0028 | |
| ResNet-50 | 92.47 | 27 | 0.0028 | |
| AlexNet | 92.95 | 21 | 0.0027 | |
| GoogleNet | 91.63 | 20 | 0.0026 | |
| SqueezeNet | 83.53 | 21 | 0.0034 | |
| ResNet-152 | 96.63 | 19 | 0.0021 | |
| Proposed | Inception-v3 | 93.63 | 15 | 0.0026 |
| VGG-19 | 93.32 | 16 | 0.0024 | |
| ResNet-50 | 94.64 | 14 | 0.0016 | |
| AlexNet | 96.27 | 16 | 0.0020 | |
| GoogleNet | 95.87 | 14 | 0.0024 | |
| SqueezeNet | 87.85 | 15 | 0.0030 | |
| ResNet-152 | 99.41 | 07 | 0.0010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhimavarapu, U.; Battineni, G. Deep Learning for the Detection and Classification of Diabetic Retinopathy with an Improved Activation Function. Healthcare 2023, 11, 97. https://doi.org/10.3390/healthcare11010097
Bhimavarapu U, Battineni G. Deep Learning for the Detection and Classification of Diabetic Retinopathy with an Improved Activation Function. Healthcare. 2023; 11(1):97. https://doi.org/10.3390/healthcare11010097
Chicago/Turabian StyleBhimavarapu, Usharani, and Gopi Battineni. 2023. "Deep Learning for the Detection and Classification of Diabetic Retinopathy with an Improved Activation Function" Healthcare 11, no. 1: 97. https://doi.org/10.3390/healthcare11010097
APA StyleBhimavarapu, U., & Battineni, G. (2023). Deep Learning for the Detection and Classification of Diabetic Retinopathy with an Improved Activation Function. Healthcare, 11(1), 97. https://doi.org/10.3390/healthcare11010097







