Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Design and Inclusion Criteria
3.2. Dosing Regimens
3.3. Primary and Secondary Outcome Measures
3.4. Efficacy of Donanemab
3.5. Safety and Tolerability of Donanemab
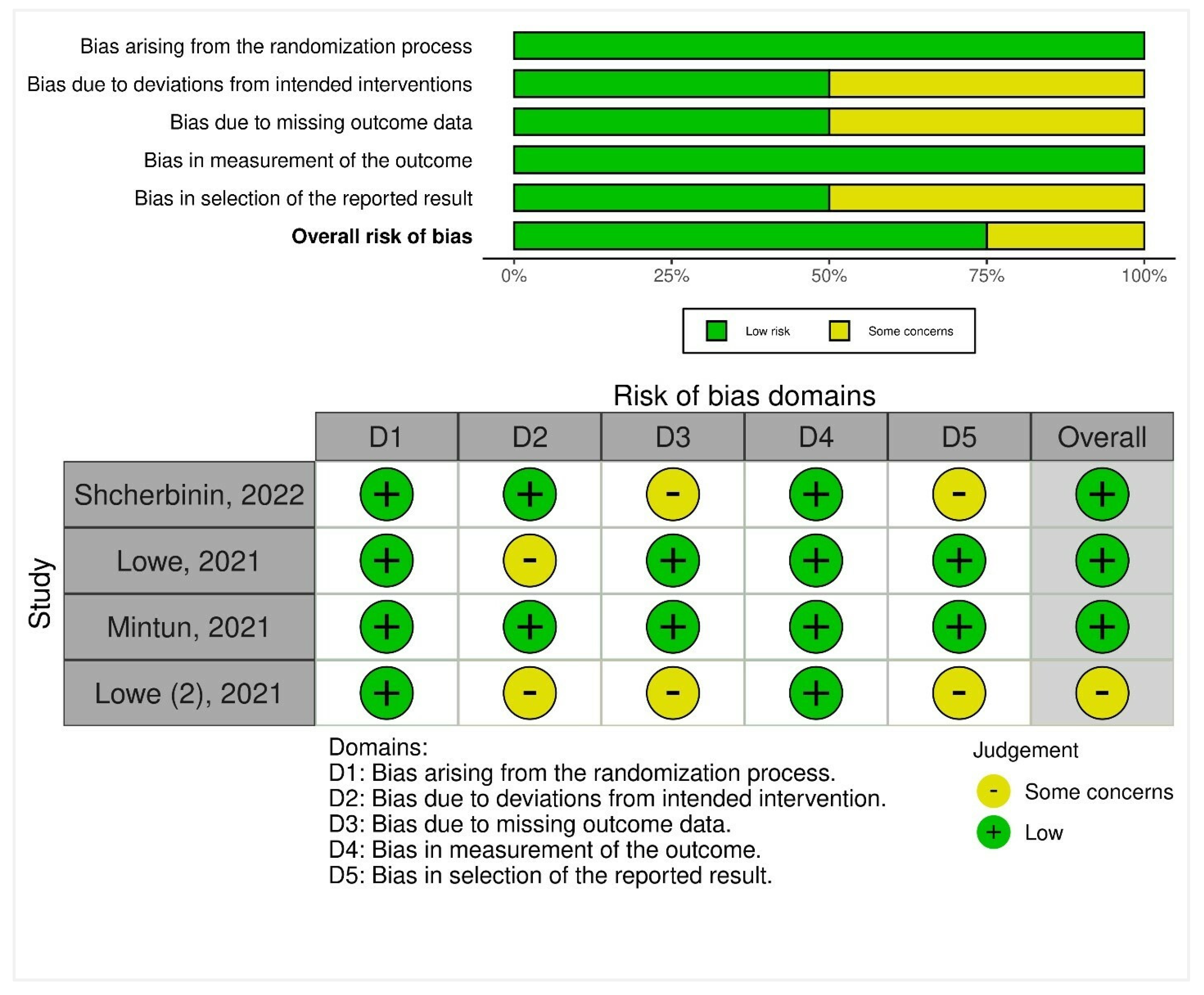
3.6. Risk-of-Bias Synthesis
4. Discussion
4.1. Limitations and Recommendations
4.2. Known Risk Factors of Donanemab
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKeown, A.; Turner, A.; Angehrn, Z.; Gove, D.; Ly, A.; Nordon, C.; Nelson, M.; Tochel, C.; Mittelstadt, B.; Keenan, A. Health Outcome Prioritization in Alzheimer’s Disease: Understanding the Ethical Landscape. J. Alzheimers Dis. 2020, 77, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, M.; Dufouil, C. Forecasting the prevalence of dementia. Lancet Public Health 2022, 7, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.-H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Frisardi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Vendemiale, G.; Capurso, A.; Imbimbo, B.P. Disease-modifying approach to the treatment of Alzheimer’s disease. Drugs Aging 2009, 26, 537–555. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New pharmacological strategies for treatment of Alzheimer’s disease: Focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers. Res. Ther. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Cole, M.A.; Seabrook, G.R. On the horizon—The value and promise of the global pipeline of Alzheimer’s disease therapeutics. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12009. [Google Scholar] [CrossRef]
- Parhizkar, S.; Holtzman, D.M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 2022, 59, 101594. [Google Scholar] [CrossRef]
- Park, G.; Nhan, H.S.; Tyan, S.-H.; Kawakatsu, Y.; Zhang, C.; Navarro, M.; Koo, E.H. Caspase activation and caspase-mediated cleavage of APP is associated with amyloid β-protein-induced synapse loss in Alzheimer’s disease. Cell Rep. 2020, 31, 107839. [Google Scholar] [CrossRef]
- Wang, D.; Kowalewski, E.K.; Koch, G. Application of Meta-analysis to Evaluate Relationships Among ARIA-E Rate, Amyloid Reduction Rate, and Clinical Cognitive Response in Amyloid Therapeutic Clinical Trials for Early Alzheimer’s Disease. Ther. Innov. Regul. Sci. 2022, 56, 501–516. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 1–17. [Google Scholar]
- Stoiljkovic, M.; Horvath, T.L.; Hajós, M. Therapy for Alzheimer’s disease: Missing targets and functional markers? Ageing Res. Rev. 2021, 68, 101318. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A. Pyroglutamate Aβ cascade as drug target in Alzheimer’s disease. Mol. Psychiatry 2022, 27, 1880–1885. [Google Scholar] [CrossRef]
- Schilling, S.; Zeitschel, U.; Hoffmann, T.; Heiser, U.; Francke, M.; Kehlen, A.; Holzer, M.; Hutter-Paier, B.; Prokesch, M.; Windisch, M. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer’s disease–like pathology. Nat. Med. 2008, 14, 1106–1111. [Google Scholar] [CrossRef]
- Frost, J.L.; Liu, B.; Kleinschmidt, M.; Schilling, S.; Demuth, H.-U.; Lemere, C.A. Passive immunization against pyroglutamate-3 amyloid-β reduces plaque burden in Alzheimer-like transgenic mice: A pilot study. Neurodegener. Dis. 2012, 10, 265–270. [Google Scholar] [CrossRef]
- Frost, J.L.; Liu, B.; Rahfeld, J.-U.; Kleinschmidt, M.; O’Nuallain, B.; Le, K.X.; Lues, I.; Caldarone, B.J.; Schilling, S.; Demuth, H.-U. An anti-pyroglutamate-3 Aβ vaccine reduces plaques and improves cognition in APPswe/PS1ΔE9 mice. Neurobiol. Aging 2015, 36, 3187–3199. [Google Scholar] [CrossRef]
- Hoffmann, T.; Meyer, A.; Heiser, U.; Kurat, S.; Böhme, L.; Kleinschmidt, M.; Bühring, K.-U.; Hutter-Paier, B.; Farcher, M.; Demuth, H.-U. Glutaminyl cyclase inhibitor PQ912 improves cognition in mouse models of Alzheimer’s disease—Studies on relation to effective target occupancy. J. Pharmacol. Exp. Ther. 2017, 362, 119–130. [Google Scholar] [CrossRef]
- DeMattos, R.B.; Lu, J.; Tang, Y.; Racke, M.M.; DeLong, C.A.; Tzaferis, J.A.; Hole, J.T.; Forster, B.M.; McDonnell, P.C.; Liu, F. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron 2012, 76, 908–920. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.L.; Willis, B.A.; Hawdon, A.; Natanegara, F.; Chua, L.; Foster, J.; Shcherbinin, S.; Ardayfio, P.; Sims, J.R. Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12112. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.L.; Duggan Evans, C.; Shcherbinin, S.; Cheng, Y.-J.; Willis, B.A.; Gueorguieva, I.; Lo, A.C.; Fleisher, A.S.; Dage, J.L.; Ardayfio, P. Donanemab (LY3002813) phase 1b study in Alzheimer’s disease: Rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J. Prev. Alzheimers Dis. 2021, 8, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Shcherbinin, S.; Evans, C.D.; Lu, M.; Andersen, S.W.; Pontecorvo, M.J.; Willis, B.A.; Gueorguieva, I.; Hauck, P.M.; Brooks, D.A.; Mintun, M.A. Association of Amyloid Reduction After Donanemab Treatment With Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Knopman, D.S.; Lowe, V.J.; Botha, H.; Graff-Radford, J.; Jones, D.T.; Vemuri, P.; Mielke, M.M.; Schwarz, C.G.; Senjem, M.L. Relationships between β-amyloid and tau in an elderly population: An accelerated failure time model. Neuroimage 2021, 242, 118440. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Janelidze, S.; Ossenkoppele, R.; Kvartsberg, H.; Brinkmalm, A.; Mattsson-Carlgren, N.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 2021, 144, 310–324. [Google Scholar] [CrossRef]
- Singh, A.; Allen, D.; Fracassi, A.; Tumurbaatar, B.; Natarajan, C.; Scaduto, P.; Woltjer, R.; Kayed, R.; Limon, A.; Krishnan, B. Functional integrity of synapses in the central nervous system of cognitively intact individuals with high Alzheimer’s disease neuropathology is associated with absence of synaptic tau oligomers. J. Alzheimers Dis. 2020, 78, 1661–1678. [Google Scholar] [CrossRef]
- Doré, V.; Krishnadas, N.; Bourgeat, P.; Huang, K.; Li, S.; Burnham, S.; Masters, C.L.; Fripp, J.; Villemagne, V.L.; Rowe, C.C. Relationship between amyloid and tau levels and its impact on tau spreading. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2225–2232. [Google Scholar] [CrossRef]
- Wang, L.; Benzinger, T.L.; Su, Y.; Christensen, J.; Friedrichsen, K.; Aldea, P.; McConathy, J.; Cairns, N.J.; Fagan, A.M.; Morris, J.C. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol. 2016, 73, 1070–1077. [Google Scholar] [CrossRef]
- Rentz, D.M.; Mormino, E.C.; Papp, K.V.; Betensky, R.A.; Sperling, R.A.; Johnson, K.A. Cognitive resilience in clinical and preclinical Alzheimer’s disease: The Association of Amyloid and Tau Burden on cognitive performance. Brain Imaging Behav. 2017, 11, 383–390. [Google Scholar] [CrossRef]
- Johnson, D.K.; Storandt, M.; Morris, J.C.; Galvin, J.E. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch. Neurol. 2009, 66, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Amieva, H.; Le Goff, M.; Millet, X.; Orgogozo, J.M.; Pérès, K.; Barberger-Gateau, P.; Jacqmin-Gadda, H.; Dartigues, J.F. Prodromal Alzheimer’s disease: Successive emergence of the clinical symptoms. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2008, 64, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Leurgans, S.E.; Boyle, P.A.; Bennett, D.A. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch. Neurol. 2011, 68, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Jack Jr, C.R.; Wiste, H.J.; Schwarz, C.G.; Lowe, V.J.; Senjem, M.L.; Vemuri, P.; Weigand, S.D.; Therneau, T.M.; Knopman, D.S.; Gunter, J.L. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 2018, 141, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Fodero-Tavoletti, M.T.; Masters, C.L.; Rowe, C.C. Tau imaging: Early progress and future directions. Lancet Neurol. 2015, 14, 114–124. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Devous, M.D.; Kennedy, I.; Navitsky, M.; Lu, M.; Galante, N.; Salloway, S.; Doraiswamy, P.M.; Southekal, S.; Arora, A.K. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer’s disease dementia. Brain 2019, 142, 1723–1735. [Google Scholar] [CrossRef]
- Whittington, A.; Gunn, R.N.; Initiative, A.D.N. TauIQ: A Canonical Image Based Algorithm to Quantify Tau PET Scans. J. Nucl. Med. 2021, 62, 1292–1300. [Google Scholar] [CrossRef]

| Sr. No. | Author | Year | Title | Journal | Phase | Design | Inclusion Criteria | Pharmacologic Intervention | Outcome Measures | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lowe (1) | 2021 | Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease | Translational Research & Clinical Interventions | Phase 1 study | Subject- and investigator-blind, randomized, placebo-controlled, parallel-group, a seven-arm study of single-dose, followed by a multiple-dose, dose-escalation study | Men or non-fertile women ≥50 years of age with evidence of memory impairment on FCSRT-IR picture version, MMSE score of 16 to 30, and a florbetapir PET scan consistent with amyloid pathology | Arm 1: 0.1 mg/kg IV (intervention: four patients, placebo: two patients); Arm 2: 0.3 mg/kg IV (intervention: seven patients, placebo: two patients) Arm 3: 1 mg/kg IV (intervention: nine patients, placebo: two patients) Arm 4: 3 mg/kg IV (intervention: 11 patients, placebo: 3 patients) Arm 5: 10 mg/kg IV (intervention: six patients, placebo: three patients) Arm 6: 3 mg/kg SC (intervention: eight patients) Arm 7: 1 mg/kg IV in six healthy volunteers | Brain amyloid plaque levels with Florbetapir-PET with SUVR; ADAS-Cog-14; MMSE; FCSRT-IR | 48-week period |
| 2 | Lowe | 2021 | Donanemab (LY3002813) Phase 1b Study in Alzheimer’s Disease: Rapid and Sustained Reduction of Brain Amyloid Measured by Florbetapir F18 Imaging | The Journal of Prevention of Alzheimer’s Disease | Phase 1b | Three-part, patient- and investigator-blind, randomized within cohort, placebo-controlled, parallel-group, six-arm, single and multiple-dose study | Men or non-fertile women ≥50 years with evidence of memory impairment on FCSRT-IR, picture version, MMSE score of 16–30, CDR of 0.5–2, memory box score ≥0.5, and a florbetapir PET scan consistent with amyloid pathology | Cohorts 1–3 (single, IC dose of Donanemab 10 mg/kg, 20 mg/kg, 40 mg/kg) or placebo; Cohort 4 (multiple IV doses of Donanemab 10 mg/kg) or placebo every 2 weeks for 24 weeks; Cohorts 5–6 (multiple IV doses of Donanemab, 10 mg/kg, 20 mg/kg) or placebo every 4 weeks for 72 weeks | Brain amyloid plaque levels with 18F-flortaucipir PET scan with SUVR values; CDR; MMSE; FCSRT-IR; ADAS-Cog-14; ADCS-MCI-ADL-24; NTB | 72 weeks (Cohorts 1 and 2); 24 weeks (Cohort 3); 48 weeks (Cohort 4); 12 weeks (Cohorts 5–6) |
| 3 | Mintun | 2021 | Donanemab in Early Alzheimer’s Disease | The New England Journal of Medicine | Phase 2 trial (NCT03367403) | Multicenter, randomized, double-blind, placebo-controlled trial | Patients 60 to 85 years of age who had early symptomatic AD, defined as prodromal AD or mild AD with dementia, and had an MMSE score of 20 to 28; flortaucipir PET scans with evidence of pathologic tau deposition but with quantitative tau levels between 1.10 and 1.46 except in advanced AD where tau levels ≤1.10 included with elevated amyloid levels (equivalent to ≥37 CL) | 1:1 ratio to receive either Donanemab (700 mg for the first three doses and 1400 mg thereafter) or placebo, administered intravenously every 4 weeks for up to 72 weeks | Change from baseline to 76 weeks in iADRS score *; Change from baseline in CDR-SB scores; ADAS-Cog13, the ADCS-iADL, and the MMSE | 76 weeks |
| 4 | Shcherbinin | 2022 | Association of Amyloid Reduction After Donanemab Treatment With Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial | JAMA Neurology | Phase 2 (NCT03367403) | Multicenter, double-blind, phase 2, placebo-controlled, randomized clinical trial | Participants with AD who had an intermediate tau level (moderate AD patterns based on visual assessment and SUVR between 1.10 and 1.46, inclusive, or advanced AD patterns and SUVR ≤1.10) plus elevated amyloid level (equivalent to ≥37 CL) | Donanemab dosing was given every 4 weeks: 700 mg for the first 3 doses, then 1400 mg for up to 72 weeks. | Change from baseline in the score on the iADRS; Change in amyloid, tau, and clinical decline after Donanemab intervention | 48-week period |
| Sr. No. | Author, Year | Sample Size | Age (Years) | Gender (Female) | Severity of AD | APOE-ε4 Carriers | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| 1 | Lowe (2), 2021 | N = 63; Donanemab, n = 51; placebo, n = 12 | 69.7 ± 16.4 years | 33 (52.4%) | MCI/Mild: 40/51 (78.4%) patients who received Donanemab and 9/12 (75%) patients who received placebo; moderate: 5/51 (9.8%) patients who received Donanemab and 3/12 (25%) who received placebo | NR | Donanemab was well-tolerated up to 10 mg/kg; Single-dose administration from 0.1 to 3.0 mg/kg yielded a mean terminal elimination half-life of ~ 4 days and this increased to ~ 10 days at 10 mg/kg; only 10-mg/kg dosage showed changes in amyloid PET, with a mean SUVR change of −0.26 (SD: −0.26) and mean CL change of −44.4 (SD: 14.2); around 90% of subjects developed anti-drug antibodies at 3 months after a single dose | No deaths or drug-related serious adverse events were reported; 6 of 37 patients (16.2%) who received IV Donanemab had mild-to-moderate infusion reactions; two patients (3.9%) in the intervention arms had asymptomatic ARIA-microhemorrhage; four participants (6.4%) across the entire cohort had serious adverse events not related to study drug, including hip fracture, cervical vertebral fracture, urinary tract infection, and noncardiac chest pain |
| 2 | Lowe, 2021 | N = 61; Donanemab, n = 46; placebo, n = 15 | 73.2 ± 8.1 years | 34 (55.7%) | Mean (SD) MMSE score: 21.1 (4.0) | 47/61 (77.0%) patients; 11 homozygotes and 36 heterozygotes | Amyloid PET mean changes: at 24 weeks, from baseline for single doses were: −16.5 CL (SE = 11.22) with 10 mg/kg, −40.0 CL (SE = 11.23) with 20 mg/kg, and −49.6 CL (SE = 15.10) with 40 mg/kg; at 24 weeks, multiple dosage cohorts had −55.8 CL (SE = 9.51) with 10 mg/kg Q2w *, −50.2 CL (SE = 10.54) with 10 mg/kg Q4w, and −58.4 CL (SE = 9.66) with 20 mg/kg Q4w **; complete amyloid clearance (threshold of below 24.2 CL) established in 11 of 46 (23.9%) patients (one patient in the 20/mg single dose, one patient in the 40 mg/kg single dose, two patients in 10 mg/kg Q2w, two patients in 10 mg/kg Q4w, and five patients in 20 mg/kg Q4w); 45 out of 46 patients had positive TE-ADA with Donanemab | Seven serious adverse events in six patients (9.8%) were reported across the entire cohort (one patient died due to non-treatmen-related myocardial infarction, one had intermittent symptomatic cerebral edema (ARIA-E), and four patients had non-treatment-related events; 12 of 46 intervened patients (26.1%) developed vasogenic edema (ARIA-E) in all dosing regimens but the 10 mg/kg single-dose arm; 10 of 46 patients (21.7%) had microhemorrhage events (ARIA-H) across all dosing arms; 2 of 46 (4.4%) patients had superficial siderosis (ARIA-H) in the 10 and 20 mg/kg Q4w arms; 2 of 46 patients (4.4%) in the 20 mg/kg Q4w arm discontinued Donanemab due to TRAE |
| 3 | Mintun, 2021 | N = 272; Donanemab, n = 131; placebo, n = 126 | 75.2 ± 5.5 years | 145 (53.3%) | Mean (SD) MMSE score: 23.5 ± 3.1 (13.0–30.0) | 197/270 (73.0%) patients; 141 heterozygotes and 56 homozygotes | Difference between the Donanemab and placebo groups in the change from baseline at 76 weeks for iADRS was 32% (p = 0.04) in favor of Donanemab, calculated through the following scores: a reduction of 6.86 from 106.2 baseline iADRS scores in the intervention group and 10.06 reduction from a baseline score of 105.9; difference between the Donanemab and placebo groups in change from baseline to 76 weeks were −0.36 for the CDR-SB score, −1.86 for the ADAS-Cog13 score, 1.21 for the ADCS-iADL score, and 0.64 for the MMSE score; there was an 84.13 CL reduction in the Donanemab group on Florbetapir PET from baseline scores of 108 CL; amyloid negative status (amyloid plaque level of < 24.10 CL) was found in 52 (40.0%) patients, 78 (59.8%) patients, and 89 (67.8%) patients at 24, 52, and 76 weeks; there was no change in global tau load from baseline to 76 weeks as assessed by flortaucipir PET; at 52 and 76 weeks, there was a greater decrease in whole-brain volume and greater increase in ventricular volume in the Donanemab group vs. the placebo group | No significant difference between the Donanemab group and the placebo group in the incidence of death or serious adverse events; 119 of 131 participants (90.8%) in the Donanemab group and 113 of 125 participants (90.4%) in the placebo group had at least one adverse event; 35 of 131 (26.7%) participants developed an ARIA-E in the Donanemab group of whom 8 (6.1%) participants were symptomatic and 1 of 125 (0.8%) participants developed an ARIA-E in the placebo group; 40 of 131 (30.5%) participants had an ARIA-H event in the Donanemab group and 9 of 125 (7.2%) participants had an ARIA-H event in the placebo group; cerebral microhemorrage was present in 10 of 131 participants (7.6%) and 3 of 125 participants (2.4%) in the Donanemab and placebo group, respectively; superficial siderosis was present in 18 of 131 (13.7%) participants in the Donanemab group and 4 of 125 (3.2%) participants in the placebo group; infusion-related reaction was present in 10 of 131 (7.6%) participants in the Donanemab group and did not occur in the placebo group |
| 4 | Shcherbinin, 2022 | N = 272; Donanemab, n = 131; placebo, n = 126; combination, n = 15 | 75.2 ± 5.5 years | 145 (53.3%) | Same as Mintun (2021) | Same as Mintun (2021) | 46 of 115 (40%) of Donanemab participants reached a complete amyloid clearance threshold of 24.1 CL (r: –0.54), placebo-treated participants did have changed amyloid clearance change appreciably (r: –0.194); achieved amyloid clearance was sustained with a mean rate of reaccumulation of 0.02 CL (SD: 7.75) over a 1-year period; those who achieved an amyloid level ≤ 11 CL at week 24 would require a mean time of 3.9 years [95% CI: 1.9–8.3 years] to regain amyloid plaque levels above the threshold (>24.1 CL); a 34% slowing of overall tau level, measured using SUVR, was observed for Donanemab compared to placebo in the entire cohort at 76 weeks | All-cause mortality was lower in Donanemab-intervened participants (n = 1, 0.76%) compared to placebo (n = 2, 1.6%); no differences were reported in serious adverse effects among Donanemab (n = 26, 19.85%) and placebo (n = 25, 20%) groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2023, 11, 32. https://doi.org/10.3390/healthcare11010032
Rashad A, Rasool A, Shaheryar M, Sarfraz A, Sarfraz Z, Robles-Velasco K, Cherrez-Ojeda I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare. 2023; 11(1):32. https://doi.org/10.3390/healthcare11010032
Chicago/Turabian StyleRashad, Areeba, Atta Rasool, Muhammad Shaheryar, Azza Sarfraz, Zouina Sarfraz, Karla Robles-Velasco, and Ivan Cherrez-Ojeda. 2023. "Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials" Healthcare 11, no. 1: 32. https://doi.org/10.3390/healthcare11010032
APA StyleRashad, A., Rasool, A., Shaheryar, M., Sarfraz, A., Sarfraz, Z., Robles-Velasco, K., & Cherrez-Ojeda, I. (2023). Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare, 11(1), 32. https://doi.org/10.3390/healthcare11010032







