The Safety and Efficacy of Psychosocial Adherence Interventions in Young People with Early Psychosis: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Review Design
2.2. Pre-Registration
2.3. Inclusion Criteria
- Population: young people (aged between 15–25 years) experiencing first-episode psychosis (defined as within the first year of treatment by mental health services).
- Intervention: any psychosocial intervention (where the focus was on enhancing medication adherence), delivered by any healthcare worker (e.g., psychologist, nurse) in any clinical setting (e.g., early psychosis service, primary care), via any medium (e.g., telephone, face-to-face).
- Comparator: any (e.g., treatment as usual, attentional control).
- Outcomes: medication adherence determined using any routine measure including, but not limited to, the drug attitude inventory, the Medication Adherence Rating Scale, pill count.
2.4. Data Sources and Search Strategy
2.5. Data Extraction and Qualitative Synthesis
2.6. Assessment of Methodological Quality
3. Results
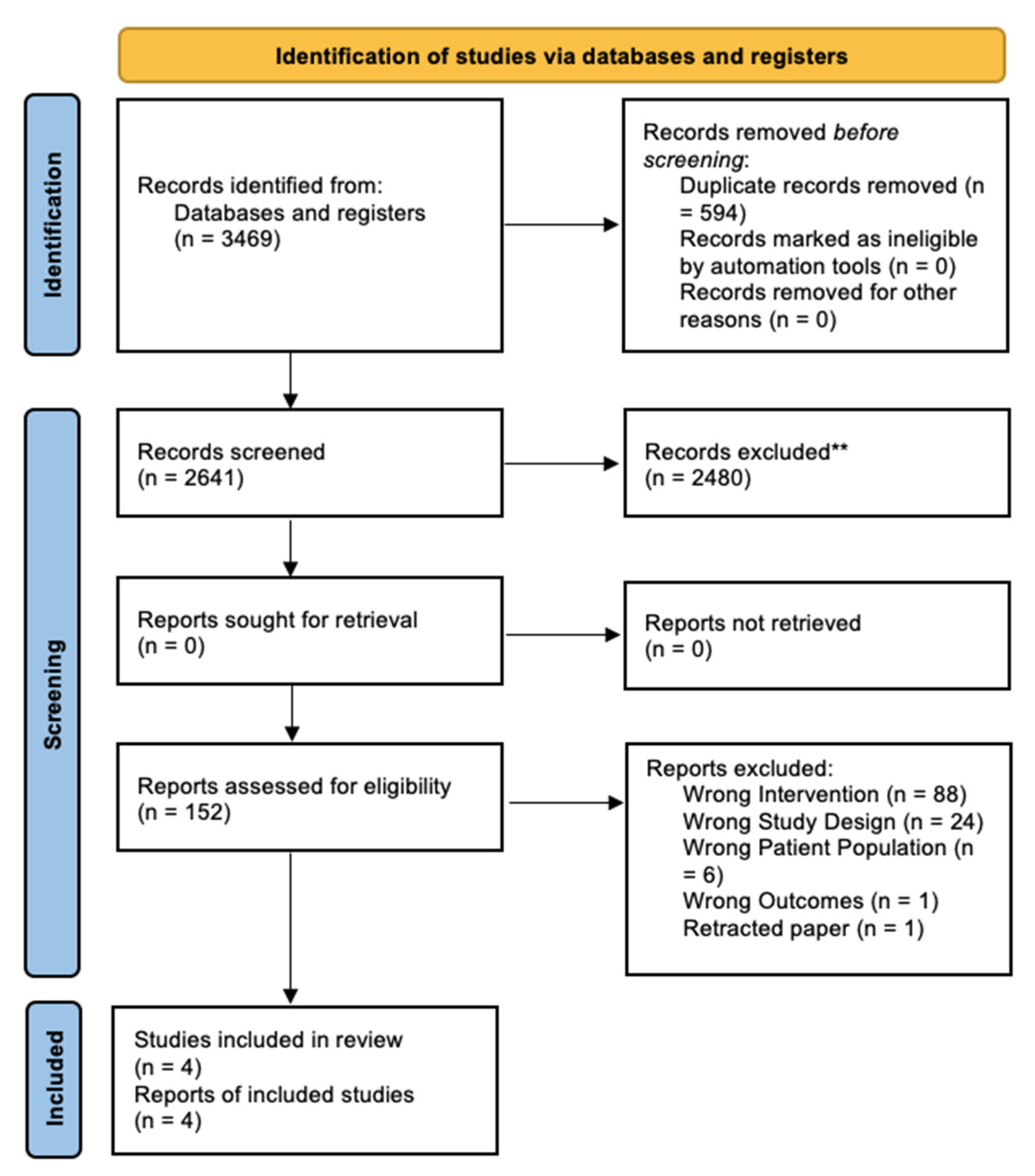
3.1. Search Results
3.2. Characteristics of Included Trials
3.3. Included Studies
3.4. Unpublished Trial
3.5. Quality of Trials
4. Discussion
Limitations of This Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.P.; Gallego, J.A.; Robinson, D.G.; Malhotra, A.K.; Kane, J.M.; Correll, C.U. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: A systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2013, 16, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Winton-Brown, T.T.; Elanjithara, T.; Power, P.; Centre, R.; Blanco-Polaina, P.; McGuire, P. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first-episode psychosis. Schizophr. Res. 2017, 179, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Conus, P.; Cotton, S.; Robinson, J.; McGorry, P.D.; Schimmelmann, B.G. Prevalence, predictors, and consequences of long-term refusal of antipsychotic treatment in first-episode psychosis. J. Clin. Psychopharmacol. 2010, 30, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Abdin, E.; Liang, W.; Poon, L.Y.; Poon, N.Y.; Verma, S. Medication adherence in first-episode psychosis patients in Singapore. Early Interv. Psychiatry 2019, 13, 780–788. [Google Scholar] [CrossRef]
- Tiihonen, J.; Haukka, J.; Taylor, M.; Haddad, P.M.; Patel, M.X.; Korhonen, P.A. Nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 2011, 168, 603–609. [Google Scholar] [CrossRef]
- Hui, C.L.M.; Poon, V.W.Y.; Kwok, V.S.K.; Chang, W.C.; Chan, S.K.W.; Lee, E.H.M.; Chen, E.Y.H. Prevalence and predictors of medication non-adherence among Chinese patients with first-episode psychosis. Psychiatry Res. 2015, 228, 680–687. [Google Scholar] [CrossRef]
- Alvarez-Jimenez, M.; Priede, A.; Hetrick, S.E.; Bendall, S.; Killackey, E.; Parker, A.G.; McGorry, P.; Gleeson, J.F. Risk factors for relapse following treatment for first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Schizophr. Res. 2012, 139, 116–128. [Google Scholar] [CrossRef]
- Brown, E.; Gray, R.; Jones, M.; Whitfield, S. Effectiveness of adherence therapy in patients with early psychosis: A mirror image study. Int. J. Ment. Health Nurs. 2013, 22, 24–34. [Google Scholar] [CrossRef]
- Yeisen, R.A.; Bjornestad, J.; Joa, I.; Johannessen, J.O.; Opjordsmoen, S. Experiences of antipsychotic use in patients with early psychosis: A two-year follow-up study. BMC Psychiatry 2017, 17, 299. [Google Scholar] [CrossRef]
- Horvitz-Lennon, M.; Predmore, Z.; Orr, P.; Hanson, M.; Hillestad, R.; Durkin, M.; El Khoury, A.; Mattke, S. The Predicted Long-Term Benefits of Ensuring Timely Treatment and Medication Adherence in Early Schizophrenia. Adm. Policy Ment. Health 2019, 47, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Bedi, G.; McGorry, P.; O’Donoghue, B. Rates and Predictors of Relapse in First-Episode Psychosis: An Australian Cohort Study. Schizophr. Bull. 2020, 1, sgaa017. [Google Scholar] [CrossRef]
- Doyle, R.; Turner, N.; Fanning, F.; Brennan, D.; Renwick, L.; Lawlor, E.; Clarke, M. First-episode psychosis and disengagement from treatment: A systematic review. Psychiatr. Ser. 2014, 65, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Lepage, M.; Bodnar, M.; Buchy, L.; Joober, R.; Malla, A. Early medication adherence and insight change in first-episode psychosis. Clin. Schizophr. Relat. Psychoses 2010, 3, 201–208. [Google Scholar] [CrossRef]
- Abdel-Baki, A.; Ouellet-Plamondon, C.; Malla, A. Pharmacotherapy challenges in patients with first-episode psychosis. J. Affect. Disord. 2012, 138, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Karson, C.; Duffy, R.A.; Eramo, A.; Nylander, A.G.; Offord, S.J. Long-term outcomes of antipsychotic treatment in patients with first-episode schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 57. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Stip, E. Oral versus long-acting injectable antipsychotics in the treatment of schizophrenia and special populations at risk for treatment non-adherence: A systematic review. Schizophr. Res. Treatment. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Kirschner, M.; Theodoridou, A.; Fusar-Poli, P.; Kaiser, S.; Jäger, M. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode of psychosis. Ther. Adv. Psychopharmacol. 2013, 3, 89–99. [Google Scholar] [CrossRef]
- Gray, R.; Bressington, D.; Ivanecka, A.; Hardy, S.; Jones, M.; Schulz, M.; Borman, S.V.; White, J.; Anderson, K.H.; Chien, W.T. Is adherence therapy an effective adjunct treatment for patients with schizophrenia spectrum disorders? A systematic review and meta-analysis. BMC Psychiatry 2016, 16, 90. [Google Scholar] [CrossRef]
- Francey, S.M.; O’Donoghue, B.; Nelson, B.; Graham, J.; Baldwin, L.; Yuen, H.P.; Kerr, M.J.; Ratheesh, A.; Allott, K.; Alvarez-Jimenez, M.; et al. Psychosocial Intervention with or without Antipsychotic Medication for First-Episode Psychosis: A Randomized Noninferiority Clinical Trial. Schizophr. Bull. 2020, 1, sgaa015. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Evans, N.; Lasen, M.; Tsey, K. Appendix A: Effective public health practice project (EPHPP) quality assessment tool for quantitative studies. In A Systematic Review of Rural Development Research: Characteristics, Design Quality and Engagement with Sustainability; Springer: New York, NY, USA, 2015; pp. 45–63. [Google Scholar]
- Uzenoff, S.R.; Perkins, D.O.; Hamer, R.M.; Wiesen, C.A.; Penn, D.L. A preliminary trial of adherence-coping-education (ACE) therapy for early psychosis. J. Nerv. Ment. Dis. 2008, 196, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.; Turkington, D.; Beaumont, J.; Mihailovic, M. Can CBT-based interventions address medication adherence in early phases of schizophrenia? Results from a pilot RCT comparing a CBT-based vs. psychoeducation-based intervention. Schizophr. Bull. 2019, 45 (Suppl. S2), S343–S344. [Google Scholar] [CrossRef]
- Turkington, D.; Kingdon, D.; Rathod, S. Back to Life, Back to Normality, Volume 1: Cognitive Therapy, Recovery and Psychosis; Cambridge University Press: Cambridge, UK, 2009; pp. 1–11. [Google Scholar]
- Wong, H.T. An Android Operating System Based mHealth Intervention on Medication Adherence in Patient with Early Psychosis: A Single-Centre Single blinded Randomised Controlled Trial. 2018. Available online: http://www.chictr.org.cn/showproj.aspx?proj=29377 (accessed on 14 November 2021).
- Kemp, R.; David, A.; Hayward, P. Compliance therapy: An intervention targeting insight and treatment adherence in psychotic patients. Behav. Cogn. Psychother. 1996, 24, 331–350. [Google Scholar] [CrossRef]
- Kemp, R.; Kirov, G.; Everitt, B.; Hayward, P.; David, A. Randomised controlled trial of compliance therapy: 18-month follow-up. Br. J. Psychiatry 1998, 172, 413–419. [Google Scholar] [CrossRef]
- Thompson, K.; Kulkarni, J.; Sergejew, A.A. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr. Res. 2000, 42, 241–247. [Google Scholar] [CrossRef]
- Hogan, T.P.; Awad, A.G.; Eastwood, R. A self-report scale predictive of drug compliance in schizophrenics: Reliability and discriminative validity. Psychol. Med. 1983, 13, 177–183. [Google Scholar] [CrossRef]
- Killikelly, C.; He, Z.; Reeder, C.; Wykes, T. Improving adherence to web-based and mobile technologies for people with psychosis: Systematic review of new potential predictors of adherence. JMIR mHealth uHealth 2017, 5, e94. [Google Scholar] [CrossRef]
- Hartung, D.; Low, A.; Jindai, K.; Mansoor, D.; Judge, M.; Mendelson, A.; Kansagara, D.; Matu’apuaka, M.; Freeman, M.; Kondo, K. Interventions to improve pharmacological adherence among adults with psychotic spectrum disorders and bipolar disorder: A systematic review. Psychosomatics 2017, 58, 101–112. [Google Scholar] [CrossRef]
- El Abdellati, K.; De Picker, L.; Morrens, M. Antipsychotic Treatment Failure: A Systematic Review on Risk Factors and Interventions for Treatment Adherence in Psychosis. Front. Neurosci. 2020, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2018, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Kannisto, K.A.; Adams, C.E.; Koivunen, M.; Katajisto, J.; Välimäki, M. Feedback on SMS reminders to encourage adherence among patients taking antipsychotic medication: A cross-sectional survey nested within a randomised trial. BMJ Open 2015, 5, e008574. [Google Scholar] [CrossRef] [PubMed]
- Barkhof, E.; Meijer, C.J.; de Sonneville, L.M.; Linszen, D.H.; de Haan, L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia–a review of the past decade. Eur. Psychiatr. 2012, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Garety, P.A.; Craig, T.K.; Dunn, G.; Fornells-Ambrojo, M.; Colbert, S.; Rahaman, N.; Reed, J.; Power, P. Specialised care for early psychosis: Symptoms, social functioning and patient satisfaction: Randomized controlled trial. Br. J. Psychiatry 2006, 188, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Oedegaard, C.H.; Davidson, L.; Stige, B.; Veseth, M.; Blindheim, A.; Garvik, L.; Sørensen, J.-M.; Søraa, Ø.; Engebretsen, I.M.S. “It means so much for me to have a choice”: A qualitative study providing first-person perspectives on medication-free treatment in mental health care. BMC Psychiatry 2020, 20, 399. [Google Scholar] [CrossRef]
| Author | Country Where Fieldwork Was Conducted | Clinical Setting | Intervention | Duration of Intervention | Adherence Measures | Primary Endpoint |
|---|---|---|---|---|---|---|
| Uzenoff et al. [22] | United States of America (USA) | Psychiatric Inpatient and Outpatient Clinics | Adherence–Coping–Education Therapy (ACE) vs. Supportive Therapy (ST) | 14 sessions over a six-months period | Patient Reports; “Need for Treatment” “Benefits of Medication “Variables in Rating of Medication Influences Scale and Insight and Treatment Attitudes Questionnaire | Adherence at the end of treatment |
| Brown et al. [8] | United Kingdom (UK) | Early Intervention in Psychosis (EIIP) | Adherence Therapy Training to EIIP Teams | 6 1-day monthly in 6-months | Relapse Rates | Relapse over twelve months from start of study |
| Weiden et al. [23] | United States of America (USA) | Psychosis Disorders Program | Health Dialogue Intervention | 6 to 12 months | All Source Verification (ASV) | Twelve months |
| Wong [25] | China | Psychiatric Outpatient Clinic | Smartphone Application | 3 months | Medication Adherence Rating Scale (MARS); Drug Attitude Inventory (DAI); Pill Count | Three months |
| Author | Trial Registration Status | Number of Participants Randomized | Number of Participants Included in the Analysis | Adherence Outcomes at the End of the Trial |
|---|---|---|---|---|
| Uzenoff et al. [22] | Not reported | n = 24 | n = 19 | ACE participants on benefits of medication scores (d = 0.59) were significantly improved in post-treatment. |
| Brown et al. [8] | Not reported | Mirror Image Study (N/A) | Patient n = 35 | Statistically significant reduction in relapses, equating to a medium effect size (0.33 [95% CI = 1.13–2.66]). |
| Weiden et al. [23] | Not reported | n = 34 | n = 34 | Experimental group stayed on medication longer than PE subjects. (46.7 weeks [95% CI 27.3–66.1] compared to 22.5 [95%CI 9.6–35.5]) |
| Wong [24] | Prospectively registered (ChiCTR1800017286) | n = 100 | Not reported | Not reported |
| Selection Bias | Study Design | Confounders | Blinding | Data Collection Method | Withdrawals/Dropouts | Global Rating | |
|---|---|---|---|---|---|---|---|
| Uzenoff et al. [22] | Strong | Strong | Strong | Moderate | Strong | Strong | Strong |
| Brown et al. [8] | Strong | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Weiden et al. [23] | Moderate | Strong | Weak | Moderate | Moderate | Weak | Weak |
| Wong [24] | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikeç, G.; Brown, E.; Bressington, D.; Thompson, A.; Gray, R. The Safety and Efficacy of Psychosocial Adherence Interventions in Young People with Early Psychosis: A Systematic Review. Healthcare 2022, 10, 1732. https://doi.org/10.3390/healthcare10091732
Dikeç G, Brown E, Bressington D, Thompson A, Gray R. The Safety and Efficacy of Psychosocial Adherence Interventions in Young People with Early Psychosis: A Systematic Review. Healthcare. 2022; 10(9):1732. https://doi.org/10.3390/healthcare10091732
Chicago/Turabian StyleDikeç, Gül, Ellie Brown, Daniel Bressington, Andrew Thompson, and Richard Gray. 2022. "The Safety and Efficacy of Psychosocial Adherence Interventions in Young People with Early Psychosis: A Systematic Review" Healthcare 10, no. 9: 1732. https://doi.org/10.3390/healthcare10091732
APA StyleDikeç, G., Brown, E., Bressington, D., Thompson, A., & Gray, R. (2022). The Safety and Efficacy of Psychosocial Adherence Interventions in Young People with Early Psychosis: A Systematic Review. Healthcare, 10(9), 1732. https://doi.org/10.3390/healthcare10091732










