Plasma Antithrombin Activity during Long-Term Magnesium Sulfate Administration for Preeclampsia without Severe Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MgSO4 Nontreatment Group
2.3. MgSO4 Treatment Group
2.4. Evaluation Points
2.5. Statistical Analysis
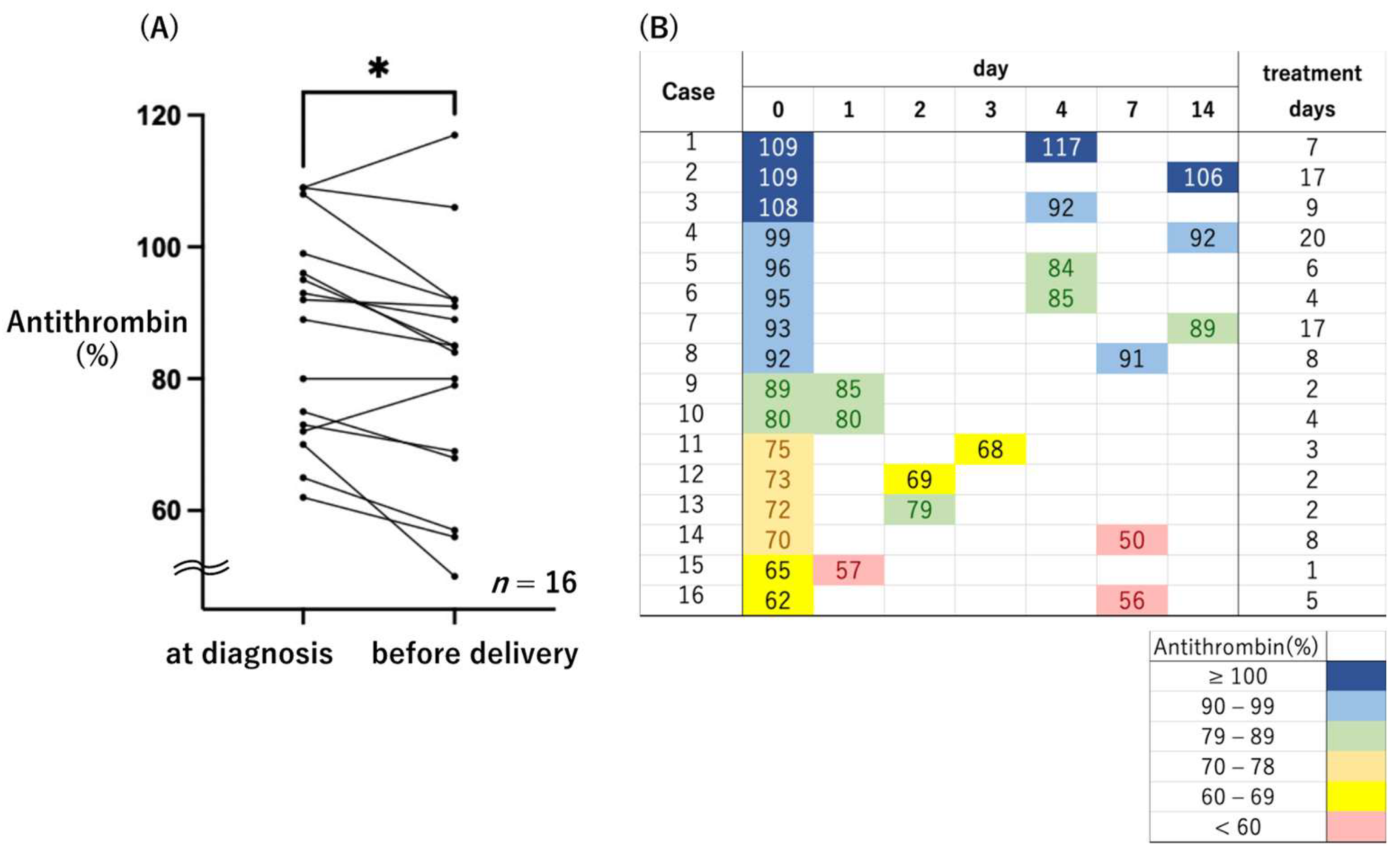
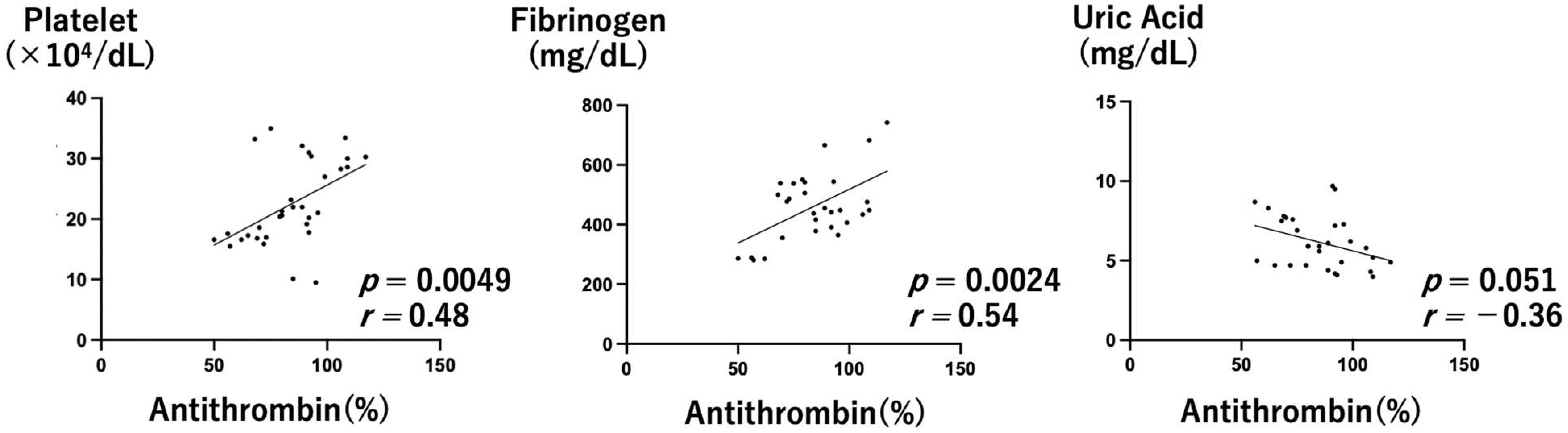
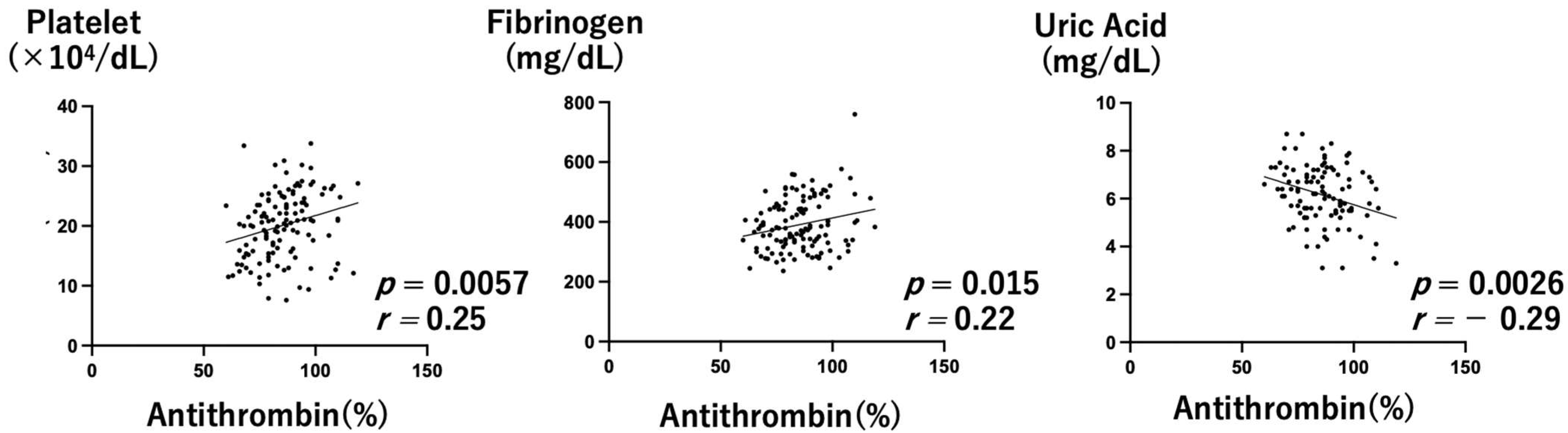
3. Results
3.1. MgSO4 Nontreatment Group
3.2. MgSO4 Treatment Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [Google Scholar] [CrossRef]
- Japan Society of Obstetrics and Gynecology (JSOG). Japan Association of Obstetricians and Gynecologists (JAOG). In Guidelines for Obstetrical Practice in Japan 2020 Edition; JSOG: Tokyo, Japan, 2020. (In Japanese) [Google Scholar]
- NICE. Hypertension in Pregnancy: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- ACOG. Committee Opinion No. 455: Magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet. Gynecol. 2010, 115, 669–671. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Sagara, M.; Mizushima, S.; Liu, L.; Ikeda, K.; Nara, Y.; CARDIAC Study Group. An inverse association between magnesium in 24-h urine and cardiovascular risk factors in middle-aged subjects in 50 CARDIAC Study populations. Hypertens. Res. 2015, 38, 219–225. [Google Scholar] [CrossRef]
- Ueda, A.; Kondoh, E.; Kawasaki, K.; Mogami, H.; Chigusa, Y.; Konishi, I. Magnesium sulphate can prolong pregnancy in patients with severe early-onset preeclampsia. J. Matern. Fetal. Neonatal. Med. 2016, 29, 3115–3120. [Google Scholar] [CrossRef]
- Fu, M.; Liu, J.; Xing, J.; Dai, Y.; Ding, Y.; Dong, K.; Zhang, X.; Yuan, E. Reference intervals for coagulation parameters in non-pregnant and pregnant women. Sci. Rep. 2022, 12, 1519. [Google Scholar] [CrossRef]
- Wickstrom, K.; Edelstam, G.; Lowbeer, C.H.; Hansson, L.O.; Siegbahn, A. Reference intervals for plasma levels of fibronectin, von Willebrand factor, free protein S and antithrombin during third-trimester pregnancy. Scand. J. Clin. Lab. Investig. 2004, 64, 31–40. [Google Scholar] [CrossRef]
- Ramalakshmi, B.A.; Raju, L.A.; Raman, L. Antithrombin III levels in pregnancy induced hypertension. Natl. Med. J. India 1995, 8, 61–62. [Google Scholar]
- Weiner, C.P.; Brandt, J. Plasma antithrombin III activity: An aid in the diagnosis of preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 1982, 142, 275–281. [Google Scholar] [CrossRef]
- Kazushi, W.; Katsuhiko, N.; Kanji, T.; Hirohito, M.; Yoshikatsu, S. Outline of Definition and Classification of “Pregnancy induced Hypertension (PIH)”. Hypertens. Res. Pregnancy 2013, 1, 3–4. [Google Scholar]
- Kazushi, W.; Keiichi, M.; Osamu, N.; Junko, U.; Akihide, O.; Keiko, K.; Shintaro, M.; Kazuya, M.; Mamoru, M.; Katsuhiko, N.; et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens. Res. Pregnancy 2018, 6, 33–37. [Google Scholar]
- Morikawa, M.; Umazume, T.; Hosokawa-Miyanishi, A.; Watari, H.; Kobayashi, T.; Seki, H.; Saito, S. Relationship between antithrombin activity and interval from diagnosis to delivery among pregnant women with early-onset pre-eclampsia. Int. J. Gynaecol. Obstet. 2019, 145, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-recommends-against-prolonged-use-magnesium-sulfate-stop-preterm#:~:text=%5B5%2D30%2D2013%5D,approved%20use%20of%20the%20drug (accessed on 15 July 2022).
- Wen, Y.H.; Wang, I.T.; Lin, F.J.; Hsu, H.Y.; Wu, C.H. Association between the prolonged use of magnesium sulfate for tocolysis and fracture risk among infants. Medicine 2021, 100, e28310. [Google Scholar] [CrossRef]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- Maier, J.A. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kondoh, E.; Chigusa, Y.; Kawamura, Y.; Mogami, H.; Takeda, S.; Horie, A.; Baba, T.; Matsumura, N.; Mandai, M.; et al. Metabolomic Profiles of Placenta in Preeclampsia. Hypertension 2019, 73, 671–679. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019, 17, 283–294. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Hirota, T.; Hiki, M.; Sato, K.; Murakami, T.; Nagaoka, I. Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis. Thromb. Res. 2018, 171, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Muraki, I.; Okada, H.; Tomita, H.; Suzuki, K.; Takada, C.; Wakayama, Y.; Kuroda, A.; Fukuda, H.; Kawasaki, Y.; et al. Recombinant Antithrombin Attenuates Acute Respiratory Distress Syndrome in Experimental Endotoxemia. Am. J. Pathol. 2021, 191, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cagidiaco, M.C.; Breschi, R. Evaluation of resin-bonded retainers with the scanning electron microscope. J. Prosthet. Dent. 1988, 59, 160–165. [Google Scholar] [CrossRef]
- D’Angelo, A.; Valsecchi, L.; Group AT-EPS. High dose antithrombin supplementation in early preeclampsia: A randomized, double blind, placebo-controlled study. Thromb. Res. 2016, 140, 7–13. [Google Scholar] [CrossRef]
- Saito, S.; Takagi, K.; Moriya, J.; Kobayashi, T.; Kanayama, N.; Sameshima, H.; Morikawa, M.; Sago, H.; Adachi, T.; Ohkuchi, A.; et al. A randomized phase 3 trial evaluating antithrombin gamma treatment in Japanese patients with early-onset severe preeclampsia (KOUNO-TORI study): Study protocol. Contemp. Clin. Trials 2021, 107, 106490. [Google Scholar] [CrossRef] [PubMed]

| n = 16 | |
|---|---|
| Patient characteristics | |
| Age (years, mean ± SD) | 30.6 ± 5.9 |
| Nullipara (%) | 15 [93.8%] |
| Onset of PE or SPE (weeks, mean ± SD) | 35.8 ± 2.6 |
| Number of PE (n, (%)) | 14 [87.5%] |
| Onset of PE (weeks, mean ± SD) | 37 ± 1.8 |
| Number of SPE (n, (%)) | 2 [12.5%] |
| Onset of SPE (weeks, mean ± SD) | 33.5 ± 2.1 |
| Obstetric outcomes | |
| Cesarean delivery (%) | 50% |
| Gestational weeks of delivery (weeks, mean ± SD) | 36.9 ± 1.7 |
| Indication for delivery (n, (%)) | |
| Fetal | 2 [12.5%] |
| Maternal | 3 [18.8%] |
| Attainment of 34–37 weeks’ gestation | 11 [68.8%] |
| Major maternal complications (n, (%)) | |
| Placental abruption | 0 |
| HELLP syndrome | 0 |
| Neonatal outcomes | |
| Neonatal and fetal death (%) | 0 |
| Birth weight (g, mean ± SD) | 2486 ± 662 |
| 1 min Apgar score | 7.8 ± 1.2 |
| 5 min Apgar score | 8.8 ± 1.0 |
| Cord pH | 7.27 ± 0.1 |
| n = 34 | |
|---|---|
| Patient characteristics | |
| Age (years, mean ± SD) | 34.8 ± 5.7 |
| Nullipara (%) | 27 [79.4%] |
| Onset of PE or SPE (weeks, mean ± SD) | 29 ± 4.7 |
| Number of PE (n, (%)) | 29 [85.3%] |
| Onset of PE (weeks, mean ± SD) | 29.7 ± 4.4 |
| Number of SPE (n, (%)) | 5 [14.7%] |
| Onset of SPE (weeks, mean ± SD) | 24.8 ± 5.1 |
| Duration of MgSO4 use (days, mean ± SD) | 10.6 ± 7.5 |
| Obstetric outcomes | |
| Cesarean delivery (%) | 73.5 |
| Gestational weeks of delivery (weeks, mean ± SD) | 31.7 ± 4.0 |
| Indication for delivery (n, (%)) | |
| Fetal | 13 [38.2%] |
| Maternal | 15 [44.1%] |
| Attainment of 34–37 weeks’ gestation | 6 [17.6%] |
| Major maternal complications (n, (%)) | |
| Placental abruption | 0 [0%] |
| HELLP syndrome | 3 [8.8%] |
| Perinatal outcomes | |
| Neonatal and fetal death (%) | 0 |
| Birth weight (g, mean ± SD) | 1389 ± 623 |
| 1 min Apgar score | 5.9 ± 2.7 |
| 5 min Apgar score | 7.7 ± 1.9 |
| Cord pH | 7.26 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriuchi, K.; Kawasaki, K.; Hayashi, M.; Ueda, A.; Yamanishi, Y.; Mogami, H.; Fujita, K.; Shiro, R.; Yo, Y.; Mandai, M.; et al. Plasma Antithrombin Activity during Long-Term Magnesium Sulfate Administration for Preeclampsia without Severe Hypertension. Healthcare 2022, 10, 1581. https://doi.org/10.3390/healthcare10081581
Moriuchi K, Kawasaki K, Hayashi M, Ueda A, Yamanishi Y, Mogami H, Fujita K, Shiro R, Yo Y, Mandai M, et al. Plasma Antithrombin Activity during Long-Term Magnesium Sulfate Administration for Preeclampsia without Severe Hypertension. Healthcare. 2022; 10(8):1581. https://doi.org/10.3390/healthcare10081581
Chicago/Turabian StyleMoriuchi, Kaori, Kaoru Kawasaki, Maako Hayashi, Akihiko Ueda, Yukio Yamanishi, Haruta Mogami, Kohei Fujita, Reona Shiro, Yoshie Yo, Masaki Mandai, and et al. 2022. "Plasma Antithrombin Activity during Long-Term Magnesium Sulfate Administration for Preeclampsia without Severe Hypertension" Healthcare 10, no. 8: 1581. https://doi.org/10.3390/healthcare10081581
APA StyleMoriuchi, K., Kawasaki, K., Hayashi, M., Ueda, A., Yamanishi, Y., Mogami, H., Fujita, K., Shiro, R., Yo, Y., Mandai, M., & Matsumura, N. (2022). Plasma Antithrombin Activity during Long-Term Magnesium Sulfate Administration for Preeclampsia without Severe Hypertension. Healthcare, 10(8), 1581. https://doi.org/10.3390/healthcare10081581







