Osteopathic Treatment for Gastrointestinal Disorders in Term and Preterm Infants: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Process
2.3. Eligibility
2.4. Study Selection and Data Collection
2.5. Outcomes
2.6. Assessment of Risk of Bias
2.7. Data Synthesis
3. Results
3.1. Studies Selection
3.2. Description of the Studies
3.3. Outcomes
3.4. Risk of Bias
3.5. Description of Results
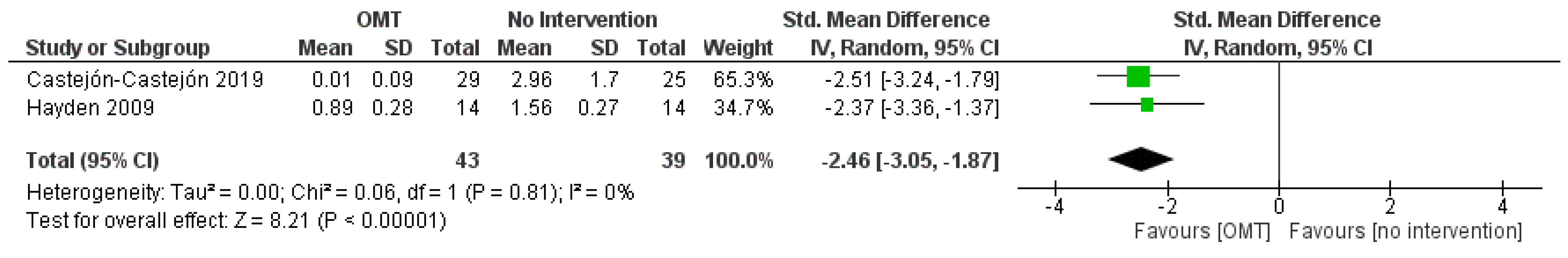
3.6. Effect of Interventions: Quantitative Synthesis
4. Discussion
4.1. Quality of Evidence
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakewell-Sachs, S.; Medoff-Cooper, B.; Escobar, G.J.; Silber, J.H.; Lorch, S.A. Infant functional status: The timing of physiologic maturation of premature infants. Pediatrics 2009, 123, e878–e886. [Google Scholar] [CrossRef] [PubMed]
- Lenfestey, M.W.; Neu, J. Gastrointestinal Development: Implications for Management of Preterm and Term Infants. Gastroenterol. Clin. North Am. 2018, 47, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Cernada, M.; Neu, J.; Pérez-Martínez, G.; Gormaz, M.; Vento, M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr. Res. 2015, 77, 726–731. [Google Scholar] [CrossRef]
- Herzhaft-Le Roy, J.; Xhignesse, M.; Gaboury, I. Efficacy of an Osteopathic Treatment Coupled with Lactation Consultations for Infants' Biomechanical Sucking Difficulties. J. Hum. Lact. 2017, 33, 165–172. [Google Scholar] [CrossRef]
- Bellaiche, M.; Oozeer, R.; Gerardi-Temporel, G.; Faure, C.; Vandenplas, Y. Multiple functional gastrointestinal disorders are frequent in formula-fed infants and decrease their quality of life. Acta. Paediatr. 2018, 107, 1276–1282. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Hauser, B.; Salvatore, S. Functional Gastrointestinal Disorders in Infancy: Impact on the Health of the Infant and Family. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 207–216. [Google Scholar] [CrossRef]
- Zeevenhooven, J.; Browne, P.D.; L'Hoir, M.P.; de Weerth, C.; Benninga, M.A. Infant colic: Mechanisms and management. Nat. Rev. Gastroenterol Hepatol. 2018, 15, 479–496. [Google Scholar] [CrossRef]
- Steutel, N.F.; Zeevenhooven, J.; Scarpato, E.; Vandenplas, Y.; Tabbers, M.M.; Staiano, A.; Benninga, M.A. Prevalence of Functional Gastrointestinal Disorders in European Infants and Toddlers. J. Pediatr. 2020, 221, 107–114. [Google Scholar] [CrossRef]
- Mahon, J.; Lifschitz, C.; Ludwig, T.; Thapar, N.; Glanville, J.; Miqdady, M.; Saps, M.; Quak, S.H.; Wijnkoop, I.L.; Edwards, M.; et al. The costs of functional gastrointestinal disorders and related signs and symptoms in infants: A systematic literature review and cost calculation for England. BMJ Open 2017, 7, e015594. [Google Scholar] [CrossRef]
- Bergna, A.; Vismara, L.; Parravicini, G.; Dal Farra, F. A new perspective for Somatic Dysfunction in Osteopathy: The Variability Model. J. Bodyw. Mov. Ther. 2020, 24, 181–189. [Google Scholar] [CrossRef]
- World Health Organization. Benchmarks for Training in Traditional/Complementary and Alternative Medicine: Benchmarks for Training in Osteopathy; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44356 (accessed on 6 February 2022).
- World Health Organization. ICD-11: International Classification of Diseases. 11th Revision; World Health Organization: Geneva, Switzerland, 2019; Available online: https://icd.who.int/ (accessed on 6 February 2022).
- Vismara, L.; Manzotti, A.; Tarantino, A.; Bianchi, G.; Nonis, A.; La Rocca, S.; Lombardi, E.; Lista, G.; Agosti, M. Timing of oral feeding changes in premature infants who underwent osteopathic manipulative treatment. Complement. Ther. Med. 2019, 43, 49–52. [Google Scholar] [CrossRef]
- Henley, C.E.; Ivins, D.; Mills, M.; Wen, F.K.; Benjamin, B.A. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: A repeated measures study. Osteopath. Med. Prim. Care 2008, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dobson, D.; Lucassen, P.L.; Miller, J.J.; Vlieger, A.M.; Prescott, P.; Lewith, G. Manipulative therapies for infantile colic. Cochrane Database Syst. Rev. 2012, 12, CD004796. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Lee, M.S.; Ernst, E. Osteopathic manipulative treatment for pediatric conditions: A systematic review. Pediatrics 2013, 132, 140–152. [Google Scholar] [CrossRef] [PubMed]
- DeMarsh, S.; Huntzinger, A.; Gehred, A.; Stanek, J.R.; Kemper, K.J.; Belsky, J.A. Pediatric Osteopathic Manipulative Medicine: A Scoping Review. Pediatrics 2021, 147, e2020016162. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane Handbook: Chichester, UK, 2022. [Google Scholar]
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2022. [Google Scholar]
- Garrett, J.; Kriegsman, W. Does Osteopathic Manipulative Treatment (OMT) Improve Length of Hospital Stay and Feeding Dysfunction in Preterm Infants? Evid. Based Pract. 2015, 18, 9. [Google Scholar] [CrossRef]
- Hayden, C.; Mullinger, B. Reprint of: A preliminary assessment of the impact of cranial osteopathy for the relief of infantile colic. Complement. Ther. Clin. Pract. 2009, 15, 198–203. [Google Scholar] [CrossRef]
- Cerritelli, F.; Pizzolorusso, G.; Ciardelli, F.; La Mola, E.; Cozzolino, V.; Renzetti, C.; D’Incecco, C.; Fusilli, P.; Sabatino, G.; Barlafante, G. Effect of osteopathic manipulative treatment on length of stay in a population of preterm infants: A randomized controlled trial. BMC Pediatr. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, F.; Pizzolorusso, G.; Renzetti, C.; Cozzolino, V.; D’Orazio, M.; Lupacchini, M.; Marinelli, B.; Accorsi, A.; Lucci, C.; Lancellotti, J.; et al. A multicenter, randomized, controlled trial of osteopathic manipulative treatment on preterms. PLoS ONE 2015, 10, e0127370. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Pimpel, B.; Kreissl, A.; Jilma, B.; Berger, A. Does visceral osteopathic treatment accelerate meconium passage in very low birth weight infants?—A prospective randomized controlled trial. PLoS ONE 2015, 10, e0123530, Erratum in PLoS ONE 2017, 12, e0187784. [Google Scholar] [CrossRef]
- Castejón-Castejón, M.; Murcia-González, M.; Gil, J.M.; Todri, J.; Rancel, M.S.; Lena, O.; Chillón-Martínez, R. Effectiveness of craniosacral therapy in the treatment of infantile colic. A randomized controlled trial. Complement. Ther. Med. 2019, 47, 102164. [Google Scholar] [CrossRef]
- Jouhier, M.D.; Boscher, C.; Roze, J.-C.; Cailleau, N.; Chaligne, F.; Legrand, A.; Flamant, C.; Muller, J.-B. Osteopathic manipulative treatment to improve exclusive breast feeding at 1 month. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 591–595. [Google Scholar] [CrossRef]
- Mills, M.V. The use of osteopathic manipulative treatment in the newborn nursery and its effect on health in the first six months of life: A retrospective observational case-control study. Complement. Ther. Clin. Pract. 2021, 43, 101357. [Google Scholar] [CrossRef]
- Tramontano, M.; Tamburella, F.; Farra, F.D.; Bergna, A.; Lunghi, C.; Innocenti, M.; Cavera, F.; Savini, F.; Manzo, V.; D’Alessandro, G. International Overview of Somatic Dysfunction Assessment and Treatment in Osteopathic Research: A Scoping Review. Healthcare 2021, 10, 28. [Google Scholar] [CrossRef]
- Browning, K.N. Excitability of nodose ganglion cells and their role in vago-vagal reflex control of gastrointestinal function. Curr. Opin. Pharmacol. 2003, 3, 613–617. [Google Scholar] [CrossRef]
- Cong, X.; Xu, W.; Romisher, R.; Poveda, S.; Forte, S.; Starkweather, A.; Henderson, W.A. Gut Microbiome and Infant Health: Brain-Gut-Microbiota Axis and Host Genetic Factors. Yale J. Biol. Med. 2016, 89, 299–308. [Google Scholar]
- Lu, J.; Claud, E.C. Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol. 2019, 61, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Freeman, B.D.; Natanson, C. Meta-Analysis of Clinical Trials. In Principles and Practice of Clinical Research, 4th ed.; Gallin, J.I., Ognibene, F.P., Johnson, L.L., Eds.; Academic Press; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]

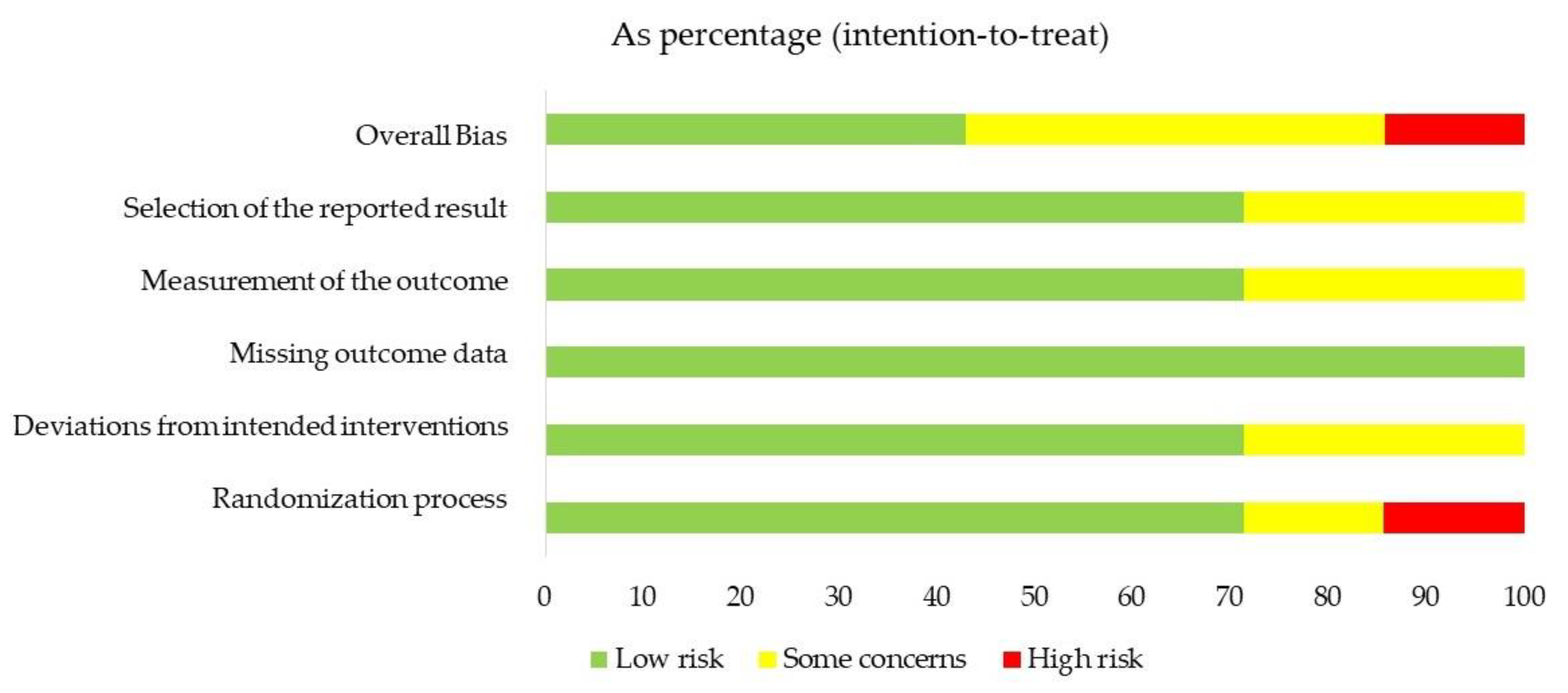
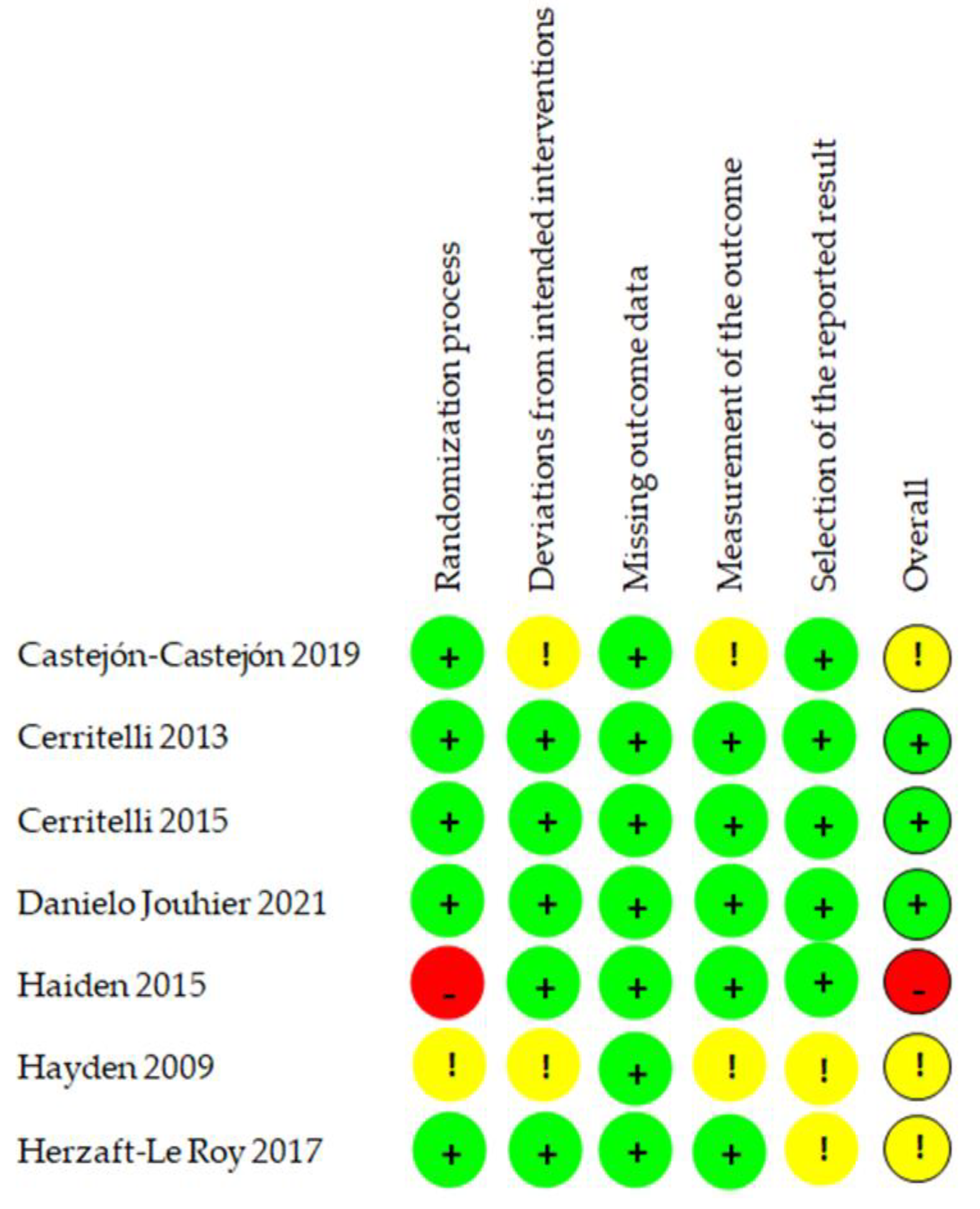
| Author Year | Study Design | Objective | Outcomes/ Variables | Population | Intervention | Comparison |
|---|---|---|---|---|---|---|
| Hayden et al. [25] 2009 | RCT | Efficacy of CST on colic in infants between 1 and 12 weeks of age |
| N = 28 Male: 79% Age at study entry (days): 45.45 ± 5.2 GA at delivery (days): 276 ± 2.35 | OMT (n = 14) Description: 5 sessions of CST for 4 weeks (first session with 60 min duration; other sessions with 30 min duration) | CG (n = 14) Description: no intervention |
| Cerritelli et al. [26] 2013 | RCT | Efficacy of OMT on LOS in preterm infants |
| N = 110 Male: 49% GA (weeks): 34 ± 2.4 Days of life: 3.4 ± 2.4 BW (g): 2161 ± 614.75 | OMT (n = 55) Description: OMT (20 min duration twice per week) plus usual care | CG (n = 55) Description: osteopathic evaluation (10 min of evaluation + 10 min without touch twice per week) plus usual care. |
| Cerritelli et al. [27] 2015 | RCT | Efficacy of OMT on LOS in preterm infants |
| N = 720 Male: 50% GA (weeks): 34.35 ± 2.25 Days of life: 3.65 ± 2.25 BW (g): 2299.5 ± 731.15 | OMT (n = 352) Description: OMT sessions twice a week until discharge plus usual care. OMT sessions lasted 30 min (10 min for evaluation and 20 min for treatment) | CG (n = 343) Description: structural evaluation (10 min of evaluation + 20 min without touch) plus usual care |
| Haiden et al. [28] 2015 | RCT | Efficacy of OMT on meconium passage in very low birth weight preterm infants |
| N = 41 GA (days): 187.5 (165–211) BW (g): 747.5 (441.5–1275) | OMT (n = 21) Description: standardized OMT algorithm within the first 48 h of life and on 3 days during the first week of life | CG (n = 20) Description: no intervention (only standard medical care) |
| Herzaft-Le Roy et al. [4] 2017 | RCT | Efficacy of OMT combined with lactation consultations on infants’ biomechanical sucking difficulties |
| N = 97 Male: 47% Age at study entry (days): 15 ± 10.41 | OMT (n = 49) Description: one session of OMT (30 min duration) plus two lactation consultations | CG (n = 48) Description: one sham manipulation (30 min duration) plus two lactation consultations |
| Castejón-Castejón et al. [29] 2019 | RCT | Effectiveness of OMT on colic in infants |
| N = 58 Male: 50% GA (weeks): 36.41 ± 18 | OMT (n = 29) Description: from one to three sessions of CST at one week distance (the number of sessions depended on the presence or not of colic) | CG (n = 29) Description: no intervention |
| Vismara et al. [13] 2019 | Retrospective cohort study | Effects of OMT on TOF in very/moderately preterm infants |
| N = 70 Male: 50% GA (weeks): 31.65 ± 1.7 BW (g): 1483.75 ± 281.7 | OMT (n = 35) Description: two OMT sessions per week since the first two weeks of life (30 min duration) plus usual care | CG (n = 35) Description: usual care |
| Danielo Jouhier et al. [30] 2021 | RCT | Efficacy of OMT on breast feeding at 1 month |
| N = 128 Male: 52% GA (weeks): 39.70 BW (g): 3466.5 ± 348 | OMT (n = 59) Description: 2 sessions of OMT (the first session before discharge and the second at one week distance) | CG (n = 59) Description: no intervention (manipulation on a doll placed next to the infant) |
| Mills et al. [31] 2021 | Case–control study | Effects of OMT on health in the first 6 months of life |
| N = 116 Male: 53% | OMT (n = 58) Description: one or two sessions, depending on the infant’s LOS (5-10 min duration) | CG (n = 58) Description: no intervention |
| Author Year | Study Design | Description of Interventions | Main Results |
|---|---|---|---|
| Hayden et al. [25] 2009 | RCT | OMT: CST After an evaluation with minimal touch, the techniques were performed until a palpable release of tensions and dysfunction was achieved. CG: no intervention. Same examination of the OMT group. 5 sessions for 4 weeks (initial visit: 60 min; following visits: 30 min). | Colic crying: There was a statistically significant difference between OMT and CG for the mean hours of colic crying in hours/24 h in favor of the OMT group (p < 0.02). AEs: None. |
| Cerritelli et al. [26] 2013 | RCT | OMT: standard medical care + OMT (myofascial release, balanced ligamentous/membranous tension, indirect fluidic and v-spread). CG: standard medical care + osteopathic evaluation. 2 sessions per week, lasting 20 min (10 min for the evaluation and 10 min for the treatment for the OMT group; 10 min for the evaluation and 10 with the osteopath standing in front of the incubator in the control group). | Weight gain: There was no statistically significant association between OMT and the average daily weight gain (p = 0.06); instead, there was a statistically significant association between birth weight and the average daily weight gain (p < 0.001) and between milk volume at study enrollment (mL) and average daily weight gain (p < 0.001). LOS reduction: There was no statistically significant reduction in LOS in the OMT group compared to CG (p < 0.03). AEs: None. |
| Cerritelli et al. [27] 2015 | RCT | OMT: standard medical care + OMT (myofascial release and balanced ligamentous/membranous tension). CG: standard medical care + osteopathic evaluation. The sessions occurred twice per week, lasting 30 min (10 min for the evaluation and 20 min for the treatment for the OMT group and 10 min for the evaluation, and 20 with the osteopath standing in front of the incubator in the control group). | Weight gain: There was no statistically significant association between OMT and the average daily weight gain (p = 0.35); instead, there was a statistically significant association between birth weight and the average daily weight gain (p < 0.01). LOS reduction: There was a statistically significant reduction of 3.9 days in LOS in the OMT group compared to CG (p < 0.01). AEs: None. |
| Haiden et al. [28] 2015 | RCT | OMT: standard medical care + OMT algorithm (global and local listening of the abdomen, release lower ribs and thoracic diaphragm, pylorus relaxation, release of the duodenum and the C-loop, small intestine diagnosis—lifting the gut and bringing it to a stillpoint, mobilization of the ileocecal valve, mobilization of colon ascendens, transversum and descendens with treatment of the Toldt fascia, root of sigmoid diagnosis and manipulation, treatment of the vagus nerve with CST via the sacrum). CG: standard medical care. There were a total of 3 sessions during the first week of life, and the OMT algorithm was repeated 3 times during each session. | Meconium excretion: There was no statistically significant difference between OMT and CG for the first meconium excretion (p = 0.16) and the last meconium excretion (p = 0.11). Feeding amount on the 14th day of life: There was no statistically significant difference between OMT and CG for the feeding amount on the 14th day of life (p = 0.74). Full enteral feeding: There was a statistically significant difference between OMT and CG for the full enteral feeding in favor of CG (p = 0.02); in fact, time to full enteral feedings was 8 days longer in the intervention group (median 34 days, 95% Cl: 30–48 days) than in the control group (median 26 days, 95% Cl: 20–31 days). Weight gain: There was no statistically significant difference between OMT and CG for the weight at discharge (p = 0.58). LOS: No statistically significant difference between OMT and CG for LOS (p = 0.14). AEs: No infant showed signs of cardiorespiratory instability, apnea, or pain. Only 1 infant (4.8%) showed agitation and signs of discomfort; however, after a 5 min break, the infant calmed down, and the treatment was continued without further problems. |
| Herzaft-Le Roy et al. [4] 2017 | RCT | OMT: lactation consultation (emotional support and better positioning of mothers and babies) + OMT (balanced membranous tension, cranial sutures, and myofascial release). CG: lactation consultation (emotional support and better positioning of mothers and babies) + sham OMT (light touch far from the osteopathic dysfunctional areas found). The OMT and sham OMT sessions lasted 30 min, while the lactation consultations lasted 60 min. | Latching: There was a statistically significant difference between OMT and CG for the infants’ ability to latch measured with the LATCH score in favor of the OMT group at Day 3 (p = 0.001). Maternal perceptions concerning feeding: There were statistically significant differences between OMT and CG regarding the infants’ ability to open the mouth widely (p < 0.016), nipple biting (p < 0.042), and the tendency for the infants’ mouth to slip on the nipple (p < 0.002) in favor of the OMT group at Day 3. AEs: None. |
| Castejón-Castejón et al. [29] 2019 | RCT | OMT: CST (balance of the pelvic and thoracic and clavicular diaphragms) + written recommendations on how to take care of a baby with infantile colic. CG: no intervention (only written recommendations on how to take care of a baby with infantile colic, the same provided to the OMT group). Infants in the OMT group received 1 to 3 CST sessions (depending on the presence of colic symptoms) lasting 30–40. The sessions occurred at Day 1 (baseline) and—when required—at Day 7 and Day 14. | Crying: There was a statistically significant difference between OMT and CG for the crying hours in favor of the OMT group at Day 7 (p < 0.0005; d = 1.73), Day 14 (p < 0.0005; d = 2.87) and Day 24 (p < 0.0005; d = 2.54). Moreover, the rANCOVA considering the respective baseline values as covariates showed statistically significant results (p = 0.000). Colic severity: There was a statistically significant difference between OMT and CG for colic severity in favor of the OMT group at Day 7 (p < 0.0005; d = 1.82), Day 14 (p < 0.0005; d = 3.07) and Day 24 (p < 0.0005; d = 3.35). AEs: None. |
| Vismara et al. [13] 2019 | Retrospective cohort study | OMT: standard medical care + OMT (treatment of the myofascial and connective tissues). Treated areas: cranial (cranial techniques) and occipital, the C1-C2-C3 areas, hyoid, sacrum, diaphragm, upper chest, scapulae, left iliac fossa and the structures connected in anatomical and physiological ways to these structures. CG: standard medical care. Sessions started in the first 2 weeks of life with a frequency of twice per week, lasting at least 30 min. | Time to oral feeding: There was a statistically significant difference between OMT and CG for the full oral feeding in favor of the OMT group (p = 0.042). Moreover, a post-hoc analysis with a stratification by body weight at birth showed a statistically significant difference between OMT and CG for the full oral feeding in VLBW infants in favor of the OMT group (p = 0.026); instead, no statistically significant difference was found in LBW infants (p = 0.096). Weight gain: There was no statistically significant difference between OMT and CG for body weight (p = 0.672). LOS: There was no statistically significant difference between OMT and CG for LOS (p = 0.065) AEs: None. |
| Danielo Jouhier et al. [30] 2021 | RCT | OMT: CST, muscular, bones, and/or visceral treatment depending on the found dysfunctional areas. CG: no intervention. The osteopath manipulated a doll placed next to the infant in order to avoid revealing to the mother that the child was not being treated. 2 sessions (the first before discharge and the second 7 days later). | Exclusive breast milk feeding: There was no statistically significant difference between OMT and CG for breast milk feeding neither at 1 month (p = 0.14) nor at 3 (p = 0.55) and 6 months (p = 0.92) in the per-protocol analysis. Moreover, there was no statistically significant difference at 1 month with the intention to treat analysis neither by imputing missing data as a breastfeeding success (p = 0.13) nor by imputing missing data as a breastfeeding failure (p = 0.15). Infant breastfeeding assessment tool (IBFAT): There was no statistically significant difference between OMT and CG for IBFAT at Day 10 (p = 0.3). Weight gain: There was no statistically significant difference between OMT and CG for weight gain at 1 month (p = 0.9). AEs: None. |
| Mills et al. [31] 2021 | Case–control study | OMT: standard medical care + OMT (articulation, direct and indirect myofascial release, balanced membranous tension, and balanced ligamentous tension). CG: standard medical care. 1 or 2 sessions depending on the length of the baby’s hospital stay, lasting 5–10 min. | Spitting/vomiting: There was a statistically significant difference between OMT and CG for spitting/vomiting at month 5 (p = 0.003). Colic suggested: There was a statistically significant difference between OMT and CG for colic suggested at month 3 (p = 0.04). AEs: None. |
| Outcome | SMD (95% CI) | N. of Subjects (Studies) | Comments | Quality of Evidence |
|---|---|---|---|---|
| Hours of crying per day (infantile colic) | −2.46 (−3.05, −1.87) | 82 (2 studies) | Downgraded by 1 level for RoB Downgraded by 1 level for Imprecision | ⊕⊕◯◯ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffone, F.; Monacis, D.; Tarantino, A.G.; Dal Farra, F.; Bergna, A.; Agosti, M.; Vismara, L. Osteopathic Treatment for Gastrointestinal Disorders in Term and Preterm Infants: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1525. https://doi.org/10.3390/healthcare10081525
Buffone F, Monacis D, Tarantino AG, Dal Farra F, Bergna A, Agosti M, Vismara L. Osteopathic Treatment for Gastrointestinal Disorders in Term and Preterm Infants: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(8):1525. https://doi.org/10.3390/healthcare10081525
Chicago/Turabian StyleBuffone, Francesca, Domenico Monacis, Andrea Gianmaria Tarantino, Fulvio Dal Farra, Andrea Bergna, Massimo Agosti, and Luca Vismara. 2022. "Osteopathic Treatment for Gastrointestinal Disorders in Term and Preterm Infants: A Systematic Review and Meta-Analysis" Healthcare 10, no. 8: 1525. https://doi.org/10.3390/healthcare10081525
APA StyleBuffone, F., Monacis, D., Tarantino, A. G., Dal Farra, F., Bergna, A., Agosti, M., & Vismara, L. (2022). Osteopathic Treatment for Gastrointestinal Disorders in Term and Preterm Infants: A Systematic Review and Meta-Analysis. Healthcare, 10(8), 1525. https://doi.org/10.3390/healthcare10081525








