Association of Hypoglycemia with Biomarkers of Oxidative Stress and Antioxidants: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement of Redox Biomarkers
2.3. Statistical Analysis
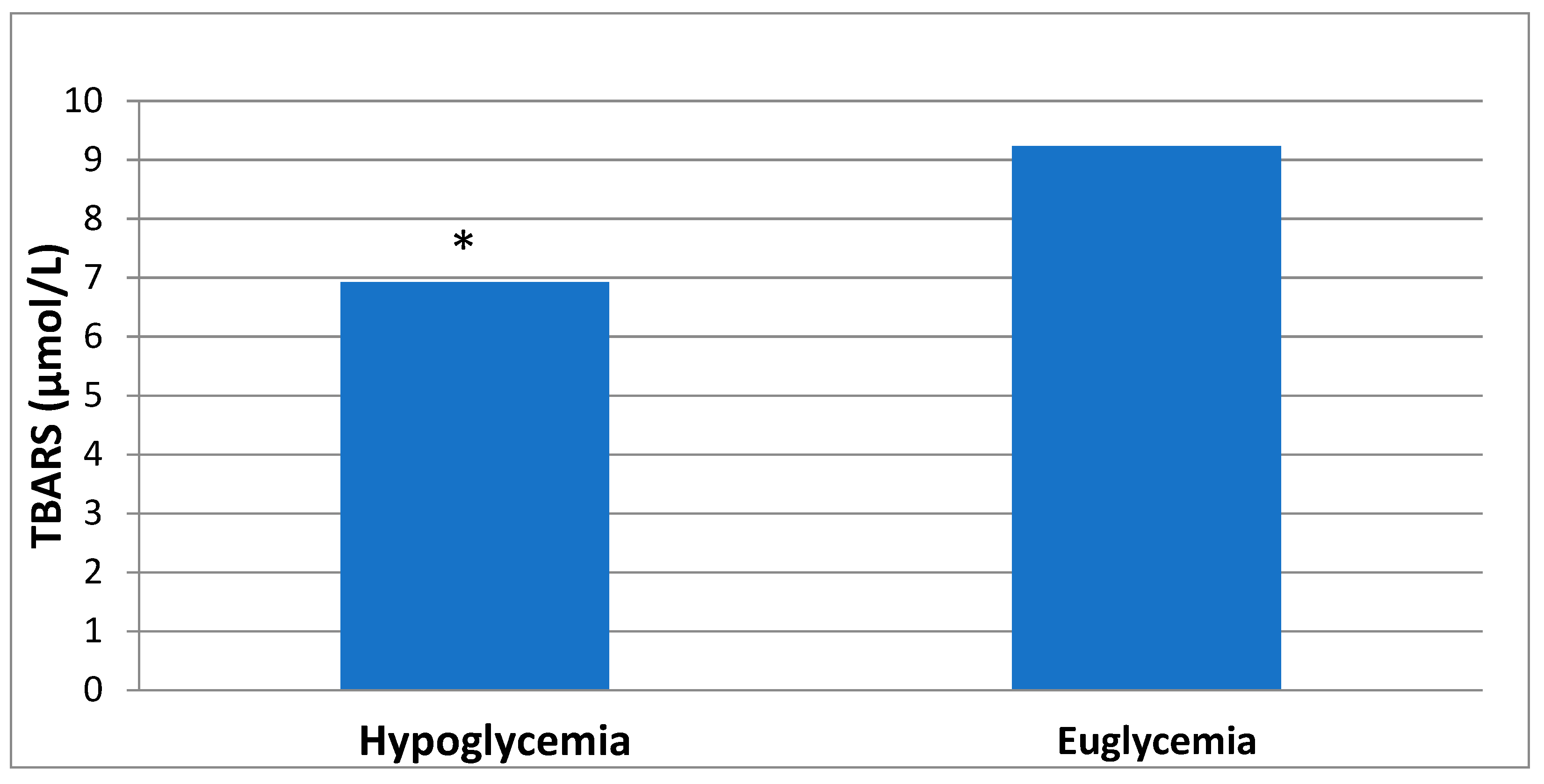
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: A cohort study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seaquist, E.R.; Miller, M.E.; Bonds, D.E.; Feinglos, M.; Goff, D.C.; Peterson, K.; Senior, P. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care 2012, 35, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Amiel, S.A.; Aschner, P.; Childs, B.; Cryer, P.E.; de Galan, B.E.; Frier, B.M.; Gonder-Frederick, L.; Heller, S.R.; Jones, T.; Khunti, K.; et al. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019, 7, 385–396. [Google Scholar] [CrossRef]
- McAulay, V.; Deary, I.J.; Frier, B.M. Symptoms of hypoglycaemia in people with diabetes. Diabet. Med. 2001, 18, 690–705. [Google Scholar] [CrossRef]
- Makrilakis, K.; Stathi, C.; Vlahodimitris, I.; Kalopita, S.; Thomakos, P.; Konstantopoulos, P.; Perrea, D.; Katsilambros, N.; Liatis, S. Hypoglycaemia causes both daytime and nighttime QTc interval prolongation in patients with type 2 diabetes receiving insulin treatment. Diabetes Metab. 2018, 44, 175–177. [Google Scholar] [CrossRef]
- Hutton, R.A.; Mikhailidis, D.; Dormandy, K.M.; Ginsburg, J. Platelet aggregation studies during transient hypoglycaemia: A potential method for evaluating platelet function. J. Clin. Pathol. 1979, 32, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Razavi Nematollahi, L.; Kitabchi, A.E.; Stentz, F.B.; Wan, J.Y.; Larijani, B.A.; Tehrani, M.M.; Gozashti, M.H.; Omidfar, K.; Taheri, E.; Taheri, E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism 2009, 58, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Newby, D.E.; Stirling, D.; Ludlam, C.A.; Macdonald, I.A.; Frier, B.M. Effects of acute insulin-induced hypoglycemia on indices of inflammation: Putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010, 33, 1591–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogitidze Joy, N.; Hedrington, M.S.; Briscoe, V.J.; Tate, D.B.; Ertl, A.C.; Davis, S.N. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010, 33, 1529–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Alexanian, A.; Ying, R.; Kizhakekuttu, T.J.; Dharmashankar, K.; Vasquez-Vivar, J.; Gutterman, D.D.; Widlansky, M.E. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: Role for AMP kinase. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 712–720. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Bofilis, E.; Zoga, A.; Constantinou, M.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. Melatonin does not prevent the protection of ischemic preconditioning in vivo despite its antioxidant effect against oxidative stress. Free Radic. Biol. Med. 2004, 37, 500–510. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Tzortzis, S.; Andreadou, I.; Paraskevaidis, I.; Katseli, C.; Katsimbri, P.; Pavlidis, G.; Parissis, J.; Kremastinos, D.; Anastasiou-Nana, M.; et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ. Cardiovasc. Imaging 2014, 7, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef]
- Daiber, A.; Hahad, O.; Andreadou, I.; Steven, S.; Daub, S.; Münzel, T. Redox-related biomarkers in human cardiovascular disease-classical footprints and beyond. Redox Biol. 2021, 42, 101875. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 2020, e61122. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Yilmaz, F.M.; Aral, Y.; Yucel, D. Levels of serum sialic acid and thiobarbituric acid reactive substances in subjects with impaired glucose tolerance and type 2 diabetes mellitus. J. Clin. Lab. Anal. 2007, 21, 260–264. [Google Scholar] [CrossRef]
- de Toledo, F.W.; Grundler, F.; Goutzourelas, N.; Tekos, F.; Vassi, E.; Mesnage, R.; Kouretas, D. Influence of Long-Term Fasting on Blood Redox Status in Humans. Antioxidants 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.R.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 2021, 768, 145288. [Google Scholar] [CrossRef] [PubMed]
- Khater, S.I.; Mohamed, A.A.R.; Arisha, A.H.; Ebraheim, L.L.M.; El-Mandrawy, S.A.M.; Nassan, M.A.; Mohammed, A.T.; Abdo, S.A. Stabilized-chitosan selenium nanoparticles efficiently reduce renal tissue injury and regulate the expression pattern of aldose reductase in the diabetic-nephropathy rat model. Life Sci. 2021, 279, 119674. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; Abdel-Rahman Mohamed, A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Nassan, M.A.; Hassan, M.E. Chitosan-stabilized selenium nanoparticles and metformin synergistically rescue testicular oxidative damage and steroidogenesis-related genes dysregulation in high-fat diet/streptozotocin-induced diabetic rats. Antioxidants 2021, 10, 17. [Google Scholar] [CrossRef]
- Griesmacher, A.; Kindhauser, M.; Andert, S.E.; Schreiner, W.; Toma, C.; Knoebl, P.; Pietschmann, P.; Prager, R.; Schnack, C.; Schemthaner, G.; et al. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am. J. Med. 1995, 98, 469–475. [Google Scholar] [CrossRef]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-Like Peptide 1 Reduces Endothelial Dysfunction, Inflammation, and Oxidative Stress Induced by Both Hyperglycemia and Hypoglycemia in Type 1 Diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Spirlandeli, A.L.; Deminice, R.; Jordao, A.A. Plasma malondialdehyde as biomarker of lipid peroxidation: Effects of acute exercise. Int. J. Sports Med. 2014, 35, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
| Variable | T1D | T2D | ND | Total |
|---|---|---|---|---|
| Number | 12 | 14 | 5 | 31 |
| Gender (male) [n (%)] | 4 (28.6) | 8 (57.1) | 2 (14.3) | 14 (45.2) |
| Age (years) | 44.3 (17.8) | 60.8 (21.3) | 47.2 (22.2) | 52.2 (21.1) |
| Weight (kg) | 69.0 (15.0) | 81.6 (14.1) | 69.4 (4.8) | 74.7 (14.5) |
| BMI (kg/m²) | 25.1 (4.5) | 29.4 (6.1) | 24.8 (0.9) | 27.0 (5.4) |
| HbA1c (%) | 7.1 (1.1) | 7.9 (1.2) | - | 7.5 (1.22) |
| DM duration (years) | 21.8 (13.9) | 18.3 (9.0) | - | 19.9 (11.4) |
| Glucose Hypo (mmol/L) | 3.12 (0.35) | 2.37 (0.71) | 2.99 (0.31) | 2.76 (0.64) |
| Glucose Eugl (mmol/L) | 5.98 (0.82) | 6.06 (0.89) | 5.47 (1.09) | 5.93 (0.89) |
| eGFR (mL/min/1.73 m²) | 90.4 (36.2) | 86.9 (44.2) | 103.0 (16.8) | 90.9 (37.4) |
| Variable | Hypoglycemia | Euglycemia | p * |
|---|---|---|---|
| ADMA (ng/mL) | 40.41 (17.09) | 44.62 (19.11) | NS |
| MDA (μΜ) | 5.11 (3.87–6.62) | 4.78 (4.11–5.58) | NS |
| 4-HΝΕ (μΜ) | 0.78 (0.58–1.11) | 0.91 (0.71–1.48) | NS |
| Protein carbonyls (nmol/L) | 12.87 (11.12–18.38) | 12.12 (10.14–18.38) | NS |
| Ox-LDL (ug/mL) | 3.05 (0.9) | 2.75 (0.85) | ΝS |
| 3-NT (ng/mL) | 66.14 (30.64) | 68.97 (30.46) | NS |
| TBARS (μmol/L) | 6.55 (4.91–8.92) | 9.23 (6.21–11.72) | 0.005 |
| ABTS (mmol/ABTS/L) | 20.72 (5.5) | 21.32 (5.75) | NS |
| Reducing power (μmol/mL) | 1.11 (1.18) | 1.13 (1.17) | NS |
| TAC (mmol DPPH/L) | 0.86 (0.1) | 0.87 (0.1) | NS |
| Superoxide scavenging capacity (mmol NBT/L) | 1.08 (0.27) | 1.01 (0.27) | NS |
| Hydroxyl-radical scavenging capacity (mmol Deoxyribose/mL) | 0.02 (0.005) | 0.01 (0.001) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papachristoforou, E.; Kountouri, A.; Maratou, E.; Kouretas, D.; Skaperda, Z.; Tsoumani, M.; Efentakis, P.; Ikonomidis, I.; Lambadiari, V.; Makrilakis, K. Association of Hypoglycemia with Biomarkers of Oxidative Stress and Antioxidants: An Observational Study. Healthcare 2022, 10, 1509. https://doi.org/10.3390/healthcare10081509
Papachristoforou E, Kountouri A, Maratou E, Kouretas D, Skaperda Z, Tsoumani M, Efentakis P, Ikonomidis I, Lambadiari V, Makrilakis K. Association of Hypoglycemia with Biomarkers of Oxidative Stress and Antioxidants: An Observational Study. Healthcare. 2022; 10(8):1509. https://doi.org/10.3390/healthcare10081509
Chicago/Turabian StylePapachristoforou, Eleftheria, Aikaterini Kountouri, Eirini Maratou, Dimitris Kouretas, Zoi Skaperda, Maria Tsoumani, Panagiotis Efentakis, Ignatios Ikonomidis, Vaia Lambadiari, and Konstantinos Makrilakis. 2022. "Association of Hypoglycemia with Biomarkers of Oxidative Stress and Antioxidants: An Observational Study" Healthcare 10, no. 8: 1509. https://doi.org/10.3390/healthcare10081509
APA StylePapachristoforou, E., Kountouri, A., Maratou, E., Kouretas, D., Skaperda, Z., Tsoumani, M., Efentakis, P., Ikonomidis, I., Lambadiari, V., & Makrilakis, K. (2022). Association of Hypoglycemia with Biomarkers of Oxidative Stress and Antioxidants: An Observational Study. Healthcare, 10(8), 1509. https://doi.org/10.3390/healthcare10081509









