COVID-19 Intensive Care—Evaluation of Public Information Sources and Current Standards of Care in German Intensive Care Units: A Cross Sectional Online Survey on Intensive Care Staff in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Participants
2.2. Questionnaire
2.3. Data Analysis
3. Results
3.1. Participants
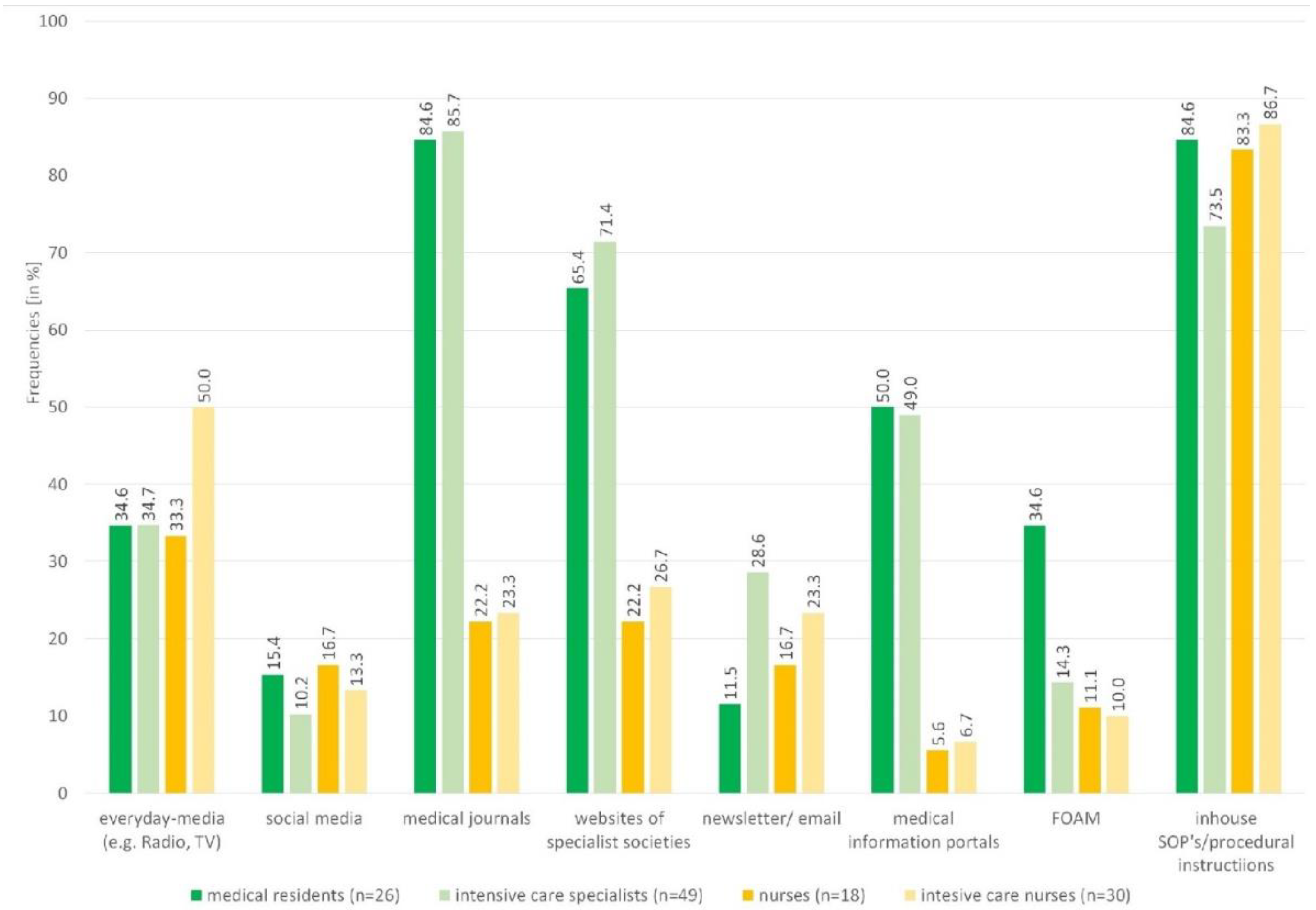
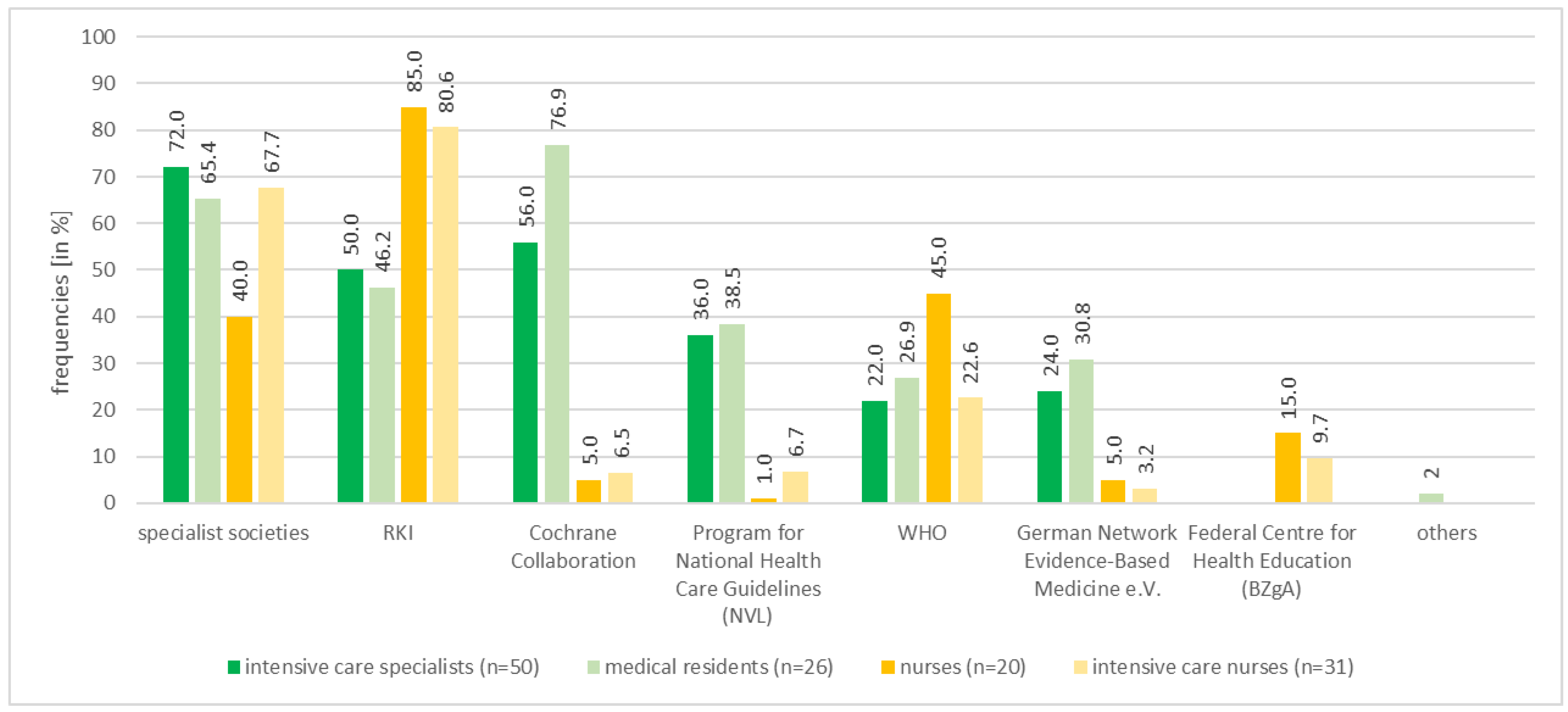
3.2. Use, Quality, Barriers and Trust in Information Sources
3.3. Evaluation of the Guideline, MagicApp and CEOsys Website
3.4. Compliance in Treatment Standards
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID Evidenz Ökosystem. Available online: https://covid-evidenz.de/ (accessed on 1 June 2022).
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie-Empfehlungen Zur Stationären Therapie von Patienten Mit COVID-19; Stand 23.02.2021; Available online: https://gth-online.org/wp-content/uploads/2021/02/AWMF-S3-LL.pdf (accessed on 1 June 2022).
- DIVI-Intensivregister. Zeitreihen zu Fallzahlen und Intensivkapazitäten der Erwachsenen-Intensivstationen. Available online: https://www.intensivregister.de/#/aktuelle-lage/zeitreihen (accessed on 1 June 2022).
- Seeber, C.; Popp, M.; Meerpohl, J.J.; Fichtner, F.; Werner, A.; Schmaderer, C.; Holzmann-Littig, C.; Dickel, S.; Grimm, C.; Moerer, O.; et al. COVID-19 pandemic: Preferences and barriers for dissemination of evidence syntheses: Survey of intensive care personnel in Germany. Der Anaesthesist 2021, 71, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. Improving the quality of Web surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef] [PubMed]
- Leiner, D.J. SoSci Survey (Version 3.1.06) [computer software]. Available online: https://www.soscisurvey.de (accessed on 1 June 2022).
- Johnson, T.P. Handbook of Health Survey Methods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Dickel, S.; Grimm, C.; Popp, M.; Struwe, C.; Sachkova, A.; Golinski, M.; Seeber, C.; Fichtner, F.; Heise, D.; Kranke, P.; et al. A Nationwide Cross-Sectional Online Survey on the Treatment of COVID-19-ARDS: High Variance in Standard of Care in German ICUs. J. Clin. Med. 2021, 10, 3363. [Google Scholar] [CrossRef] [PubMed]
- COVID-19: MAGIC Making a Difference and MAGICapp Now Available to Develop Living Guidelines. Available online: https://magicevidence.org/ (accessed on 1 June 2022).
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie-Empfehlungen Zur Stationären Therapie von Patienten Mit COVID-19; Stand 05.10.2021; Available online: https://www.divi.de/aktuelle-meldungen-intensivmedizin/neue-empfehlungen-zur-stationaeren-therapie-von-covid-19-erkrankten (accessed on 1 June 2022).
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie-Empfehlungen Zur Stationären Therapie von Patienten Mit COVID-19; Stand. 17.05.2021; Available online: https://www.grc-org.de/files/ArticleFiles/document/S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19__17.05.2021.pdf (accessed on 1 June 2022).
- Cheese, F.; Coulton, H. Predicting the COVID-19 Pandemic: The Perceptions of Healthcare Workers and the General Public. Cureus 2021, 13, e12615. [Google Scholar] [CrossRef] [PubMed]
- Tunnecliff, J.; Ilic, D.; Morgan, P.; Keating, J.; Gaida, J.E.; Clearihan, L.; Sadasivan, S.; Davies, D.; Ganesh, S.; Mohanty, P.; et al. The acceptability among health researchers and clinicians of social media to translate research evidence to clinical practice: Mixed-methods survey and interview study. J. Med. Internet Res. 2015, 17, e119. [Google Scholar] [CrossRef] [Green Version]
- Falcone, R.; Sapienza, A. How COVID-19 Changed the Information Needs of Italian Citizens. Int. J. Environ. Res. Public Health 2020, 17, 6988. [Google Scholar] [CrossRef]
- Jordan, P.; Mpasa, F.; Ham-Baloyi, W.T.; Bowers, C. Implementation strategies for guidelines at ICUs: A systematic review. Int. J. Health Care Qual. Assur. 2017, 30, 358–372. [Google Scholar] [CrossRef]
- Mostofian, F.; Ruban, C.; Simunovic, N.; Bhandari, M. Changing physician behavior: What works? Am. J. Manag. Care 2015, 21, 75–84. [Google Scholar]
- NHS Centre for Reviews and Dissemination. Getting Evidence into Practice. Available online: https://www.york.ac.uk/media/crd/ehc51.pdf (accessed on 1 June 2022).
- Narayanaswami, P.; Gronseth, G.; Dubinsky, R.; Penfold-Murray, R.; Cox, J.; Bever, C.; Martins, Y.; Rheaume, C.; Shouse, D.; Getchius, T.S.D. The Impact of Social Media on Dissemination and Implementation of Clinical Practice Guidelines: A Longitudinal Observational Study. J. Med. Internet Res. 2015, 17, e193. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.K.; Boreskie, P.; Luckett-Gatopoulos, S.; Gysel, L.; Lanktree, M.B.; Chan, T.M. Quality Appraisal and Assurance Techniques for Free Open Access Medical Education (FOAM) Resources: A Rapid Review. Semin. Nephrol. 2020, 40, 309–319. [Google Scholar] [CrossRef]
- Thurtle, N.; Banks, C.; Cox, M.; Pain, T.; Furyk, J. Free Open Access Medical Education resource knowledge and utilisation amongst Emergency Medicine trainees: A survey in four countries. Afr. J. Emerg. Med. Rev. Afr. De La Med. D’urgence 2016, 6, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownson, R.C.; Eyler, A.A.; Harris, J.K.; Moore, J.B.; Tabak, R.G. Getting the Word Out: New Approaches for Disseminating Public Health Science. Journal of public health management and practice. JPHMP 2018, 24, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabana, M.D.; Rand, C.S.; Powe, N.R.; Wu, A.W.; Wilson, M.H.; Abboud, P.A.; Rubin, H.R. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999, 282, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.M.; Eccles, M.P.; Lavis, J.N.; Hill, S.J.; Squires, J.E. Knowledge translation of research findings. Implement. Sci. IS 2012, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Lange, K.; Klose, K.; Greiner, W.; Kraemer, A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umsetzung von Leitlinien–hinderliche und förderliche Faktoren. Available online: https://www.iqwig.de/download/v12-04_abschlussbericht_umsetzung-von-leitlinien.pdf (accessed on 1 June 2022).
- Price, L.C.; McCabe, C.; Garfield, B.; Wort, S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020, 56, 2001608. [Google Scholar] [CrossRef]
- McCormack, B.; Kitson, A.; Harvey, G.; Rycroft-Malone, J.; Titchen, A.; Seers, K. Getting evidence into practice: Mean. ‘context’. J. Adv. Nurs. 2002, 38, 94–104. [Google Scholar] [CrossRef]
- Schubert, I. Implementierung von Leitlinien: Ansätze zur Evaluation:Vortrag auf der Arbeitstagung AWMF-DNVF; Workshop, Leitlinien, Attraktivität, Implementierung und Evaluation: Frankfurt, Germany, 2012. [Google Scholar]
- Kirchner, H.; Fiene, M.; Ollenschläger, G. Bewertung und Implementierung von Leitlinien. Die Rehabil. 2003, 42, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Ina Kopp. Messen von Leitlinienkonformität-Was Bedeutet Das? Workshop, Leitlinien, Attraktivität, Implementierung und Evaluation: Frankfurt, Germany, 2012. [Google Scholar]
- Kumpf, O.; Braun, J.-P.; Brinkmann, A.; Bause, H.; Bellgardt, M.; Bloos, F.; Dubb, R.; Greim, C.; Kaltwasser, A.; Marx, G.; et al. Quality indicators in intensive care medicine for Germany-third edition 2017. Ger. Med. Sci. GMS E-J. 2017, 15, 10. [Google Scholar] [CrossRef]
- Wardropper, C.B.; Dayer, A.A.; Goebel, M.S.; Martin, V.Y. Conducting conservation social science surveys online. Conserv. Biol. J. Soc. Conserv. Biol. 2021, 35, 1650–1658. [Google Scholar] [CrossRef]
- Rosenberg, H.; Syed, S.; Rezaie, S. The Twitter pandemic: The critical role of Twitter in the dissemination of medical information and misinformation during the COVID-19 pandemic. CJEM 2020, 22, 418–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Medical Journals | |||||
|---|---|---|---|---|---|
| International Journals | Physicians | Nursing Staff | Physicians | Nursing Staff | |
| n | n | German Journals | n | n | |
| NEJM | 21 | 1 | Deutsches Ärzteblatt | 16 | |
| The Lancet | 17 | AINS | 11 | ||
| JAMA | 15 | Anästhesiologie & Intensivmedizin | 10 | ||
| Critical Care | 7 | Der Anästhesist | 6 | 1 | |
| Intensive Care Medicine | 7 | Intensiv | 2 | ||
| The BMJ | 3 | Die Schwester der Pfleger | 2 | ||
| Critical Care Medicine | 3 | Notfall + Rettungsmedizin | 1 | ||
| European Journal of Anaesthesiology | 2 | PflegenIntensiv | 1 | ||
| Anesthesiology | 2 | Intensivpflege | 1 | ||
| Nature | 2 | Intensiv news | 1 | ||
| Anesthesia & Analgesia | 1 | ||||
| AJRCCM | 1 | ||||
| Journal of Intensiv Care | 1 | ||||
| NEJM The New England Journal of Medicine, JAMA Journal of the American Medical Association, AJRCCM American Journal of Respiratory and Critical Care Medicine, Anesthesiology, AINS Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie | |||||
| Websites of scientific medical societies a | n | ||||
| DIVI | 27 | ||||
| DGAI | 20 | ||||
| AWMF | 13 | ||||
| DIVI German Interdisciplinary Association for Intensive and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin), DGAI German Society of Anaesthesiology and Intensive Care Medicine (Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin), AWMF Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V.), | |||||
| everyday-media b | |||||
| Public broadcasters (e.g., ARD, ZDF, BBC) | 14 | ||||
| Daily/weekly newspapers (incl. online editions) (e.g., Der Spiegel, New York Times, FAZ, Die Zeit, regional daily newspaper, Süddeutsche Zeitung) | 14 | ||||
| TV in general | 12 | ||||
| Social media c | |||||
| 6 | |||||
| 5 | |||||
| 2 | |||||
| FOAM d | |||||
| EmCrit.org | 6 | ||||
| nerdfallmedizin.blog | 6 | ||||
| pin-up-docs.de | 4 | ||||
| Medical information portals e | |||||
| www.uptodate.com | 14 | ||||
| AWMF | 7 | ||||
| AMBOSS | 5 | ||||
| Physicians (n = 76) | Nursing Staff (n = 51) | Total (n = 127) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Use | Quality * | Use | Quality * | Use | Quality * | ||||
| % | MD | IQR | % | MD | IQR | % | MD | IQR | |
| Social media | 12.0 | 1 | 0–3 | 16.6 | 2 | 0–3 | 13.0 | 1 | 0–3 |
| Everyday media (e.g., Radio, TV) | 34.7 | 2 | 1–4 | 43.8 | 4 | 1–6 | 38.2 | 2.5 | 1–5 |
| Newsletter/email | 22.7 | 5 | 4–7 | 20.8 | 7 | 6–8 | 22.0 | 6 | 4–8 |
| FOAM | 21.3 | 7 | 5–7.5 | 10.4 | 7 | 5–8 | 17.1 | 7 | 5–8 |
| Medical information portals | 49.3 | 7 | 6–9 | 6.3 | 8 | 6–8.75 | 32.5 | 7 | 6–9 |
| Websites of the scientific medical societies | 69.3 | 8 | 7–9 | 25.0 | 8 | 6–9 | 52.0 | 8 | 7–9 |
| Medical journals | 85.3 | 8 | 8–9 | 22.9 | 8 | 7–10 | 61.0 | 8 | 7–9 |
| Inhouse SOP’s/procedural instructions | 77.3 | 8 | 7–9 | 85.4 | 8 | 7–9 | 80.5 | 8 | 7–9 |
| p | r | |
|---|---|---|
| Social media (n = 98) | <0.001 * | −0.49 |
| Everyday media (e.g., Radio, TV) (n = 116) | <0.001 * | −0.43 |
| Newsletter/email (n = 94) | <0.001 * | −0.38 |
| FOAM (n = 56) | <0.001 * | −0.45 |
| Medical information portals (n = 87) | 0.075 | −0.19 |
| Websites of the scientific medical societies (n = 100) | 0.012 | −0.25 |
| Medical journals (n = 106) | 0.006* | −0.27 |
| Inhouse SOP’s/procedural instructions (n = 119) | 0.206 | −0.12 |
| Disturbance of Conscious-ness | Respiratory Rate | Clinical Assessment of Respiratory Work | Rapid-Shallow-Breathing-Index | CO2-Elimination Disorder | Horovitz-Index/Oxygenationindex | Work-of-Breathing-Index | I Don’t Know | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | |
| % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| Medical residents | 78.3 | 72.5 | 47.8 | 40.6 | 87.0 | 62.3 | 4.3 | 10.1 | 56.5 | 53.6 | 91.3 | 66.7 | 13.0 | 11.6 | 4.3 | 8.7 |
| Intensive care specialists | 81.0 | 82.1 | 61.9 | 53.0 | 61.9 | 65.8 | 16.7 | 13.7 | 66.7 | 62.4 | 69.0 | 70.1 | 21.4 | 12.0 | 4.8 | 2.6 |
| Nurses * | 83.3 | 61.5 | 8.3 | 28,2 | 50.0 | 46.2 | 16.7 | 5.1 | 25.0 | 51.3 | 75.0 | 48.7 | 16.7 | 25.6 | 8.3 | 12.8 |
| Intensive care nurses | 74.1 | 58.2 | 11.1 | 44.3 | 77.8 | 51.9 | 7.4 | 20.3 | 51.9 | 51.9 | 66.7 | 55.7 | 18.5 | 21.5 | 3.7 | 7.6 |
| Total | 78.8 | 71.1 | 39.4 | 44.7 | 70.2 | 58.9 | 11.5 | 13.5 | 55.8 | 56.3 | 74.0 | 62.8 | 18.3 | 16.1 | 4.8 | 6.5 |
| Leading ICU physicians 2020 [7] | 87.9 | 81.8 | 85.5 | 27.9 | 77.6 | 82.4 | 13.9 | |||||||||
| Recommended in Guideline… [1,8,9] | Survey 2021 a | Rationale for Decision Making in % (for n Selections) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Feb 2021 | May 2021 | Oct 2021 | n (%) | Evidence Based S3 Guideline | Own Literature Research | Determined by Supervisors | Good Experience with This Medication | I Do Not Know Why | |
| Corticosteroids (e.g., dexa-methasone) | p | p | p | 97 (93.3) | 63.9 | 3.1 | 23.7 | 2.2 | 7.2 |
| IL-6 receptor blockers (e.g., tocilizumab, sarilumab) | n | p *** | p | 29 (27.9) | 70 | 10 | 13. 3 | - | 6.7 |
| Vitamin D | n | n | n | 24 (23.4) | 33.3 | 12.5 | 41.7 | - | 12.5 |
| Specific antibodies | n * | n * | n ** | 23 (22.1) | 54.5 | 18.2 | 18. 2 | - | 9.1 |
| Remdesivir | - | nn | nn | 17 (16.3) | 47.1 | 17.6 | 17.6 | 5.9 | 11.8 |
| JAK inhibitors (e.g., baricitinib) | - | - | p | 9 (8.7) | 66.7 | 11.6 | 22.2 | - | - |
| Convalescent plasma | n | n | n | 3 (2.9) | 33.3 | 0 | 66.7 | - | - |
| Ivermectin | n | n | n | 2 (1.9) | 50 | 50 | - | - | - |
| Hydroxy-chloroquine | n | n | - | 1 (1.0) | 0 | 100 | - | - | - |
| Lopinavir/ritonavir | n | n | - | 2 (1.9) | 50 | 50 | - | - | - |
| others b | 6 (5.8) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, A.; Popp, M.; Fichtner, F.; Holzmann-Littig, C.; Kranke, P.; Steckelberg, A.; Lühnen, J.; Redlich, L.M.; Dickel, S.; Grimm, C.; et al. COVID-19 Intensive Care—Evaluation of Public Information Sources and Current Standards of Care in German Intensive Care Units: A Cross Sectional Online Survey on Intensive Care Staff in Germany. Healthcare 2022, 10, 1315. https://doi.org/10.3390/healthcare10071315
Werner A, Popp M, Fichtner F, Holzmann-Littig C, Kranke P, Steckelberg A, Lühnen J, Redlich LM, Dickel S, Grimm C, et al. COVID-19 Intensive Care—Evaluation of Public Information Sources and Current Standards of Care in German Intensive Care Units: A Cross Sectional Online Survey on Intensive Care Staff in Germany. Healthcare. 2022; 10(7):1315. https://doi.org/10.3390/healthcare10071315
Chicago/Turabian StyleWerner, Anne, Maria Popp, Falk Fichtner, Christopher Holzmann-Littig, Peter Kranke, Anke Steckelberg, Julia Lühnen, Lisa Marie Redlich, Steffen Dickel, Clemens Grimm, and et al. 2022. "COVID-19 Intensive Care—Evaluation of Public Information Sources and Current Standards of Care in German Intensive Care Units: A Cross Sectional Online Survey on Intensive Care Staff in Germany" Healthcare 10, no. 7: 1315. https://doi.org/10.3390/healthcare10071315
APA StyleWerner, A., Popp, M., Fichtner, F., Holzmann-Littig, C., Kranke, P., Steckelberg, A., Lühnen, J., Redlich, L. M., Dickel, S., Grimm, C., Moerer, O., Nothacker, M., & Seeber, C. (2022). COVID-19 Intensive Care—Evaluation of Public Information Sources and Current Standards of Care in German Intensive Care Units: A Cross Sectional Online Survey on Intensive Care Staff in Germany. Healthcare, 10(7), 1315. https://doi.org/10.3390/healthcare10071315






