Implementation and Evaluation of Two Nudges in a Hospital’s Electronic Prescribing System to Optimise Cost-Effective Prescribing
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Context
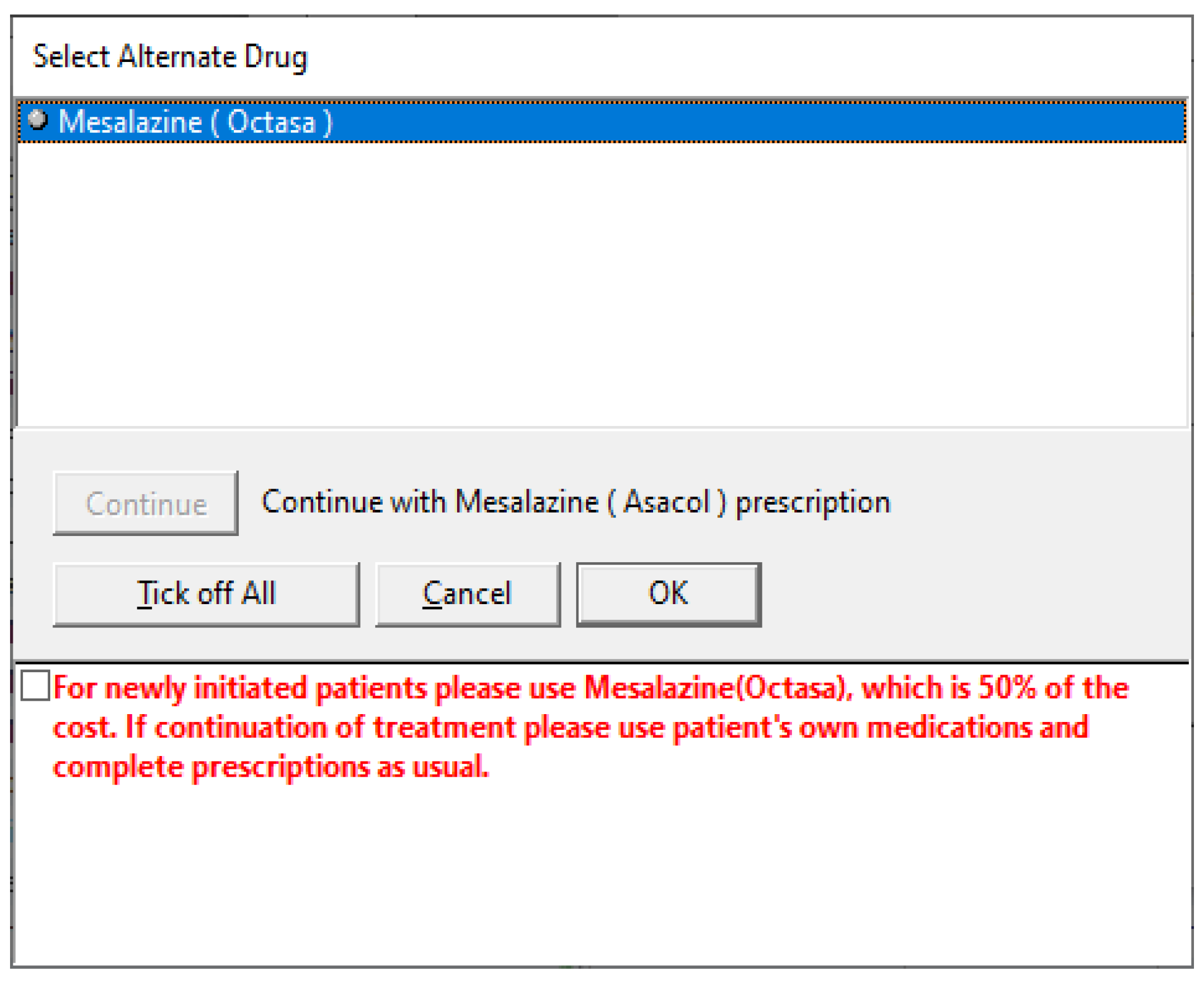
2.3. Development of Interventions
2.4. Implementation of the Interventions
2.5. Outcomes and Measurements
2.6. Statistical Analysis
3. Results
3.1. Usage of Asacol®
3.1.1. Simple Cost Nudge
3.1.2. Aggregated Cost Nudge
3.2. Octasa® Usage
3.2.1. Simple Cost Nudge
3.2.2. Aggregated Cost Nudge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health and Social Care Information Centre. Prescribing Costs in Hospital and the Community: England 2019/20; NHS Digital: London, UK, 2020.
- Martini, E.M.; Garrett, N.; Lindquist, T.; Isham, G.J. The Boomers Are Coming: A Total Cost of Care Model of the Impact of Population Aging on Health Care Costs in the United States by Major Practice Category. Health Serv. Res. 2007, 42, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Sood, A.; Ghosh, A.K. Non-Evidence-Based Variables Afecting Physicians’ Test-Ordering Tendencies: A Systematic Review. Neth. J. Med. 2007, 65, 167–177. [Google Scholar] [PubMed]
- Fogarty, A.W.; Sturrock, N.; Premji, K.; Prinsloo, P. Hospital Clinicians’ Responsiveness to Assay Cost Feedback: A Prospective Blinded Controlled Intervention Study. JAMA Intern. Med. 2013, 173, 1654–1655. [Google Scholar] [CrossRef]
- Evans, J.S.B.T. In Two Minds: Dual-Process Accounts of Reasoning. Trends Cogn. Sci. 2003, 7, 454–459. [Google Scholar] [CrossRef] [PubMed]
- PrescQIPP. Bulletin 249: Branded Mesalazine. 2018. Available online: https://www.prescqipp.info/our-resources/bulletins/bulletin-249-branded-mesalazine/ (accessed on 10 April 2022).
- Sinnott, C.; Mercer, S.W.; Payne, R.A.; Duerden, M.; Bradley, C.P.; Byrne, M. Improving medication management in multimorbidity: Development of the MultimorbiditY COllaborative Medication Review And DEcision making (MY COMRADE) intervention using the Behaviour Change Wheel. Implement. Sci. 2015, 10, 132. [Google Scholar] [CrossRef]
- Schmidtke, K.A.; Nightingale, P.G.; Reeves, K.; Gallier, S.; Vlaev, I.; Watson, S.I.; Lilford, R.J. Randomised controlled trial of a theory-based intervention to prompt front-line staff to take up the seasonal influenza vaccine. BMJ Qual. Saf. 2020, 29, 189–197. [Google Scholar] [CrossRef]
- University Hospitals Birmingham NHS Foundation Trust. Queen Elizabeth Hospital Birmingham. 2022. Available online: https://www.uhb.nhs.uk/about/sites/queen-elizabeth-hospital-birmingham.htm (accessed on 7 April 2022).
- Birmigham City Council. Index of Deprivation 2019. 2022. Available online: https://www.birmingham.gov.uk/downloads/file/2533/index_of_deprivation_2019 (accessed on 12 April 2022).
- University Hospitals Birmingham NHS Foundation Trust. Informatics. 2022. Available online: http://www.tya.uhb.nhs.uk/international-informatics.htm (accessed on 7 April 2022).
- Michie, S.; Atkins, L.; West, R. The behaviour change wheel. In A Guide to Designing Interventions, 1st ed.; Silverback Publishing: Great Britain, UK, 2014; pp. 1003–1010. [Google Scholar]
- Barker, F.; Atkins, L.; de Lusignan, S. Applying the COM-B behaviour model and behaviour change wheel to develop an intervention to improve hearing-aid use in adult auditory rehabilitation. Int. J. Audiol. 2016, 55, S90–S98. [Google Scholar] [CrossRef]
- Courtenay, M.; Rowbotham, S.; Lim, R.; Peters, S.; Yates, K.; Chater, A. Examining influences on antibiotic prescribing by nurse and pharmacist prescribers: A qualitative study using the theoretical domains framework and COM-B. BMJ Open 2019, 9, e029177. [Google Scholar] [CrossRef]
- Félix, I.B.; Guerreiro, M.P.; Cavaco, A.; Cláudio, A.P.; Mendes, A.; Balsa, J.; Carmo, M.B.; Pimenta, N.; Henriques, A. Development of a complex intervention to improve adherence to antidiabetic medication in older people using an anthropomorphic virtual assistant software. Front. Pharmacol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Ojo, S.O.; Bailey, D.P.; Brierley, M.L.; Hewson, D.J.; Chater, A.M. Breaking barriers: Using the behavior change wheel to develop a tailored intervention to overcome workplace inhibitors to breaking up sitting time. BMC Public Health 2019, 19, 1126. [Google Scholar] [CrossRef]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, K.H.; Hauser, A.; Hauser, M.A. Package CausalImpact; Google LLC: Mountain View, CA, USA, 2017. [Google Scholar]
- Ding, W.; Li, Y.; Bai, Y.; Li, Y.; Wang, L.; Wang, Y. Estimating the effects of the COVID-19 outbreak on the reductions in tuberculosis cases and the epidemiological trends in China: A causal impact analysis. Infect. Drug Resist. 2021, 14, 4641. [Google Scholar] [CrossRef]
- Kurz, C.F.; Rehm, M.; Holle, R.; Teuner, C.; Laxy, M.; Schwarzkopf, L. The effect of bariatric surgery on health care costs: A synthetic control approach using Bayesian structural time series. Health Econ. 2019, 28, 1293–1307. [Google Scholar] [CrossRef]
- Pinilla, J.; Barber, P.; Vallejo-Torres, L.; Rodríguez-Mireles, S.; López-Valcárcel, B.G.; Serra-Majem, L. The economic impact of the SARS-COV-2 (COVID-19) pandemic in Spain. Int. J. Environ. Res. Public Health 2021, 18, 4708. [Google Scholar] [CrossRef] [PubMed]
- Takyi, P.O.; Bentum-Ennin, I. The impact of COVID-19 on stock market performance in Africa: A Bayesian structural time series approach. J. Econ. Bus. 2021, 115, 105968. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Taylor, D.; Ovchinikova, L.; Heaney, A.; Morgan, T.; Dartnell, J.; Holbrook, R.; Humphreys, L.; Weekes, L.; Blogg, S. Relationship between antimicrobial-resistance programs and antibiotic dispensing for upper respiratory tract infection: An analysis of Australian data between 2004 and 2015. J. Int. Med. Res. 2018, 46, 1326–1338. [Google Scholar] [CrossRef]
- Talat, U.; Schmidtke, K.; Khanal, S.; Chan, A.; Turner, A.; Horne, R.; Chadborn, T.; Gold, N.; Sallis, A.; Vlaev, I. A systematic review of nudge interventions to optimize medication prescribing. Front. Pharmacol. 2022, 13, 798916. [Google Scholar] [CrossRef]
- Abellan, J.M.; Jimenez-Gomez, D. Behavioral economics to improve lifestyle choices and to reduce risk factors. Gac. Sanit. 2020, 34, 197–199. [Google Scholar] [CrossRef]
- Ancker, J.S.; Gossey, J.T.; Nosal, S.; Xu, C.; Banerjee, S.; Wang, Y.; Veras, Y.; Mitchell, H.; Bao, Y. Effect of an Electronic Health Record “Nudge” on Opioid Prescribing and Electronic Health Record Keystrokes in Ambulatory Care. J. Gen. Intern. Med. 2021, 36, 430–437. [Google Scholar] [CrossRef]
- Gong, E.; Chukwuma, A.; Ghazaryan, E.; de Walque, D. Invitations and incentives: A qualitative study of behavioral nudges for primary care screenings in Armenia. BMC Health Serv. Res. 2020, 20, 1110. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.R.; Sexton, M.E.; Robichaux, C.; Wiley, Z.; Varkey, J.B.; Suchindran, S.; Albrecht, B.; Ashley Jones, K.; Fridkin, S.K.; Jacob, J.T. The impact of an electronic medical record nudge on reducing testing for hospital-onset Clostridioides difficile infection. Infect. Control Hosp. Epidemiol. 2020, 41, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Reynolds, T.L.; Zheng, K. Medication safety alert fatigue may be reduced via interaction design and clinical role tailoring: A systematic review. J. Am. Med. Inform. Assoc. 2019, 26, 1141–1149. [Google Scholar] [CrossRef]
- Sunstein, C.R. Nudges that fail. Behav. Public Policy 2017, 1, 4–25. [Google Scholar] [CrossRef]
- Brehm, S.S.; Brehm, J.W. Psychological Reactance: A Theory of Freedom and Control; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Capuzzo, M.; Rhodes, A. Should cost considerations be included in medical decisions? Not so sure…. Intensive Care Med. 2015, 41, 1844–1846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McNulty, S. The tragedy of the commons for the NHS? BMJ 2013, 346, f2238. [Google Scholar] [CrossRef]
- Castañeda-Orjuela, C.; García-Molina, M.; De la Hoz-Restrepo, F. Is There Something Else Beyond Cost-Effectiveness Analysis for Public Health Decision Making? Value Health Reg. Issues 2020, 23, 1–5. [Google Scholar] [CrossRef]
- Dyer, C. Let pharmacists make minor substitutions in event of drug shortages, say stakeholders. BMJ 2020, 370, m3657. [Google Scholar] [CrossRef]
- Pawson, R.; Tilley, N.; Tilley, N. Realistic evaluation; SAGE Publication: Thousand Oaks, CA, USA, 1997. [Google Scholar]

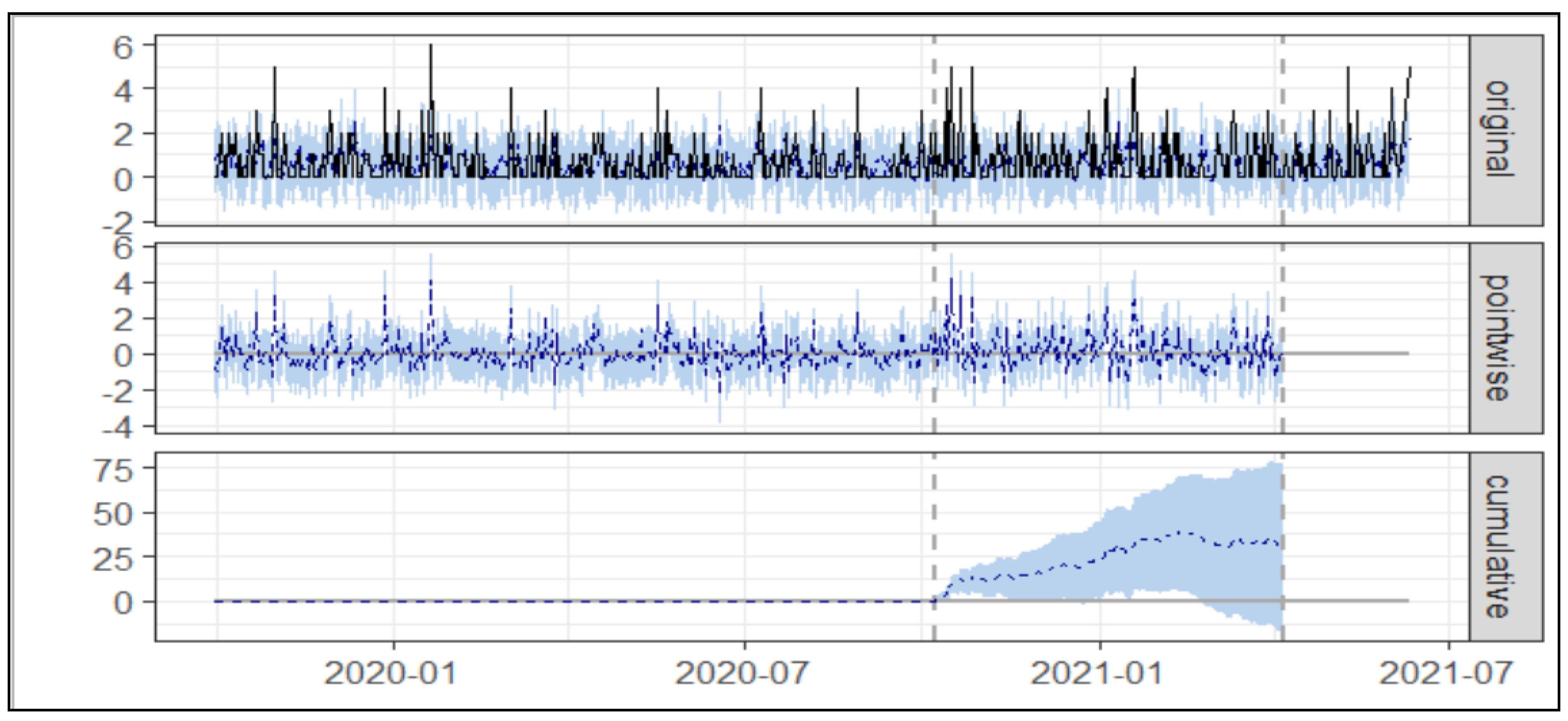
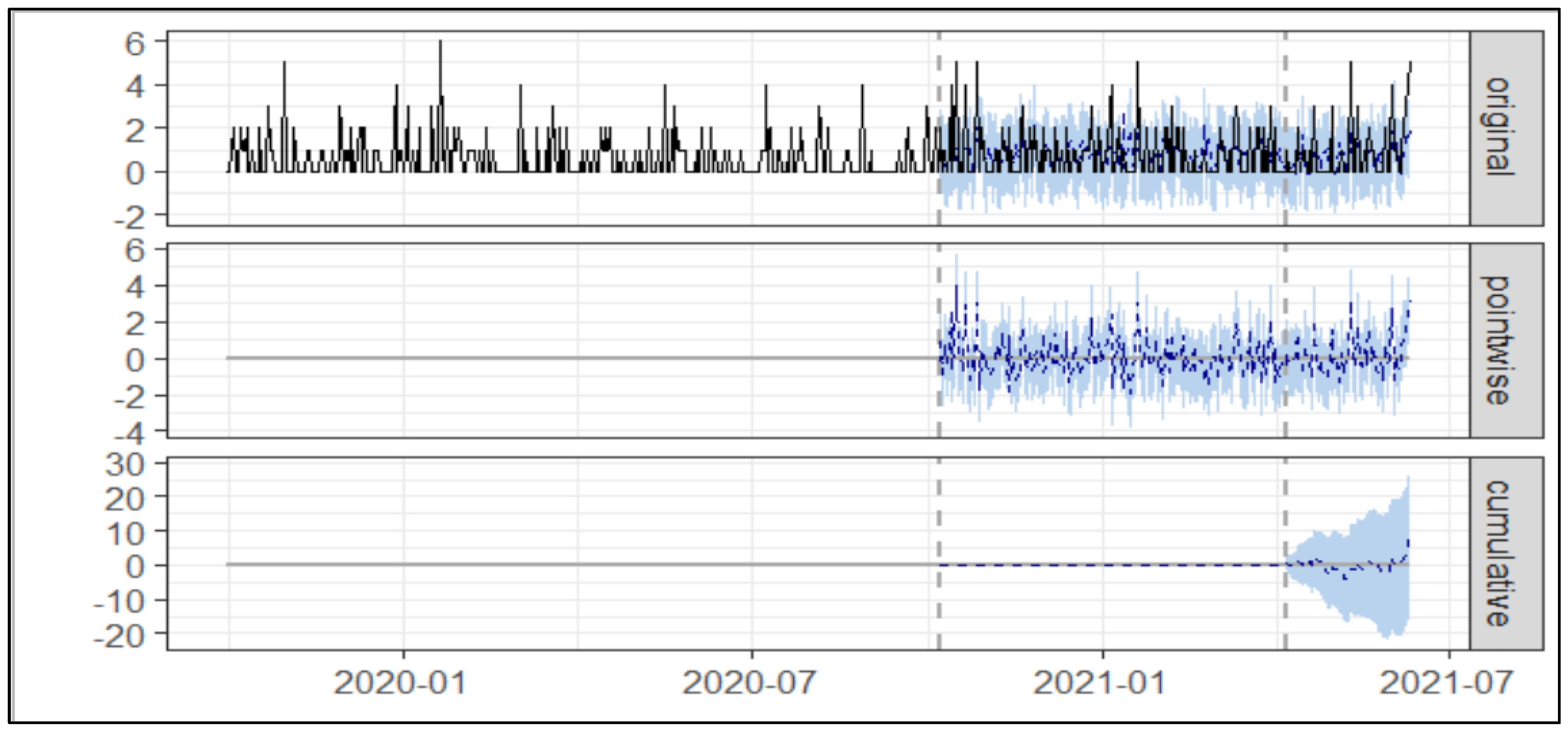


| Average No of Prescription (Asacol®) | Cumulative No of Prescription (Asacol®) | |
|---|---|---|
| Actual | 0.77 | 140.00 |
| Prediction (SD) | 0.6 (0.13) | 108.6 (23.19) |
| 95% CI | [0.34, 0.85] | [62.22, 154.23] |
| Absolute effect (SD) | 0.17 (0.13) | 31.35 (23.19) |
| 95% CI | [−0.08, 0.43] | [−14.23, 77.78] |
| Relative effect (SD) | 29% (21%) | 29% (21%) |
| 95% CI | [−13%, 72%] | [−13%, 72%] |
| Average No of Prescription (Asacol®) | Cumulative Number of Prescription (Asacol®) | |
|---|---|---|
| Actual | 0.80 | 52.00 |
| Prediction (SD) | 0.67 (0.17) | 43.28 (10.89) |
| 95% CI | [0.36, 1.00] | [23.21, 64.86] |
| Absolute effect (SD) | 0.13 (0.17) | 8.72 (10.89) |
| 95% CI | [−0.20, 0.44] | [−12.9, 28.79] |
| Relative effect (SD) | 20.00% (25.00%) | 20% (25%) |
| 95% CI | [−30.00%, 67.00%] | [−30%, 67%] |
| Average No of Prescription (Octasa®) | Cumulative No of Prescription (Octasa®) | |
|---|---|---|
| Actual | 3.22 | 582.0 |
| Prediction (SD) | 3.40 (0.24) | 614.60 (43.86) |
| 95% CI | [2.94, 3.91] | [532.48, 706.87] |
| Absolute effect (SD) | −0.18 (0.24) | −32.60 (43.86) |
| 95% CI | [−0.69, 0.27] | [−124.87, 49.52] |
| Relative effect (SD) | −5.30% (7.10%) | −5.30% (7.10%) |
| 95% CI | [−20.00%, 8.10%] | [−20.00%, 8.10%] |
| Average No of Prescription (Octasa®) | Cumulative No of Prescription (Octasa®) | |
|---|---|---|
| Actual | 3.10 | 200.00 |
| Prediction (SD) | 3.30 (0.24) | 215.00 (15.70) |
| 95% CI | [2.80, 3.80] | [183.80, 245.50] |
| Absolute effect (SD) | −0.23 (0.24) | −14.96 (15.62) |
| 95% CI | [−0.69, 0.22] | [−45.08, 14.00] |
| Relative effect (SD) | −7.00% (7.30%) | −7.00% (7.30%) |
| 95% CI | [−21.00%, 6.50%] | [−21.00%, 6.50%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, S.; Schmidtke, K.A.; Talat, U.; Sarwar, A.; Vlaev, I. Implementation and Evaluation of Two Nudges in a Hospital’s Electronic Prescribing System to Optimise Cost-Effective Prescribing. Healthcare 2022, 10, 1233. https://doi.org/10.3390/healthcare10071233
Khanal S, Schmidtke KA, Talat U, Sarwar A, Vlaev I. Implementation and Evaluation of Two Nudges in a Hospital’s Electronic Prescribing System to Optimise Cost-Effective Prescribing. Healthcare. 2022; 10(7):1233. https://doi.org/10.3390/healthcare10071233
Chicago/Turabian StyleKhanal, Saval, Kelly Ann Schmidtke, Usman Talat, Asif Sarwar, and Ivo Vlaev. 2022. "Implementation and Evaluation of Two Nudges in a Hospital’s Electronic Prescribing System to Optimise Cost-Effective Prescribing" Healthcare 10, no. 7: 1233. https://doi.org/10.3390/healthcare10071233
APA StyleKhanal, S., Schmidtke, K. A., Talat, U., Sarwar, A., & Vlaev, I. (2022). Implementation and Evaluation of Two Nudges in a Hospital’s Electronic Prescribing System to Optimise Cost-Effective Prescribing. Healthcare, 10(7), 1233. https://doi.org/10.3390/healthcare10071233






